Abstract
This study aimed to study the impact of a combination of maternal and post-weaning high-fat diets and whether resveratrol was beneficial. Sprague-Dawley dams were fed either chow or a high-fat diet, before mating, during pregnancy, and into lactation. At weaning, their offspring were randomly fed chow or a high-fat diet. Four experimental groups were generated: CC (maternal/postnatal chow diet), HC (maternal high-fat/postnatal chow diet), CH (maternal chow/postnatal high-fat diet), and HH (maternal/postnatal high-fat diet). A fifth group consisted of HH plus resveratrol. The 4 month-old offspring of HH group had higher body weight, higher levels of plasma triglycerides, leptin, angiotensin I and angiotensin II and abnormal intraperitoneal glucose tolerance test results, which fulfilled the features of metabolic syndrome. The dysregulation of the renin-angiotensin system was seen in multiple organs. Sirtuin 1 expression/abundance was reduced by a maternal/postnatal high-fat diet, in all the organs examined. Resveratrol ameliorated most of the features of metabolic syndrome and molecular alterations. The administration of a high-fat diet in both periods showed interactive metabolic effects in the plasma and many organs. Our results suggest that a maternal high-fat diet sensitizes offspring to the adverse effects of subsequent high-fat intake on multiple organs.
Similar content being viewed by others
Introduction
Over 2.1 billion adults are estimated to be overweight or obese, at present, of whom 38% are women of childbearing age1. Maternal obesity/a high-fat diet may predispose offspring to altered energy balance, obesity, and metabolic syndrome2,3,4,5. Metabolic syndrome is a compilation of risk factors, including hypertension, dyslipidemia, obesity and insulin resistance. Metabolic syndrome is now considered a disease related to developmental disorder6. Previously conducted studies showed that a maternal high-fat diet, followed by a postnatal high-fat diet, increased the risk of metabolic syndrome7,8. However, the mechanistic link between mothers who are obese/on a high-fat diet and offspring with metabolic syndrome is not yet completely understood.
Sirtuin 1 (SIRT1) is a prototype mammalian NAD(+)-dependent protein deacetylase that has emerged as a key metabolic sensor in various metabolic tissues. Growing evidence suggests that SIRT1 regulates glucose and lipid metabolism through its deacetylase activity9. Moreover, accumulating evidence supports that obesity with chronic inflammation is associated with low levels of NAD+ and SIRT110. SIRT1 might be a new therapeutic target for the prevention of diseases related to insulin resistance, such as metabolic syndrome and type 2 diabetes mellitus.
The renin-angiotensin system (RAS) contains many angiotensin peptides and can locally act in almost all the tissues of the body11. The RAS is closely associated with metabolic syndrome, and the inhibitors of the RAS have been shown to be able to prevent the onset of type 2 diabetes, in high-risk populations12. Previously conducted studies showed that SIRT1 and the RAS are mutually regulated in various cells/tissue13,14,15,16. Guberman et al. suggested that a maternal high-fat diet induced persistent alterations in the adipose RAS components of offspring, and this was further exacerbated by a postnatal high-fat diet17.
Resveratrol, a natural polyphenolic compound produced by plants in response to environmental stress, is found in red grape skin, peanuts, a variety of berries and medical plants, and has gained special interest as a calorie-restriction mimetic, based on data from rodents. The biological role of resveratrol is to initiate the activation of SIRT1, which epigenetically modifies and inactivates the acetylation of inflammatory proteins. When rodents were fed a high-fat diet, resveratrol treatment improved glucose homeostasis, mitochondrial function, lipid parameters, body weight, and survival18.
In the current study, we aimed to study the long-term effects of a combined maternal and postnatal high-fat diet on multiple organs, including adipose tissue, the pancreas, and the dorsal hippocampus. Particularly, we evaluated SIRT1, the RAS, and the effects of resveratrol.
Results
Body weight, calorie intake and biochemistry
Female rats on a high-fat diet were heavier than those in the control group since 1 week after taking a different diet (Supplemental Fig. 1A). Mating was arranged after 5 weeks on a different diet. At this time, female rats taking a high-fat diet had higher plasma aspartate aminotransferase (AST), alanine transaminase (ALT) and leptin levels (Table 1). There was no significant difference in the plasma total cholesterol (Chol), high-density lipoprotein (HDL), glucose, or triglyceride (TG) levels (Table 1).
