Abstract
Reversible colour change of leuco dye-based composites is in general closely related to their phase change, thus the two phenomena should occur at around the same temperature and should be influenced similarly. However, spatial confinement of the analysed sample affects the change in colour differently compared to its phase transition and the most pronounced effects can be observed during cooling. The bulk composite is coloured while still liquid and the colour hysteresis does not exhibit a loop. In an open-porous medium the colouration coincides well with the crystallization and the colour hysteresis widens to about 4 °C. Microencapsulated composite exhibits two crystallization processes, one of them taking place at the bulk crystallization temperature and the other one at about 20 °C lower. Under such conditions the composite is coloured just before the onset of the second crystallization, i.e. about 15 °C below crystallization in the bulk, and the corresponding colour hysteresis widens to 18 °C. The two crystallization forms are thermally independent and have the same crystalline structure. These effects should be taken into account when designing future applications where the phase-changing materials are implemented.
Similar content being viewed by others
Introduction
Organic thermochromic (TC) materials are applied in a wide range of commercial products such as in colour-changing textiles1,2,3,4, for temperature control of a foldable paper or textile5 and even for colour-changing wood veneers6. In these applications the TC material is used in a spatially confined form. Most frequently, microencapsulation is used to produce colour-changing “pigments” suitable for printing inks.
During the last decades, the leuco dye-based TC materials have received substantial attention from both researchers and industry. In these materials, the temperature-induced colour change occurs due to molecular rearrangement of colour formers (leuco dyes) in the presence of a developer and this process is controlled by a phase change of the co-solvent. The molecular mechanisms were thoroughly studied in fully coloured as well as in totally decoloured states, i.e. in solid and liquid states of the composite, respectively7,8,9,10,11,12,13,14. However, a leuco dye-based TC composite can solidify without changing its colour, and the colourless solid state can exist within a reasonably wide temperature region15. Such a state has not yet been studied in more detail.
The colour change as a function of temperature produces a hysteresis which is very narrow for TC composites and much wider for printing inks where these are microencapsulated to protect the phase-changing material (PCM) from its surrounding16,17,18,19. The shape of the colour hysteresis provides the first evidence of changes in the TC composite, such as poor fastness against light and high temperatures18. To the best of our knowledge, the relationship between the phase- and colour changes has not yet been analysed.
The phase changes of leuco dye-based TC composites are controlled by the applied co-solvent, which can be a long-chain alkyl alcohol, ester, ketone, ether, or acid1,2. Such PCMs were extensively investigated to determine the relationship between their structure and energy storage properties in eutectic mixtures for the solid-liquid transition20,21,22,23,24. PCMs are commonly used in a microencapsulated form1,25,26,27,28,29,30 but this can change their thermal properties when measured in the bulk31. In many cases, supercooling lowers crystallization temperature, depending on the type of polymer shell and the size of microcapsules32,33,34,35. A greatly reduced number of nucleation sites induces an independent crystallization within isolated parts of the material, known as homogeneous nucleation. Different nucleation efficiency in microcapsules of various sizes may result in multiple crystallization peaks31. Supercooling of 10 °C and more was investigated during the crystallization of mini-emulsion droplets (immiscible liquid drops of 100–500 nm) of alkanes36,37. Mini-emulsions of immiscible polymer blends with a broad size distribution of droplets were shown to have multiple crystallization peaks; a broader size distribution leads to a larger number of peaks38.
Large effects were also observed in porous media. Crystallization of a fluid in a nanometer-sized porous material resulted in a much lower freezing temperature, i.e. in a more pronounced supercooling effect, which was described as a layer-by-layer crystal growth39. Freezing of n-alcohols in mesoporous silicon was obtained at 28 °C lower temperature than in the bulk40.
The most important research of a TC composite in spatially confined conditions was done for its application as the active core in fibres made by melt coaxial electrospinning. It was shown that the thermal and colouration properties of the active core were only slightly affected in fibres with a diameter of 0.5–2 µm3, but many changes were found in fibres with a diameter of 1.7–5.7 µm4. Multiple solidification peaks observed in dynamic DSC curves were contributed to different diameters of the fibre’s core and the change in freezing temperature was linked to possible reactions between the polymer shell and the active core inside the microcapsules. It was also concluded that the phase transition temperature might not be the only parameter governing the colour transition temperature4.
The above results were a strong motivation for our research. If the colour of leuco dye-based TC composites follows their phase transition temperatures, their colour and phase changes should be equally affected by a spatial micro-arrangement. To analyse this assumption, the same TC composite was studied both in the bulk and in a confined form. Because TC composites can only seldom be applied in the bulk, the obtained results should be important for a design of the functional properties of the final application.
