Abstract
Geologic storage of carbon dioxide (CO2) is considered a viable strategy for significantly reducing anthropogenic CO2 emissions into the atmosphere; however, understanding the flow mechanisms in various geological formations is essential for safe storage using this technique. This study presents, for the first time, a two-phase (CO2 and brine) flow visualization under reservoir conditions (10 MPa, 50 °C) for a highly heterogeneous conglomerate core obtained from a real CO2 storage site. Rock heterogeneity and the porosity variation characteristics were evaluated using X-ray computed tomography (CT). Multiphase flow tests with an in-situ imaging technology revealed three distinct CO2 saturation distributions (from homogeneous to non-uniform) dependent on compositional complexity. Dense discontinuity networks within clasts provided well-connected pathways for CO2 flow, potentially helping to reduce overpressure. Two flow tests, one under capillary-dominated conditions and the other in a transition regime between the capillary and viscous limits, indicated that greater injection rates (potential causes of reservoir overpressure) could be significantly reduced without substantially altering the total stored CO2 mass. Finally, the capillary storage capacity of the reservoir was calculated. Capacity ranged between 0.5 and 4.5%, depending on the initial CO2 saturation.
Similar content being viewed by others
Introduction
Atmospherically released carbon dioxide (CO2) is considered to be a major deriver behind climate change, and as such geologic CO2 storage is considered a key technology in climate change mitigation strategies1,2. Both industry and research communities are currently evaluating the safety and feasibility of long-term CO2 sequestration, and a number of pilot- and demonstration-scale projects have been conducted as a part of this effort to test, monitor, and verify technologies in various subsurface geological environments3,4,5.
Previous research related to geologic CO2 storage has until now focused on evaluating sandstone formations, as their relatively high porosity and permeability suggest greater economic viability than other rock formations. For this reason, the reservoir lithology of most pilot-scale (e.g., Frio, Nagaoka, Ketzin and Otway) and demonstration- or commercial-scale projects (e.g., MGSC Decatur, Sleipner, Snøhvit, In Sala and Gorgon) has been sandstone3,5. The average reservoir porosity has been 5–35% and reservoir permeabilities have ranged from as low as 5 mD (In Salah) to 5,000 mD (Sleipner).
One exception was the MRSCP Gaylord project (a Midwest regional carbon sequestration partnership), where the targeted storage formation consisted of dolomite. This was characterized by interbedded, laminated algal dolomudstone, represented by a mean porosity and permeability of 13% and 22.6 mD, respectively6. In an effort to assess the geologic storage potentials, basalt formations have also been considered as alternative storage formations7,8.
Although a number of CO2 storage projects are being conducted worldwide, the need for additional safe storage verification experiments using various geological formations cannot be overemphasized. The fact that only limited data has been collected during the injection and post-injection phases demonstrates the necessity of additional field assessments of the processes leading to plume stabilization and long term trapping9. Followed by on-going international efforts, the first onshore pilot-scale (~10,000 ton) CO2 storage project is being conducted in South Korea at the Janggi sedimentary formation, located on a south-eastern portion of the Korean Peninsula (Fig. 1(a)). One distinctive feature differing from other pilot-scale projects is that the targeted formation is a conglomerate, consisting of gravel and cobbles in a silty sand matrix. Figure 1(b) shows a thickness-permeability cross-plot of values selected from various existing storage projects. This plot reveals the injectivity at Janggi falls between 0.1 and 1 darcy-meter, similar to the estimated injectivities at the Nagaoka and In Salah sites.
In addition to field-demonstration studies, well-controlled laboratory experiments are essential for obtaining a fundamental understanding of physical10,11 and chemical processes12,13 occurring in potential storage formations, as well as predicting their CO2 trapping capacities14. A number of studies have conducted reactive transport experiments to understand the complex processes of CO2-water-mineral interactions and their coupling effects on the CO2-water multiphase flow at in-situ reservoirs conditions15,16,17,18. These experimental data can be utilized to validate the numerical studies and build-up confidence in the modeling tools. For example, Smith et al.18 used detailed experimental data to constrain three-dimensional reactive transport models in order to describe and predict the evolution of pore-space and permeability during geologic CO2 storage in carbonate reservoirs.