The body weights of 2-day-old male offspring were lower in the group born to mothers taking a high-fat diet (Supplemental Fig. 1B). The offspring were weaned at 0.75 month of age, and were assigned to either the chow diet or high-fat diet group, from weaning until 4 months of age., We found that the body weight of the 2-month-old offspring was affected by postnatal high-fat diet. At 4 months of age, we found that the body weight and the plasma leptin level of the offspring were affected by both a maternal high-fat diet and postnatal high-fat diet. In addition, an interaction between maternal high-fat and postnatal high-fat diet, in terms of body weight and plasma leptin levels, was observed Post hoc analysis showed higher body weight and plasma leptin levels in the HH group than the CH group. These data indicated that maternal obesity/a high-fat diet led to increased body weight and plasma leptin levels, and sensitized offspring to a second hit, i.e., a postnatal high-fat diet. A Mann-Whitney U test showed that resveratrol treatment significantly reduced the plasma leptin level and body weight in the HH group. In regards to calorie intake per day from 2 to 4 months of age, a postnatal high-fat diet effect was observed; however, no effect of maternal high-fat diet was noted. Two-way analysis of variance (ANOVA) showed no significant interaction about calorie intake per day between maternal high-fat diet and postnatal high-fat diet. Mann-Whitney U test showed that rats in the resveratrol treatment group had lower daily calorie intake then the HH group. The plasma total Chol and TG levels were affected by both a maternal and postnatal high-fat diet. Resveratrol reversed the increased total Chol and TG levels in the HH group. In terms of plasma AST, ALT and HDL levels, a postnatal high-fat diet treatment effect was observed. Mann-Whitney U test showed that resveratrol treatment could reverse the increased plasma ALT and HDL levels in the HH group (Table 2).
Blood pressure
The systolic, diastolic and mean BP increased through the consumption of a postnatal high-fat diet but were not affected by a maternal high-fat diet. Two-way ANOVA showed no significant interaction between maternal obesity/a high-fat diet and a postnatal high-fat diet. Resveratrol could reverse the increased systolic, diastolic and mean BP in the HH group (Table 2).
Intraperitoneally injected glucose tolerance test (IPGTT)
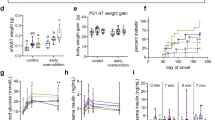
In terms of glucose AUC, two-way ANOVA showed no effect of maternal high-fat diet but a significant effect on postnatal high-fat diet. Resveratrol could reverse the sugar AUC value in the HH group. In terms of insulin AUC, two-way ANOVA showed both significant effects of maternal high-fat diet and postnatal high-fat diet. Resveratrol could reverse the insulin AUC value in the HH group (Fig. 1).
Intraperitoneal glucose tolerance test (IPGTT). (A) Serum glucose kinetics (B) Area under glucose curve (C) Serum insulin kinetics (D) Area under insulin curve during the IPGTT. Five experimental groups (n = 13–14 per group): CC: maternal rat chow diet + postnatal chow diet; HC: maternal obesity/high-fat diet + postnatal chow diet; CH: maternal rat chow diet + postnatal high-fat diet; HH: maternal obesity/high-fat diet + postnatal high-fat diet; HHR: maternal obesity/high-fat diet + postnatal high-fat diet + resveratrol. Hit 1, maternal high-fat diet; Hit 2, postnatal high-fat diet. Two-way ANOVA was used to assess the statistical significance of differences among groups and the therapeutic effect of resveratrol was evaluated by Mann-Whitney U test. **P < 0.01.
Plasma angiotensin I and angiotensin II
The plasma angiotensin I levels were affected (they increased) both by a maternal high-fat diet and postnatal diet. Resveratrol could reverse the increased plasma angiotensin I levels in the HH group. An interaction between maternal high-fat and postnatal high-fat diet, for the 4-m plasma angiotensin II levels, was observed. Post hoc analysis showed higher plasma angiotensin II levels in the HH group than in the CH group. These data indicate that maternal obesity/high-fat diet increased the plasma angiotensin II levels, and sensitized offspring to a second hit, i.e., a postnatal high-fat diet. Resveratrol could reverse the increased plasma angiotensin I and II levels in the HH group (Fig. 2).