Results and Discussion
Colour properties
The shape of the hysteresis was described by the loop width and four characteristic temperatures, describing the onset (T1 and T3) and termination (T2 and T4) of the TC effect: discolouration at heating (T1 and T2) and colouration at cooling (T3 and T4), respectively (Fig. S1, Supplementary information).
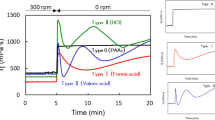
The temperature-dependent colour and thermal properties of the three forms of the TC composite are shown in Fig. 1 and the data describing the colour hysteresis in Table 1. The same heating and cooling rates (5 °C/min and 10 °C/min, respectively) were used to enable comparison between the two measurements.
Thermal (DSC, black curve) and colorimetric properties (blue curve with circles) of the (a) TC composite in the bulk, (b) inside the chromatographic paper and (c) in microcapsules. Heating and cooling rates of 5 °C/min and 10 °C/min, respectively, were used for both measurements. Pictures of the corresponding holders are included. The bulk composite is shown in the fully coloured and totally discoloured states (a).
In the bulk state (C6), the composite discolours before the melting is completed (transition A) but it re-colours well above the freezing point (transition B). This confirms that colouration/discolouration of a TC composite may not follow the phase changes15, however, a coloured liquid is formed before solidification and colourless solid before melting. The corresponding colour hysteresis possesses an unusual shape where the cooling and heating curves intersect. This is in agreement with our previous research - TC composites have a very narrow colour hysteresis and in some cases the two curves may also cross each other41. When the composite is inside the paper (pC6), transitions A and B remain practically unaffected, but the colour hysteresis broadens to about 4 °C and becomes less steep than in the bulk. Moreover, discolouration of the sample at heating and re-colouration at cooling coincide well with the temperatures where melting (A) and crystallization (B) processes are completed. The µC6 sample shows two phase transitions at cooling, one at crystallization of its bulk form (B) and another one at much lower temperatures (C) and the colour hysteresis is very broad (∼18 °C). This sample discolours at higher temperatures than in the bulk form (C6) and inside the paper (pC6), but still at about 6 °C below the temperature at which the active core inside microcapsules completely melts. Re-colouration of the sample occurs at considerably lower temperatures than in the other two forms and completes just before the onset of the transition C.
Discolouration upon heating starts at virtually the same temperature in all three samples (T1) but completes at a higher temperature (T2) in the paper (pC6) and especially in the microcapsules (µC6). A stronger influence of the spatial conditions of the TC composite on its colouration and phase changes was recorded at cooling, the most pronounced in the microencapsulated form. A direct comparison of the thermal and colouration properties of the analysed TC composite is shown in Fig. S2 (Supplementary information).
Thermal properties
A slow DSC experiment (Fig. 2a) resolved the details obscured by the rapid scanning used to obtain the colorimetric measurements (Fig. 1). The results were compared with those for the ML co-solvent in the same three forms (Fig. 2b). The corresponding phase change temperatures and transition enthalpies are given in Table 2. The mass of the host material (paper and microcapsule’s shells) was not taken into account, therefore thermograms were normalized according to the melting peak (A) and transition enthalpies can be compared only among samples with the same holding material.
DSC thermograms of the TC composite and of the applied co-solvent show the same features with systematically larger transition enthalpies with the latter, thus these transitions are driven by the co-solvent. Two (or even three) transition peaks were resolved at bulk crystallization (transition B), lower in µML and higher in µC6. The µML sample shows peaks also between transitions B and C where practically no features were observed in the µC6 sample (Fig. S3, Supplementary information).
The supercooling effect of transition B is very small, about 1 °C for the composite and about 2 °C for the co-solvent. Thus, the spatial separation of the PCM is not critical and transition B can be regarded as having a bulk nature. The size effect of the microcapsules is discussed later in the article.
To understand the measured crystallisation properties, two questions have to be answered – first, are the transitions B and C thermally related and, second, if the two transitions lead to the same crystal structure (i.e. to the same crystalline polymorph). The first question was addressed by combining static and dynamic DSC measurements and the second one by performing powder XRD measurements.