Core-flooding is a technique that has been used to conduct various laboratory experiments on core samples under conditions closely imitating those of subsurface environments19. Due to this advantage, core-flooding tests have been utilized for characterizing the dynamic behaviour of CO2 under reservoir conditions. Such tests have focused on resolving various problems related to important factors controlling the behaviours of two-phase fluids, including permeability heterogeneity and multiphase flow20,21,22, measuring relative permeabilities23,24,25, interfacial tension26,27, and capillary pressures28,29,30. Additionally, the core-flooding experiments have been applied to assessing residually trapped CO2 amounts under reservoir conditions23,25,31,32,33. Still other research topics have included multiphase fluid behaviour in fractured porous media34,35, salt-precipitation dynamics36,37 and the non-equilibrium dissolution of CO2 in a heterogeneous core32,38.
Despite these numerous core-scale studies, there has been little investigation on how CO2 migrates in a highly heterogeneous porous media. This study presents, for the first time, a two-phase flow visualization under reservoir conditions for a highly heterogeneous conglomerate core that has been obtained from the real CO2 storage site. The three-dimensional (3D) distribution of clasts within the matrix was determined utilizing X-ray computed tomography (CT). Additionally, real-time X-ray scanning techniques (conducted during the CO2 and brine flow tests) captured the dynamic distribution of CO2 saturation in the conglomerate core. Multiphase transport simulations were also conducted to assess the effects of heterogeneity on pressure build-up as well as spatial variation of CO2 saturation. Finally, based on the initial-residual (IR) characteristic curve, the storage potential of the conglomerate formation is discussed.
Results and Discussion
Heterogeneity characterization
A core-plug obtained from the Janggi conglomerate formation was used to investigate the spatial distribution of clasts within a silty sand matrix. Figure 2(a) presents two cross-sectional CT images taken along the longitudinal axis at different angles. In addition, Fig. 2(b) shows the binary image generated from the 8-bit CT images, where the boundaries between the clasts and matrix are distinguished more clearly and discontinuities within the clasts are highlighted. The clast fractioning profiles along the longitudinal core-axis are shown in Fig. 2(c). Nine cross-sectional images, taken along the white-dashed lines appearing in Fig. 2(a), are presented in Fig. 2(d). In the upstream region (30 mm) of the core, the constituents are relatively homogeneous and only a small fraction of clasts were preserved (f < 0.3). In the midstream portion (70 mm), both medium size of clasts and matrix existed together, whereas larger clast sizes were present in the downstream region (100 mm). Figure 1(e) shows the three-dimensional distribution of clasts in the core-plug.
(a) Two CT cross-sectional images along the longitudinal axis taken from different angles. (b) Binary image of the core-plug for clarification of the discontinuities in the clasts. (c) The profile for clasts fraction along the longitudinal core-axis. (d) 2D cross-section images along the white-dashed lines shown in (a). (e) 3D distribution of clasts in the core-plug.
The pore size distribution were analysed for both the matrix and clasts (Fig. 3(a)). The clast regime was characterized by a skewed uni-modal pore-size distribution dominated by micropores (<1 μm diameter). The silty sand matrix regime demonstrated a bi-modal pore size distribution dominated by micropores (<1 μm diameter) and mesopores (>2 μm diameter) (Fig. 3(a)). The bi-modal pore size distribution characteristics of the matrix were also reflected in the shape of the capillary pressure (Pc) curve, appearing as a superposition of two uni-modal pore size distribution characteristic curves. The Pc (Sw = 0.5) of the clasts was more than two orders of magnitude greater than that of the matrix (Fig. 3(b)).
Multiphase flow experiments
This experimental study aimed to characterize the spatio-temporal distribution of CO2 saturation build-up during the multiphase flow tests. Two different tests were conducted: (i) CO2 injection into a brine-saturated core, simulating the CO2 injection period (drainage) conditions; and (ii) brine injection after the CO2 injection test, reproducing the post-injection period (imbibition) conditions.