Plasma angiotensin I and II levels. (A) Angiotensin I level (B) Angiotensin II level. Two-way ANOVA was used to assess the statistical significance of differences among groups and the therapeutic effect of resveratrol was evaluated by Mann-Whitney U test. n = 13 per group in (A) and 6–8 per group in (B). *P < 0.05.
SIRT1
The SIRT1 mRNA expressions of adipose tissue were affected by a postnatal high-fat diet but not by a maternal high-fat diet (Fig. 3). By Western blot (WB), it was found that the fat SIRT1 abundance was not affected either by a maternal high-fat diet or postnatal high-fat diet. Resveratrol did not change the fat SIRT1 mRNA expressions or abundance in the HH group (Fig. 3). The SIRT1 mRNA expressions in the pancreas were not significantly affected by maternal or postnatal high-fat diets. By WB, the SIRT1 expressions of the pancreas were found to be affected by a maternal high-fat diet but not a postnatal high-fat diet. Resveratrol could increase the pancreas SIRT1 mRNA expressions and abundance in the HH group (Fig. 3). We checked the SIRT1 expressions of the dorsal hippocampus because previous studies reported that saturated fat and refined sugar intake was associated with poorer hippocampal-dependent memory function19, while the dorsal hippocampus is related to memory processes20.We found the SIRT1 mRNA expressions of the dorsal hippocampus were not affected by maternal or postnatal high-fat diets. By WB, the SIRT1 abundance of the dorsal hippocampus was found to be affected (decreased) by a postnatal high-fat diet but not by a maternal high-fat diet. Resveratrol could reverse the decrease in the dorsal hippocampus SIRT1 abundance, in the HH group (Fig. 3).
Sirtuin 1 (SIRT1) mRNA expression and abundance. (A) fat (B) pancreas (C) dorsal hippocampus. Two-way ANOVA was used to assess the statistical significance of differences among groups and the therapeutic effect of resveratrol was evaluated by Mann-Whitney U test. Representative immunoblots and densitometric quantification of SIRT-1 are presented. Values are mean ± SEM. Represented full blots are presented in Supplementary Figure S2. n = 11–13 per group in SIRT1 mRNA expression and n = 6–8 in SIRT1 protein amount. There were one outlier in the pancreas and two outliers in the dorsal hippocampus SIRT1 protein abundance, respectively. *P < 0.05; **P < 0.01.
RAS
The AT1R, ACE and ACE2 expressions of adipose tissue were affected (decreased) by a postnatal high-fat diet but not by a maternal high-fat diet. The AT2R expressions of the adipose tissue were affected (decreased) by a maternal high-fat diet but not a postnatal high-fat diet. Resveratrol could reverse the decreased AT2R and ACE2 expressions, and increase the MAS expressions in the HH group (Fig. 4). The ACE expressions of the pancreas were affected (increased) by a postnatal high-fat diet but not by a maternal high-fat diet. The ACE2 expressions were affected (but decreased) by a maternal high-fat diet but not by a postnatal high-fat diet. Resveratrol could reverse the increased ACE expression in the HH group (Fig. 5). The AT1R and AT2R expressions of the dorsal hippocampus were both affected (decreased) by a postnatal high-fat diet but not by a maternal high-fat diet. Resveratrol could reverse the expressions of AT1R and AT2R in the HH group (Fig. 6).
AT1R, AT2R, ACE, ACE2 and MAS in fat. Two-way ANOVA was used to assess the statistical significance of differences among groups and the therapeutic effect of resveratrol was evaluated by Mann-Whitney U test. n = 11–13 per group. *P < 0.05. AT1R, angiotensin II type I receptor; AT2R, angiotensin II type II receptor; ACE, Angiotensin-converting enzyme; ACE2, Angiotensin-converting enzyme 2.
Discussion
We report, here, that maternal obesity/high-fat diet interacts with postnatal high-fat diet to induce features of metabolic syndrome, and resveratrol could alleviate most of the symptoms. Our study showed that: (1) a combination of a maternal high-fat diet and postnatal high-fat diet led to the greatest metabolic disruption; (2) there was an interaction between maternal high-fat and postnatal high-fat diets, in terms of the body weight at 4 months of age, serum leptin level and angiotensin II level; (3) resveratrol could ameliorate the increased body weight, levels of serum ALT, Chol, TG, HDL, leptin, angiotensin I and II, and glucose and insulin AUC values in the HH group; (4) Resveratrol could increase the SIRT1 abundance of the pancreas and dorsal hippocampus in the HH group; (5) the AT2R expressions of fat were decreased through a maternal high-fat diet and the AT2R expressions of the dorsal hippocampus were decreased through a postnatal high-fat diet. Resveratrol could increase the AT2R expression of fat and the dorsal hippocampus in the HH group; (6) the ACE2 expressions of fat were decreased by a postnatal high-fat diet. Resveratrol could increase the ACE2 expression of fat in the HH group; (7) the ACE expressions of the pancreas were increased by a postnatal high-fat diet. Resveratrol could decrease the ACE expression of the pancreas in the HH group.