The crystallization properties of the microencapsulated samples were analysed by applying a 20 minute isothermal regime between transitions B and C, i.e. at −10 °C (Fig. 3 and Table 3). When a 20 min isothermal DSC was used after cooling to −10 °C followed by the samples’ heating back to 15 °C, the melting enthalpy (transition A) was found to correspond reasonably well with the crystallization one (transition B). When after 20 min isothermal DSC at −10 °C the sample was cooled down to −50 °C, the peaks at transition C were similar to those when only a dynamic DSC was applied. Moreover, almost an identical curve was produced upon heating (compare blue and red curves in Fig. 3). The 20 minutes isothermal regime used at −10 °C caused a negligible crystallization, thus the transition A remained practically unaffected when cooling was stopped. Similar results were obtained for the TC composite and for the co-solvent. It is therefore safe to conclude that the transitions B and C are thermally independent.
DSC thermograms of (a) the composite and (b) the co-solvent in the bulk (C6, ML) and microencapsulated forms (µC6, µML). Green curve: 20 min isothermal DSC was used after cooling to −10 °C, followed by heating. Blue curve: after 20 min isothermal DSC taken at −10 °C, the dynamic regime continued to −50 °C, followed by heating. Both heating and cooling rates in the dynamic regime were 2 °C/min.
Crystalline phase
The crystalline phase, resulting from the transitions B and C was checked by powder XRD of the samples µML and µC6 cooled down to 10 °C, −10 °C and −50 °C. At 10 °C, both samples are amorphous, as expected – according to the DSC results, they are still liquid at this temperature. However, at −10 °C and −50 °C the diffraction patterns show the appearance of peaks, confirming that for both samples, the thermal effects are due to crystallization. The appearance of diffraction peaks at the same diffraction angles and having the same intensity ratios clearly indicates that the crystalline phase is apparently the same in all cases (both samples and both temperatures, just its amount is larger at −50 °C in both samples, see Fig. 4). We can conclude that the presence of the dye does not affect the crystallization to the extent that another polymorph of ML or co-crystal would crystallize. It has to be noted that no crystal structure data for ML are available so that structure-related discussion of the powder patterns is neither possible nor needed for the purpose of this work.
Spatial confinement effect
The microencapsulated samples have a broad size distribution, ranging from 1.6 to 25.4 µm with the median 9.4 µm for µC6 (Fig. 5) and similar for µML (Fig. S4a). The samples have the same thickness as microcapsules’ shell (Table 4).
To investigate the influence of the microcapsules’ size on the colorimetric and thermal properties, the µC6 and µML dispersions were gravimetrically separated into fractions with smaller (µC6-s, µML-s) and larger microcapsules (µC6-l, µML-l), respectively; see Figs 6a,b, and S4a,b. The size of the microcapsules in the obtained samples is given in Table 4 and the corresponding DSC thermograms are shown in Figs 6c and S4d (Supplementary information) with the corresponding thermal data displayed in Table 5.
The transition B does not appear in DSC thermograms for smaller microcapsules (<11 µm, µC6-s and µML-s), but only for larger ones. The transition C consists of multiple peaks (Figs 6c, S4), which is typical for crystallization in mini-emulsion droplets36,37,38.
Thermal properties of the TC composite and of the applied co-solvent are very similar, leading to the conclusion that the phase changes of the TC composite are predominantly driven by the co-solvent while the other two components (leuco dye and developer) have only a minor influence. However, the colouration-discolouration ability is subject to the dye-developer interactions in the phase-changing co-solvent, and discolouration requires the interaction between a developer and a co-solvent. This interaction might strongly depend on the host material, especially on its morphology and the latter can be of considerable importance. For a deeper understanding of this topic, spectroscopic investigations would be necessary; however, the contribution of the host material will inevitably obscure the properties of the composite. In addition, good temperature stability during measurements must be assured. We will try to do some progress in this direction in our future work.
Conclusions
Colour changes of the analysed leuco dye-based TC composite become loosely related to its phase transitions when the material is restricted within micrometer dimensions of a holding medium. The strongest effects are observed in microcapsules when cooling where their colour changes well before crystallization and the corresponding hysteresis is extremely wide. Two thermally independent exothermic transitions with the same crystalline structure were found – a weak one at bulk crystallization temperature and a much stronger one at a more than 15 °C lower temperature. Apparently, the low-temperature crystallization shifts the colouration of the microencapsulated composite to much lower temperatures, but not all the way to the phase change temperature. On the other hand, the open nature of the porous structure with narrower particle sizes than the smallest microcapsules shows only the bulk crystallization and the colour changes follow the phase transitions reasonably well. Here, the colour hysteresis exhibits a standard loop shape.