CO2 saturation distribution snapshots revealed the propagation of CO2 saturation throughout the conglomerate core with increases in pore volume (PV) (Supplementary Figs S1 and S2). Figure 4(a) presents the vertically averaged CO2 saturations from the five different locations (30, 60, 70, 110, and 120 mm) delineated in Fig. 2(a). Although the averaging is smoothing out the fluctuation of CO2 saturation related to small-scale heterogeneity, it clearly presents the increasing tendency within the core sample. All profiles showed logarithmic increases with the PVs. The CO2 saturation profile at 30 mm increased slowly when approaching the asymptote during the flow tests; however, the other profile (such as at 110 mm) approached their asymptotes more rapidly, implying that CO2 saturation was quickly stabilized throughout the experiment. It is interesting to note that CO2 saturation at the downstream side of the core was greater than that at the upstream side in early stages (<1.0 PV). The spatial inversion of CO2 saturation distribution was attributed to the heterogeneous pore space along the conglomerate core, including the presence of complex discontinuities in clasts (Fig. 2(b)). On the upstream side where the matrix was dominant, CO2 saturation was low due to the presence of relatively large sized pores, which accommodated the injected CO2. Equal amounts of CO2 flowing through the downstream side, dominated by clasts, showed elevated CO2 saturation because clast discontinuities provided only small pore-spaces.
(a) Vertically averaged CO2 saturation at five different locations (30, 60, 70, 110, and 120 mm) versus pore volume during the CO2 injection test. (b) Three subdomains (20 × 20 mm) of the core located at upstream (domain I), midstream (domain II), and downstream (domain III) region that are mainly composed of sand, small clasts and large clasts, respectively. (c) Snapshots of CO2 saturation at three different domains during the CO2 injection test. (d) Vertically averaged CO2 saturation at five different locations (30, 60, 70, 110, and 120 mm) versus pore volume during the brine injection test. (e) Snapshots of CO2 saturation at three different domains during the brine injection test.
To perform a detailed assessment of the spatial distribution of CO2 saturation, three subdomains (20 × 20 mm) were selected, one each located in the upstream (domain I), midstream (domain II), and downstream (domain III) regions as shown in Fig. 4(b). These domains primarily composed of sand, small clasts, and large clasts, respectively. Domain I (primarily sand) was characterized by a relatively homogeneous CO2 saturation distribution over time (Fig. 4(c)) with a small (sub-core) scale heterogeneous CO2 saturation observed due to the effect of the bi-modal pore-size distribution preserved in the matrix (Fig. 3(a)). Domain II, which was composed of irregularly spaced small clast fragments, demonstrated heterogeneous CO2 saturation caused by irregularly spaced small clast fragments. Low CO2 saturations resulted from the presence of clasts, whereas high CO2 saturations were observed in high porosity sand. Highly heterogeneous CO2 saturation was observed in domain III, caused by a dense network of discontinuities within the large clasts where multiple discontinuities with relatively large apertures (110 mm) were located, as seen in Fig. 4(c). This discontinuity network provided well-connected pathways for CO2 flow, resulting in a rapid increase in CO2 saturation.
The vertically averaged CO2 saturation was also plotted for the brine injection tests (Fig. 4(d)). As discussed in Oh et al.32, the dynamic transition of CO2 trapping could be assessed during the imbibition test. Three regimes of CO2 trapping mechanisms were characterized: (i) immediate mobile CO2 displacement by injected brine (R1), (ii) immobile CO2 preservation as residual trapping (R2), and (iii) the gradual dissolution of residually trapped CO2 into the fresh brine (R3). The dynamic transition of CO2 trapping at 30 mm displayed initial decrease in CO2 saturation from 0.35 to 0.27 during the early stage (0.01–0.1 PV) primarily resulted from the mobile CO2 displacement (Regime 1). In Regime 2, the CO2 saturation was relatively consistent and stabilized at 0.27 between 0.1 and 1.0 PV, which implies the preservation of immobile CO2 (residually trapped). This is possible because the brine that enters the core will initially dissolve a small amount of CO2 at the inlet of the core and becomes fully-saturated preventing it from dissolving any more CO2. However, as more fresh brine was injected into the core, the residually trapped CO2 dissolved into fresh brine (Regime 3).