An increasing number of studies in rodents has demonstrated that offspring-exposure to maternal obesity/overnutrition, during both pregnancy and lactation, leads to a predisposition to a greater increase in adiposity and metabolic dysregulation, than in the case of control dams in which the offspring themselves are challenged with a high-fat diet, after weaning2,3,7,21,22,23,24. Here, we present a wide spectrum of the metabolic syndrome in rat offspring born to high-fat dams, and having a postnatal high-fat diet. Moreover, we showed that resveratrol can ameliorate most of the features of metabolic syndrome in the HH group. These are consistent with previous reports that resveratrol can improve glucose tolerance and insulin sensitivity25,26. Resveratrol is well-known to have a wide variety of effects including anti-oxidant, anti-inflammatory properties and increased mitochondrial biogenesis thereby ameliorates diabetes27,28. Interestingly, we also found resveratrol treatment group rats had lower daily calories intake. Therefore, the improvement of blood pressure, leptin, glucose intolerance, etc. may be attributable to appetite suppression related weight loss.
SIRT1 is an important modulator of the maturation and remodeling of adipose tissues29. Recently conducted studies also showed that SIRT1 promotes fat mobilization, and stimulates brown remodeling of white fat in white adipose tissue29, and the genetic ablation of SIRT1 in adipose tissue leads to increased adiposity and insulin resistance25. In addition, a high-fat diet was also reported to trigger the inflammation-induced cleavage of SIRT1 in adipose tissue, to promote metabolic dysfunction25. SIRT1 has been also shown to be a positive regulator for pancreatic insulin secretion, which also triggers glucose uptake and utilization. The promotion of insulin secretion was thought to occur through the transcriptional repression of uncoupling protein 229. The activation of SIRT1 by its activators (e.g. resveratrol) may protect against high-fat-induced obesity and insulin resistance25,27,30. We previously showed a combination of a maternal and lactation high-fat diet and postnatal high-fat diet resulted in cognition deficit and derangement of the mediators involved in cognition, in the dorsal hippocampus of the adult offspring31. Heyward et al. showed that mice maintained on a high-fat diet present with impaired hippocampus-dependent spatial memory that may be mediated by the neuroepigenetic dysregulation of SIRT1, within the hippocampus32. In our study, the SIRT1 mRNA expression was decreased in the fat tissue by a postnatal high-fat diet and the SIRT1 abundance of the pancreas and dorsal hippocampus were decreased by a maternal high fat diet and postnatal high-fat diet, respectively. The change of SIRT1 mRNA expression was not all the same across groups may be due to different sensitivity to insult among organs. In addition, resveratrol could restore the SIRT1 abundance of the pancreas and dorsal hippocampus. The divergent effect of resveratrol on SIRT1 mRNA expression and protein abundance across the organs may be related with the dose and duration of resveratrol treatment.
White adipose tissue expresses all the critical elements of the RAS, and is a predominant source of circulating angiotensinogen33,34. It can generate local angiotensin II to stimulate AT1R and AT2R on adipocytes and other cells within adipose tissue, and contribute to angiotensin II activity on more distant tissues12. AT2R is involved in early adipocyte differentiation. AT2R activation can restore normal adipocyte morphology, and improve insulin sensitivity35. RAS activation is also closely correlated to both insulin resistance and β cell dysfunction36. The underlying mechanism behind this deleterious effect was thought to be related to the negative regulation exerted by angiotensin II through AT1R of several steps of the insulin signaling cascade37. The increase in the ACE2/Ang (1–7)/Mas receptor axis could be associated with diminished insulin resistance, through the induction of the activation of insulin-signaling pathways and counteraction of the inhibitory effects of ACE/Ang II/AT1R38. The central nervous system (CNS) plays an integral role in maintaining this balance, as it receives and integrates peripheral signals regarding the status of the energy stores and responds to these signals39,40,41. Numerous neuropeptides and other factors influence energy balance by acting directly in the CNS, and accumulating evidence implicates the RAS in this process12,40.