To the best of our knowledge, this is the first report of the influence of spatial confinement on the colour- and phase changes of a leuco dye-based TC composite. Both phenomena depend on the conditions in which the PCM is captured, but are only loosely related to each other. The results presented in the paper are important for designing different applications where such materials are used.
Methods
Crystal violet lactone leuco dye (CVL, >95%, Tokyo Chemical Industry), benzyl 4-hydroxybenzoate (B4HB, >99%, Tokyo Chemical Industry) and methyl laurate (ML, 96%, Acros OrganicsTM, Fisher Scientific) were used as received. The TC composite (C6) was prepared by dissolving B4HB and CVL in the ML co-solvent in molar ratio CVL:B4HB:ML = 0.5:6:100.
The chromatography paper No. 232-674-98 (85 g/m², 2-4 µm pore size, Machery-Nagel GmbH & Co. KG) was used to spread the material into the porous paper (pC6, pML).
Microencapsulation (µC6, µML) was done by in situ polymerization of amino-aldehyde prepolymers42,43. All materials for the microcapsule shell originated from the continuous aqueous phase of the oil-in water emulsion. A modifying agent was added to enable polymerization of the aminoaldehyde precondensate only at the surface of the emulsified microcapsule cores. Under controlled polymerization conditions (pH, temperature) total mass of the shell material precipitated and was distributed evenly over the surface of droplets in the emulsion. This resulted in a uniform microcapsule shell thickness, regardless of the microcapsule size. Such result was also reported in previous studies44,45.
The size distribution of microcapsules was obtained by image analysis of SEM micrographs (Karl Zeiss Supra 35 C) using ImageJ image processing software with suitable numerical procedures46.
Temperature-dependent colour of the C6 was measured inside a cylindrical groove (11.2 mm diameter, 0.3 mm depth) in the 2.9 mm thick white-coated copper plate. The colour of the µC6 was measured on a thin layer of microcapsules prepared over a white paper using the Film-Applicator (Byk Additives&Instruments) with 200 µm clearance gap and dried at room temperature. All samples were cooled by dry ice (−78.5 °C) down to −30 °C and heated on a laboratory table at room temperature, producing cooling- and heating rates of about 10 °C/min and 5 °C/min, respectively. Temperature of the sample surface was measured by Ni Cr-Ni thermocouple connected to IR thermometer (Fluke 561). Colour of the samples was measured by the i1 Pro spectrometer (X-Rite, USA). The CIELAB colour values with D50 illuminant and 2° standard colorimetric observer were calculated by KeyWizzard software47. The colour change was expressed by the CIE colour difference ΔEab* with respect to the highest measured temperature and normalized according to the totally coloured state taken at the lowest temperature.
Thermal properties were analysed by differential scanning calorimetry (DSC) using Mettler Toledo DSC1 apparatus at heating/cooling rates of 2 °C/min, 5 °C/min, and 10 °C/min from −50 °C to 15 °C. For this purpose, the water dispersions of microcapsules were dried at room temperature.
The XRD patterns were collected on the Rigaku SuperNova diffractometer, equipped with Atlas CCD detector, using CuKα radiation and the Powder power tool within the CrysalisPro data collection software (version 171.37.34t). The cooling of the samples was done using an Oxford Instruments CryoJet device.
References
Aitken, D., Burkinshaw, S. M., Griffiths, J. & Towns, A. D. Textile applications of thermochromic systems. Rev. Prog. Coloration 26, 1–8 (1996).
White, M. A. & LeBlanc, M. Thermochromism in Commercial Products. J. Chem. Educat. 76, 1201–1205 (1999).
Li, F. et al. Thermochromic Core-Shell Nanofibers Fabricated by melt Coaxial Electrospinning. J. Appl. Polym. Sci. 112, 269–274 (2009).
Malherbe, I., Sanderson, R. D. & Smit, E. Reversibly thermo-chromic microfibres by coaxial electrospinning. Polymer 51, 5037–5043 (2010).
Busuioc, C., Evanghelidis, A., Galatanu, A. & Enculescu, I. Direct and contactless electrical control of temperature of paper and textile foldable substrates using electrospun metallic-web transparent electrodes. Sci. Rep. 6, 34584, https://doi.org/10.1038/srep34584 (2016).
Zhu, X., Liu, Y., Dong, N. & Li, Z. Fabrication and characterization of reversible thermochromic wood veneers. Sci. Rep. 7, 16933, https://doi.org/10.1038/s41598-017-17238-9 (2017).