Figure 4(e) shows CO2 saturation snapshots from the three different domains during the brine injection test. In domain I where the matrix dominated, CO2 was distributed uniformly at the end of drainage test. Brine displacement tests induced similar uniform distributions in residually trapped CO2, which eventually dissolved into the brine. However, in domains II and III, a larger fraction of pores was initially filled with CO2, resulting in more snap-off and trapping as brine invaded the pore space. Under these conditions, CO2 dissolution was highlighted only within the matrix and discontinuities.
Multiphase transport simulations
The above experiments were conducted with fluid injection at the left boundary, but CO2 injection from the opposite boundary would cause difference in the distribution of CO2 saturation due to nature in highly heterogeneous conglomerate core. We used TOUGH239 simulator to model multiphase and multicomponent fluid transport to assess directional effect of fluid flow on pressure build-up as well as CO2 saturation distribution along the core sample. A two-dimensional model was constructed based on the CT image of the core sample to reflect the distribution of clasts. Then, two different cases were simulated (case 1: left-to-right flow; case 2: reverse flow).
Figure 5 presents simulation results of pressure (a and f) and CO2 saturation map (b and g), profiles for CO2 pressure (c and h) and saturation (d and i) along the center of the model domain, and pressure build-up (e and j) for the two different cases. Both cases showed a significant pressure drop between points A and B, which lie on a boundary of a large sized clast. The heterogeneous pressure distribution resulted in different CO2 saturation within the model for the two cases. For example, case 1 showed relatively high CO2 saturation (Sg~0.5) at point A and low CO2 saturation (Sg~0.3) at point B, while case 2 showed relatively high CO2 saturation (Sg~0.5) at point B. This implies that the distribution of CO2 saturation is dependent on the flow direction. The pressure difference between the inlet and outlet (ΔP) showed ~150 kPa during the early stage of injection and stabilized at ~30 kPa for both cases (Fig. 5(e and j)).
Simulation results for the two different flow directions (case 1: left-to-right flow (a–e); case 2: right-to-left flow (f–j)). The results presents pressure (a and f) and CO2 saturation map (b and g), profiles for CO2 pressure (c and h) and saturation (d and i) along the center of the model domain, and pressure build-up (e and j).
CO2 mass at different capillary numbers
A dimensionless capillary number (\({{\rm{N}}}_{{\rm{c}}}\)), representing the relative contributions of viscous versus capillary forces, was set to reflect the reservoir conditions. Two CO2 injection tests (qco2 = 1.0 and 0.1 ml/min) were performed in this study, producing associated \({{\rm{N}}}_{{\rm{c}}}\) values of ~10−1 and ~10−2, respectively. The high \({{\rm{N}}}_{{\rm{c}}}\) case was observed in a transition regime between the capillary and viscous limits, whereas the low \({{\rm{N}}}_{{\rm{c}}}\) case was under capillary dominated flow conditions. The transient pressure difference (ΔP) values across the core for the two different cases are shown in Fig. 6. The ΔP build-up under a high injection rate (\({{\rm{N}}}_{{\rm{c}}}\) ~ 10−1) reached 2,300 kPa in early injection stages before stabilizing to ~700 kPa; however, the low injection rate (\({{\rm{N}}}_{{\rm{c}}}\) ~ 10−2) displayed a small buildup of ΔP build-up (~150 kPa) in early injection stages before ΔP stabilized to ~65 kPa for the remainder of the multiphase flow experiment.
Figure 7 displays the CO2 saturation distribution maps for the two injection rates (qco2 = 1.0 and 0.1 ml/min) and the CO2 saturation contrast (ΔSco2 = Sq=1.0−Sq=0.1) maps between the high and low injection rate cases. The measured ΔSco2 displayed a strong dependency on core heterogeneity, showing negative values on the upstream side (implying lower CO2 saturation with a greater injection rate (Sq=1.0 < Sq=0.1)). The high injection rate created excessed pressure on CO2 (nonwetting phase), enabling CO2 to transport across the high Pc region on the midstream side. This had a negative impact on the build-up of CO2 mass on the upstream side. At low injection rates, however, additional CO2 occupied the pore-spaces on the upstream side because CO2 transport across the high Pc region on the midstream side was hindered. The CO2 mass within the core was calculated for both \({{\rm{N}}}_{{\rm{c}}}\) cases to be 2.24 and 2.18 g, respectively, once a steady-state condition was reached. This demonstrated a 10× reduction in injection rate caused only a 2% decrease in CO2 mass, suggesting that injection rates, which may cause reservoir overpressure when high, can be significantly reduced without substantially altering the total CO2 mass.