In our study, the AT2R expressions were decreased in the fat tissue by maternal high fat diet. The ACE expression of the pancreas was increased by a postnatal high-fat diet and the AT2R expression was decreased in dorsal hippocampus by a postnatal high-fat diet. In addition, resveratrol could reverse them which suggested that resveratrol may ameliorate high-fat diet-induced glycemic dysregulation through regulating ACE/ACE2/AT2R axis.
Oliveira Andrade et al. reported that high-fat feed mice had an interaction between angiotensin-(1–7)/Mas axis and sirtuins in the adipose tissue42. Clarke et al. found that SIRT1 was up-regulated after 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) treatment, but, conversely, was down-regulated after IL-1β treatment. Chromatin immunoprecipitation analysis demonstrated that SIRT1 was bound to the ACE2 promoter, and that while the binding increased after AICAR treatment, it decreased after IL-1β treatment43. In our study, the AT2R and ACE2 expressions were corrected by resveratrol, and thus further explained the close relationship between SIRT1 and RAS.
Many studies have reported the important role angiotensin II plays in connecting insulin resistance and the RAS44. Richey et al. indicated that an intravenous infusion of angiotensin II induced insulin resistance45 and Furuhashi et al. reported that the blockade of the RAS improved insulin sensitivity46. Moreover, Santos and colleagues reported that Mas-knockout mice presented with dyslipidemia, as well as increased levels of insulin and leptin, and that Mas deletion led to glucose intolerance and reduced insulin sensitivity47. In our study, we found that the plasma angiotensin I and angiotensin II levels were increased in the 4 m/o offspring of maternal high-fat and postnatal high-fat diets, and that resveratrol can reverse these, which hint resveratrol may improve high fat-diet-induced metabolic syndrome by decreasing the levels of angiotensin I and angiotensin II.
Our study has a few limitations. First, we did not examine different doses or therapeutic durations of resveratrol. Given that gene–diet interactions vary during different developmental windows, it would be interesting to study whether various therapeutic protocols of resveratrol lead to differential protection on maternal or postnatal high fat-induced adult diseases. Moreover, we haven’t ruled out a reduction in food intake with resveratrol contributing the “normalization” of blood pressure, body weight and leptin, etc. In this connection, a pair-feeding group would allow us to assess the effect of resveratrol independent of food intake. Next, we did IPGTT but not oral glucose tolerance test; this will neglect the effect of incretins. In addition, further study is necessary to measure SIRT1 deacetylase activity and other pathways activated by resveratrol e.g. AMPK.
In conclusion, exposure to a maternal high-fat diet and a post-weaning, high-fat diet appears to “poise” offspring to be hyper-responsive to a high-fat diet, thereby promoting the development of the features related to metabolic syndrome. High-fat diets during both periods resulted in supplementary effects on body weight and plasma leptin and angiotensin II levels. Our findings present important points in the understanding of the complexity of the fetal programming process, and might be particularly useful in the search for efficient therapies against malprogramming. Further studies should be performed to clarify multiple-organ crosstalk, and the interaction of pathogenic mediators in the process of nutritional programming.
Methods
Animals and experimental design
This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committee of the Kaohsiung Chang Gung Memorial Hospital approved the protocol. Virgin Sprague-Dawley (SD) rats (BioLASCO Taiwan Co., Ltd., Taipei, Taiwan) were housed and maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The rats were housed in standard rat cage at 22 °C, with a relative humidity of 55%, in a 12 h light/12 h dark cycle.
Two months-old female rats were weight-matched and assigned to receive either a normal diet of regular rat chow (control diet; Fwusow Taiwan Co., Ltd., Taichung, Taiwan; (59.7% carbohydrates, 27.5% protein, 12.6% fat by energy, 3.25 kcal/gm) or a high-fat hypercaloric diet (high-fat diet; D12331, Research Diets, Inc., New Brunswick, NJ, USA; 58% fat [hydrogenated coconut oil], 16.4% protein plus high sucrose [25% carbohydrate] by energy, 5.56 kcal/gm) ad libitum, for 5 weeks, before mating as well as during gestation and lactation. Body weight and food intake were measured daily.