Burkinshaw, S. M., Griffiths, J. & Towns, A. D. Reversibly thermochromic systems based on pH-sensitive spirolactone-derived functional dyes. J. Mater. Chem. 8, 2677–2683 (1998).
MacLaren, D. C. & White, M. A. Dye–developer interactions in the crystal violet lactone–lauryl gallate binary system: implications for thermochromism. J. Mater. Chem. 13, 1695–1700 (2003).
MacLaren, D. C. & White, M. A. Competition between dye–developer and solvent–developer interactions in a reversible thermo-chromic system. J. Mater. Chem. 13, 1701–1704 (2003).
Luthern, J. & Peredes, A. Determination of the stoichiometry of a thermochromic color complex via the method of continuous variation. J. Mater. Sci. Lett. 22, 881–884 (2003).
Zhu, C. F. & Wu, A. B. Studies on the synthesis and thermo-chromic properties of crystal violet lactone and its reversible thermochromic complexes. Thermochim. Acta 425, 7–12 (2005).
MacLaren, D. C. & White, M. A. Design rules for reversible thermochromic mixtures. J. Mater. Sci. 40, 669–676 (2005).
Tang, H., MacLaren, D. C. & White, M. A. New insights concerning the mechanism of reversible thermochromic mixtures. Can. J. Chem. 88, 1063–1070 (2010).
Borque, A. N. & White, M. A. Control of thermochromic behaviour in crystal violet lactone (CVL)/alkyl gallate/alcohol ternary mixtures. Can. J. Chem. 93, 22–31 (2015).
Hajzeri, M., Bašnec, K., Bele, M. & Klanjšek Gunde, M. Influence of developer on structural, optical and thermal properties of a benzofluoran-based thermochromic composite. Dyes Pigm. 113, 754–762 (2015).
Kulčar, R., Friškovec, M., Hauptman, N., Vesel, A. & Klanjšek Gunde, M. Colorimetric properties of reversible thermochromic printing inks. Dyes Pigm. 86, 271–277 (2010).
Kulčar, R., Friškovec, M., Klanjšek Gunde, M. & Knešaurek, N. Dynamic colorimetric properties of mixed thermochromic printing inks. Color. Technol. 127, 411–417 (2011).
Friškovec, M., Kulčar, R. & Klanjšek Gunde, M. Light fastness and high-temperature stability of thermochromic printing inks. Color. Technol. 129, 214–222 (2013).
Panák, O., Držková, M. & Kaplanová, M. Insight into the evaluation of colour changes of leuco dye based thermochromic systems as a function of temperature. Dyes & Pigm. 120, 279–287 (2015).
Zhang, J. J., Zhang, J. L., He, S. M., Wu, K. Z. & Liu, X. D. Thermal studies on the solid-liquid phase transition in binary systems of fatty acids. Thermochim. Acta 369, 157–160 (2001).
Ventolà, L. et al. Solid state equilibrium in the n-alkanols family: the stability of binary mixed samples. Phys. Chem. Chem. Phys. 5, 947–752 (2003).
Ventolà, L., Calvet, T., Cuevas-Diarte, M. A., Oonk, H. A. J. & Mondieig, D. Solid–solid and solid–liquid equilibria in the n-alkanols family: C18H37OH–C20H41OH system. Phys. Chem. Chem. Phys. 6, 3726–3731 (2004).
Zuo, J., Li, W. & Weng, L. Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change materials (PCM). Energy Build. 43, 207–210 (2011).
Liston, L. C. et al. Binary mixtures of fatty acid methyl esters as phase change materials for low temperature applications. Appl. Therm. Engn. 96, 501–507 (2016).
Nelson, G. Application of microencapsulation in textiles. Int. J. Pharmaceuticals 242, 55–62 (2002).
Guo, X., Cao, J., Peng, Y. & Liu, R. Incorporation of microencapsulated dodecanol into wood flour/high density polyethylene composite as a phase change material for thermal energy storage. Materials and Design 89, 1325–1334 (2016).
Li, W. et al. Design, controlled fabrication and characterization of narrow-disperse macrocapsules containing Micro/nanoPCMs. Materials and Design 99, 225–234 (2016).
Meng, F., Wang, S., Liu, H., Xu, X. & Ma, H. Microencapsulation of oxalic acid (OA) via coacervation induced by polydimethylsiloxane (PDMS) for the sustained release performance. Materials and Design 116, 31–41 (2017).