Storage capacity
During the post-injection stage, capillary forces at the pore scale are responsible for breaking up the CO2 phase into the form of blobs or ganglia, becoming effectively immobile40,41. The capillary trapping potential was evaluated in these conditions based on the performed multiphase flow experiments. The initial-residual (IR) characteristic curves have commonly been used to describe the residual trapping potentials of rock, with numerous developed models describing this relationship. This study applied the Land model42, one of the earliest and most widely used trapping models.
Figure 8(a) presents the relationship between the initial (Sco2,i) and residual saturations (Sco2,r), where Sco2,i refers to the initial CO2 saturation prior to brine injection testing whereas Sco2,r refers to the residually trapped CO2 saturation. For analysis, highly heterogeneous conglomerate core was divided into 50 equal subdomains. Then, based on the brine injection testing, the residual CO2 saturation achieved at 0.1 PV (R2) was determined (Fig. 4(d)), and subsequently, the amount of capillary trapped CO2 was assessed using the Land model given by \({{\rm{S}}}_{{{\rm{CO}}}_{2,{\rm{r}}}}=({{\rm{S}}}_{{{\rm{CO}}}_{2,{\rm{i}}}})/(1+{\rm{C}}\cdot {{\rm{S}}}_{{{\rm{CO}}}_{2,{\rm{i}}}})\). Here, C is the dimensionless constant known as the Land coefficient. In this study, the calculated C was calculated as 0.9, showing a monotonic increase in the residual saturation as a function of Sco2,i. This was in agreement with numerous previous works investigating water-wet porous medias.
(a) A trapping curve relating initial CO2 saturation (Sco2,i) to residual CO2 saturation (Sco2,r). The Sco2,i refers to the initial CO2 saturation prior to imbibition test while the Sco2,r refers to the residually trapped CO2 saturation. Red circles represent the relationship between the Sco2,i and Sco2,r within equally divided subdomains; error bars represent one standard deviation. (b) Trapping capacity (φSco2,r) versus initial CO2 saturation (Sco2,i); colors represent different ranges of porosity.
The capillary-trapping capacity, defined as φSco2,r (φ is porosity) is a rescaling of the traditional IR saturation plot43,44. This quantity is of great importance in geologic CO2 storage applications because it states how much CO2 can be stored securely per unit volume of a rock. Figure 8(b) presents the relationship between Sco2,i and φSco2,r at different porosities (blue, red and orange circle symbols). The IR relationships of other core samples (Berea and Pohang sandstones) are plotted alongside for comparison. Core sample details are described in the Supplementary Table S1. The trapping capacities of both Berea and Pohang sandstones showed more or less linear increases with Sco2,i, whereas the φSco2,r values for heterogeneous conglomerate cores were widely spread and dependent on the size of porosity. Although the overall capacity of the conglomerate core was smaller than those of the Berea or Pohang sandstones, the conglomerate high porosity region displayed a similar capacity to the sandstones (e.g., φSco2,r ~4.5% at Sco2,i = 0.3).
Conclusions
Although sandstone formations are considered to be the most appropriate for CO2 sequestration, other types of geologic formations are being investigated for this purpose. For example, the target rock formation in MRSCP Gaylord project was dolomite45, and the Midale project (an extension of the Weyburn project) demonstrated the possibility for CO2 containment in a fractured reservoir5. Basaltic formations have also been considered as alternative storage formations7,8. In South Korea, a conglomerate formation is being tested for geologic CO2 storage.