We elected to study only male offspring because hypertension, an important component of metabolic disease, occur at an earlier age and at a higher rate in males than females48,49. The offspring were weaned at 0.75 month of age, and onto either a normal diet or high-fat diet ad libitum, from weaning until 4 months of age. Five experimental groups (n = 13–14 per group) were generated: maternal control diet/postnatal rat chow normal fat diet (CC), maternal high-fat diet/postnatal rat chow normal fat diet (HC), maternal control diet/postnatal high-fat diet (CH), and maternal high-fat diet/postnatal high-fat diet (HH); in addition, a therapeutic group, with resveratrol in drinking water from 2 to 4 months of age, on a maternal high-fat diet/postnatal high-fat diet was raised for comparison (HHR). Resveratrol was prepared twice weekly by dissolving the drug (50 mg) in 5.5 ml of 20% cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA). This solution was then diluted with water. The final concentration is 50 mg/L. Water bottles were wrapped with aluminum foil to protect from light50. The mean dose of resveratrol ingested was 10 mg/kg/day.
IPGTT
After an 8-h fast at postnatal days ~114, blood samples were collected at five time points: before injection and at 15, 30, 60, and 120 min after the intraperitoneal (i.p.) injection of glucose (2 g/kg body weight). Plasma glucose levels were immediately measured using the enzymatic (hexokinase) method, with a glucose assay kit. Serum insulin levels were checked using enzyme-linked immunosorbent assay (Crystal Chem Inc., Downers Grove, IL, USA), as previously reported51.
Blood pressure
Blood pressure was determined in conscious 4 months of age male offspring using an indirect tail-cuff method (BP-2000; Visitech Systems, Inc., Apex, NC, USA) after being systematically trained as previously described52.
Tissue collection and blood sampling
At the age of 4 months, animals were weighed, and then euthanized under isoflurane, by cervical dislocation. The retroperitoneal adipose tissue, pancreas and brain’s dorsal hippocampus were collected. Enzyme-linked immunosorbent assays for the plasma, including TG, Chol, HDL AST, ALT, angiotensin I, angiotensin II and leptin, were performed according to the manufacturers’ protocols.
Real-time PCR analysis
Briefly, RNA was extracted using TRI Reagent (Sigma, St. Louis, MO, USA), treated with DNase I (Ambion, Austin, TX, USA) to remove DNA contamination, and 2 µg was reverse transcribed (SuperScript II RNase H–-Reverse Transcriptase, Invitrogen, Bethesda, MD, USA) with random primers (Invitrogen), in a total volume of 40 µl, as we have already published53. Control RT reactions were performed by omitting the RT enzyme, and PCR was amplified to ensure that DNA did not contaminate the RNA. Two-step quantitative real-time PCR was conducted using Quantitect SYBR Green PCR Reagents (Qiagen, Valencia, CA, USA), according to the manufacturer’s protocol on a LightCycler® 480 Real-Time PCR System (Roche Diagnostics Ltd., Taipei, Taiwan). Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as a reference. (Supplemental Table 1 shows the primer sequences used in real-time PCR. All samples were run in duplicate (2.5 µl of cDNA/well in a 96-well format). For the relative quantification of gene expression, the comparative threshold cycle (Ct) method was employed. The averaged CT was subtracted from the corresponding averaged GADPH value for each sample, resulting in ΔCt. ΔΔCt was achieved by subtracting the average control ΔCt value from the average experimental ΔCT. The fold-increase was established by calculating the 2−ΔΔCT for the experimental vs. control samples.
WB
WB analysis was done as previously described54. Total protein extracts from homogenized cultured cells and liver were lysed in ice-cold RIPA buffer with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). After centrifugation, the protein concentrations in the supernatants were determined by the DC protein assay kit (Bio-Rad, Hercules, CA, USA). The Western blotting technique was performed to quantify the protein density of SIRT1. We used rabbit anti-rat SIRT-1 antibody (Millipore, Billerica, MA, USA), followed by secondary goat anti-mouse antibody. Bands of interest were visualized using enhanced chemiluminescence (ECL) reagents (PerkinElmer, Waltham, MA, USA) and quantified by densitometry (Quantity One Analysis software; Bio-Rad, Hercules, CA, USA) as the integrated optical density after subtraction of background. The integrated optical density was normalized to Ponceau red staining (Pon S) to correct for any variations in total protein loading. The protein abundance was represented as integrated optical density/Pon S.