Shams, T., Parhizkar, M., Illangakoon, U. E., Orlu, M. & Edirisinghe, M. Core/shell microencapsulation of indomethacin/paracetamol by co-axial electrohydrodynamic atomization. Materials and Design 136, 204–213 (2017).
Nomura, T., Zhu, C., Sheng, N., Saito, G. & Akiyama, T. Microencapsulation of Metal-based Phase Change Material for High-temperature Thermal Energy Storage. Sci. Rep. 5, 9117, https://doi.org/10.1038/srep09117 (2015).
Chaiyasat, P., Noppalit, S., Okubo, M. & Chaiyasat, A. Do encapsulated heat storage materials really retain their original thermal properties? Phys. Chem. Chem. Phys. 17, 1053–1059 (2015).
Zhang, Y. X., Fan, Y. F., Tao, X. M. & Yick, K. L. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Coll. Interf. Sci. 281, 299–306 (2005).
Zhang, S. & Niu, J. Experimental investigation of effects of supercooling on microencapsulated phase-change material (MPCM) slurry thermal storage capacities. Sol. En. Mat. Sol. Cells 94, 1038–1048 (2010).
Tang, X., Li, W., Zhang, X. & Shi, H. Fabrication and characterization of microencapsulated phase change material with low supercooling for thermal energy storage. Energy 68, 160–166 (2014).
Cao, F. & Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 113, 1512–1518 (2014).
Montenegro, R., Antonietti, M., Mastai, Y. & Landfester, K. Crystallization in Miniemulsion Droplets. J. Phys. Chem. B 107, 5088–5094 (2003).
Montenegro, R. & Landfester, K. Metastable and Stable Morphologies during Crystallization of Alkanes in Miniemulsion Droplets. Langmuir 19, 5996–6003 (2003).
Tol, R. T., Mathot, V. B. F. & Groeninckx, G. Confined crystallization phenomena in immiscible polymer blends with dispersed micro- and nanometer sized PA6 droplets, part 2: reactively compatibilized PS/PA6 and (PPE/PS)/PA6 blends. Polymer 46, 369–382 (2005).
Alba-Simionesco, C. et al. Effects of confinement on freezing and melting. J. Phys.: Condens. Matter. 18, R15–68 (2006).
Berwanger, R., Schumacher, C., Huber, P. & Pelster, R. Size-dependent freezing of n-alcohols in silicon nanochannels, Eur. Phys. J. Special Topics 189, 239–249 (2010).
Panák, O., Hauptman, N., Klanjšek Gunde, M. & Kaplanová, M. Colorimetric characterization of thermochromic composites with different molar ratios of components. JPMTR 1, 113–120 (2012).
Šumiga, B. et al. B, Production of Melamine-Formaldehyde PCM Microcapsules with Ammonia Scavenger used for Residual Formaldehyde Reduction. Acta Chim. Slov. 58, 14–25 (2011).
Boh, B., Knez, E. & Starešinič, M. Microencapsulation of higher hydrocarbon phase change materials by in situ polymerization. Journal of microencapsulation 22, 715–735 (2005).
Pretzl, M. et al. Formation and mechanical characterization of aminoplast core/shell microcapsules. ACS applied materials & interfaces 4, 2940–2948 (2012).
Yuan, Y. C., Rong, M. Z. & Zhang, M. Q. Preparation and characterization of microencapsulated polythiol. Polymer 49, 2531–2541 (2008).
Gonzalez, R. C., Woods, R. E. RE: Digital Image Processing. 3rd Ed. Prentice-Hall: Upper Saddle River, New Jersey 2008.
J. Shanda, CIE colorimetry. In: Shanda J, editor. Colorimetry; understanding the CIE system. Hoboken: Wiley, p. 25–78 (2007).
Acknowledgements
The financial support from the state budget by the Slovenian Research Agency (Project No. L2-5571) and the EN-FIST Centre of Excellence, Slovenia for use of the Supernova diffractometer are acknowledged.
Author information
Authors and Affiliations
Contributions
K.B. made the formulation and preparation of the active material, L.S.P. contributed with colorimetric and SEM measurements, B.Š. and B.B.P. made microencapsulation of the composite, M.H. provided DSC analysis and size separation of microcapsules, A.M. made XRD measurements and explanations, A.H. provided the particle size distribution analysis of SEM micrographs, and M.K.G. lead the entire research and wrote the article.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bašnec, K., Perše, L.S., Šumiga, B. et al. Relation between colour- and phase changes of a leuco dye-based thermochromic composite. Sci Rep 8, 5511 (2018). https://doi.org/10.1038/s41598-018-23789-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23789-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.