Statistical analysis
Results were analyzed using two-way ANOVA (maternal diet x postweaning diet), followed by LSD post hoc tests if the interaction was significant in the first four groups. The therapeutic effect of resveratrol was evaluated by Mann-Whitney U test between HH and HHR group. For all the variables measured, outliers which lay 1.5 interquartile ranges (IQRs) below the first quartile or 1.5 IQRs above the third quartile were removed from the analysis. All analyses were performed using Statistical Package for the Social Sciences (SPSS) software. Values were expressed as mean ± SEM. Significance was defined as P < 0.05 for all tests.
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 384, 766–81 (2014).
Bayol, S. A., Farrington, S. J. & Stickland, N. C. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 98, 843–51 (2007).
Chen, H., Simar, D. & Morris, M. J. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One. 4, e6259 (2009).
Ainge, H., Thompson, C., Ozanne, S. E. & Rooney, K. B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond). 35, 325–35 (2011).
Ryckman, K. K., Borowski, K. S., Parikh, N. I. & Saftlas, A. F. Pregnancy Complications and the Risk of Metabolic Syndrome for the Offspring. Curr Cardiovasc Risk Rep. 7, 217–23 (2013).
Gluckman, P. D. & Hanson, M. A. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 15, 183–7 (2004).
Parente, L. B., Aguila, M. B. & Mandarim-de-Lacerda, C. A. Deleterious effects of high-fat diet on perinatal and postweaning periods in adult rat offspring. Clin Nutr. 27, 623–34 (2008).
Ito, J. et al. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 151, 70–5 (2016).
Colak, Y. et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 17, HY5–9 (2011).
Moschen, A. R. et al. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. J Hepatol. 59, 1315–22 (2013).
Skov, J., Persson, F., Frøkiær, J. & Christiansen, J. S. Tissue Renin-Angiotensin systems: a unifying hypothesis of metabolic disease. Front Endocrinol (Lausanne). 5, 23 (2014).
de Kloet, A. D., Krause, E. G. & Woods, S. C. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 100, 525–34 (2010).
Miyazaki, R. et al. Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 28, 1263–9 (2008).
Pantazi, E. et al. Losartan activates sirtuin 1 in rat reduced-size orthotopic liver transplantation. World J Gastroenterol. 21, 8021–31 (2015).
Yang, S. Y. et al. Downregulation of angiotensin type 1 receptor and nuclear factor-κB by sirtuin 1 contributes to renoprotection in unilateral ureteral obstruction. Sci Rep. 6, 33705 (2016).
Gu, J. et al. Olmesartan prevents microalbuminuria in db/db diabetic mice through inhibition of angiotensin II/p38/SIRT1-induced podocyte apoptosis. Kidney Blood Press Res. 41, 848–64 (2016).
Guberman, C., Jellyman, J. K., Han, G., Ross, M. G. & Desai, M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol. 209, 262 (2013).
Diaz-Gerevini, G. T. et al. Beneficial action of resveratrol: How and why? Nutrition. 32, 174–8 (2016).
Francis, H. M. & Stevenson, R. J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 125, 943–55 (2011).
Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65, 7–19 (2010).
Page, K. C., Malik, R. E., Ripple, J. A. & Anday, E. K. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physio Reg Integr. 297, R1049–57 (2009).
Rajia, S., Chen, H. & Morris, M. J. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J Neuroendocrinol. 22, 905–14 (2010).
Torrens, C. et al. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS One. 7, e50671 (2012).
Desai, M. et al. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol. 211, 237 (2014).
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444, 337–42 (2006).
Um, J. H. et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 59, 554–63 (2010).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 127, 1109–22 (2006).
Szkudelski, T. & Szkudelska, K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 1852, 1145–54 (2015).
Li, X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai). 45, 51–60 (2013).
Milne, J. C. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 450, 712–6 (2007).
Li, S. W. et al. Combined maternal high-fat diet in pregnancy and lactation and a postnatal high-fat diet leads to metabolic syndrome with spatial deficit: the effects of resveratrol. Oncotarget. 8, 111998–2013 (2017).
Heyward, F. D. et al. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. J Neurosci. 36, 1324–35 (2016).
Chalkiadaki, A. & Guarente, L. High-fat diet triggers inflammation induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–8 (2012).
Mallow, H., Trindl, A. & Loffler, G. Production of angiotensin II receptors type one (AT1) and type two (AT2) during the differentiation of 3T3-L1 preadipocytes. Horm Metab Res. 32, 500–3 (2000).
Shum, M. et al. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab. 304, E197–210 (2013).
Leung, P. S. Pancreatic renin-angiotensin system: a novel target for the potential treatment of pancreatic diseases? JOP. 4, 89–91 (2003).
Velloso, L. A., Folli, F., Perego, L. & Saad, M. J. The multi-faceted crosstalk between the insulin and angiotensin II signaling systems. Diabetes/Metab Res Rev. 22, 98–107 (2006).
Giani, J. F. et al. Chronic infusion of angiotensin-(1–7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab. 296, E262–71 (2009).
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature. 404, 661 (2000).
Woods, S. C., Seeley, R. J., Porte, D. Jr. & Schwartz, M. W. Signals That Regulate Food Intake and Energy Homeostasis. Science. 280, 1378–83 (1998).
Seeley, R. J. & Woods, S. C. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 4, 901 (2003).
Oliveira Andrade, J. M. et al. Cross talk between angiotensin-(1–7)/Mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides. 55, 158–65 (2014).
Clarke, N. E., Belyaev, N. D., Lambert, D. W. & Turner, A. J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond). 126, 507–16 (2014).
Matayoshi, T. et al. Relationship between insulin resistance and the renin-angiotensin system: analysis for patients with essential and renovascular hypertension. Clin Exp Hypertens. 29, 479–87 (2007).
Richey, J. M. et al. Angiotensin II induces insulin resistance independent of changes in interstitial insulin. Am J Physiol. 277, 920–6 (1999).
Furuhashi, M. et al. Blockade of the renin-angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J Hypertens. 22, 1977–82 (2004).
Santos, S. H. et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 57, 340–7 (2008).
Reckelhoff, J. F. Gender differences in the regulation of blood pressure. Hypertension. 37, 1199–208 (2011).
Tain, Y. L. et al. High fat diets sex-specifically affect the renal transcriptome and program obesity, kidney injury, and hypertension in the offspring. Nutrients. 9, E357 (2017).
Tain, Y. L. et al. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J Nutr Biochem. 48, 120–7 (2017).
Chen, Y. C. et al. Prenatal Dexamethasone Exposure Programs the Development of the Pancreas and the Secretion of Insulin in Rats. Pediatr Neonatol. 58, 135–44 (2017).
Tain, Y. L. et al. Maternal Melatonin Therapy Rescues Prenatal Dexamethasone and Postnatal High-Fat Diet Induced Programmed Hypertension in Male Rat Offspring. Front Physiol. 6, 377 (2015).
Sheen, J. M. et al. Programming Effects of Prenatal Glucocorticoid Exposure with a Postnatal High-Fat Diet in Diabetes Mellitus. Int J Mol Sci. 7, 533 (2016).
Sheen, J. M. et al. Combined intraperitoneal and intrathecal etanercept reduce increased brain tumor necrosis factor-alpha and asymmetric dimethylarginine levels and rescues spatial deficits in young rats after bile duct ligation. Front Cell Neurosci. 10, 167 (2016).
Acknowledgements
This work was supported by grants CMRPG8F0121 (J.M. Sheen), CMRPG8F0111 and CMRPG8G0631 (L.T. Huang), CMRPG8F0161 (Y.L. Tain), CMRPG8F0141, and CMRPG8G0261 (H.R. Yu), from Chang Gung Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Contributions
S.J.M. and Yu. H.R. wrote the first draft of the manuscript. T.Y.L., T.W.L., and T.M.M., revised the subsequent drafts. L.I.C and T.C.C. performed statistical analyses. L.Y.J. and H.L.T. critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheen, JM., Yu, HR., Tain, YL. et al. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: a multiple-organ study. Sci Rep 8, 5607 (2018). https://doi.org/10.1038/s41598-018-24010-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24010-0
This article is cited by
-
Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet
Lipids in Health and Disease (2023)
-
Maternal and offspring high-fat diet leads to platelet hyperactivation in male mice offspring
Scientific Reports (2021)
-
Sexual dimorphism in rats exposed to maternal high fat diet: alterations in medullary sympathetic network
Metabolic Brain Disease (2021)
-
Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue
Lipids in Health and Disease (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.