This study reported a thorough core-scale investigation of a highly heterogeneous conglomerate formation. The rock heterogeneity was investigated with the use of X-ray CT and MICP analysis revealing that the targeted storage formation was composed of a silty sand matrix and irregularly spaced clasts of various sizes. Laboratory multiphase flow tests with flow visualization under reservoir conditions provided important information on CO2 migration behaviours as well as the storage capacity of the highly heterogeneous conglomerate. Variations in clast spatial configurations gave rise to different storage features. Dense networks of discontinuities within clasts were observed to provide well-connected CO2 flow pathways, causing a rapid increase in CO2 saturation, and potentially helping to reduce overpressure. Two flow tests, one in the capillary-dominated condition and the other in a transition regime between the capillary and viscous limits, revealed that a 10× reduction in injection rate caused only a 2% decrease in the CO2 mass. This implies the heterogeneity positively influenced pressure management because high injection rates, which may cause overpressure in the reservoirs, could be significantly reduced without substantially altering the total stored CO2 mass. We note that although chemical reactions are not considered in this study, the complex process of CO2-water-mineral interactions may play important roles on multiphase flow in subsurface reservoirs and trapping mechanisms.
Among the four potential trapping mechanisms, capillary trapping is a rapid and effective mechanism for rendering an injected fluid immobile and reducing the need to ensure caprock integrity. The capillary-trapping capacity of the conglomerate core investigated in this study was calculated using imbibition test results and the IR characteristic curve. While the Berea and Pohang sandstone cores showed approximately linear increases in capacity with initial CO2 saturation, the Janggi conglomerate displayed significant variations due to its highly heterogeneous porosity distribution. The conglomerate capacity ranged between 0.5 and 4.5%, depending on the initial saturation (0–0.4): for an initial saturation of 0.4, the conglomerate capacity ranged between 3–4%. Despite the fact that the trapping analyses showed lower overall capacities relative to homogeneous sandstone, the high porosity region of the conglomerate core displayed a similar capacity to sandstones, implying conglomerate formations could be an alternative for CO2 storage formation. CO2 injection and comprehensive monitoring in conglomerate formations will validate the feasibility of CO2 storage in the highly heterogeneous conglomerate formations.
Methods
Field site and geological setting
An onshore pilot-scale geologic CO2 storage project was launched in South Korea in 2011. The primary tasks (Phase I, 2012–2015) were the site-selection and initial characterization of potential pilot-scale storage sites. The Janggi conglomerate, composed of both clasts and silty sand matrix, was chosen as the target storage formation for the project. More recently, Phase II (2015–2017) was completed, including the drilling of seven boreholes to investigate the lithology of targeted and capping formations. The representative lithological log for this is illustrated in Fig. 1(a)46. The average porosity and permeability of the storage formation were 16.1% and 8.5 mD, respectively47. The overlying Seongdongri formation, composed of thick mudstone and massive dacitic tuffs, forms the regional sealing formation for CO2 storage, and a monitoring well (~40 m up-gradient from the injection well) was drilled. A comprehensive monitoring system was installed including a down-hole pressure and temperature measurements, vertical seismic profile (VSP), electrical resistivity tomography (ERT), distributed temperature sensing (DTS) and distributed acoustic sensing (DAS) along the well via an optical fiber, and U-tube downhole sampler.
X-ray micro CT
The dimensions of the core-plug obtained from the Janggi conglomerate formation were 130 × 48 mm (length × diameter). X-ray computed tomography (CT), a non-destructive and non-invasive method, was used to investigate the spatial distribution of clasts within the silty sand matrix. The CT images used in this study were obtained at 140 kV and 200 μA. A total of 2,000 cross-sectional images were taken at 0.065 mm intervals with a micrometer-order pixel size. Subsequently, two-dimensional (2D) images were stacked to generate 3D core sample images.
Multiphase flow tests with in-situ imaging technology
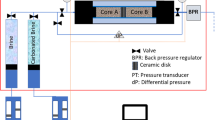
The apparatus for core-flooding experiments was designed to conduct multiphase flow tests. It was comprised of fluid (supercritical CO2 and brine) injection, core-holder, temperature controlling, confining, and back-pressure control systems (Supplementary Fig. S5). A 2D X-ray scanning system, consisting of an X-ray tube and a detector, was integrated into the core-flooding system in order to capture the real-time distribution of CO2 saturation distributions along the core sample. The detector board had two arrays of 128 channels each, corresponding to 256 detectors with a detector pitch of 0.4 mm. The array of the X-ray detector arrays were perpendicular to the core-axis, performing scans parallel to core-axis. The data output was in 16-bit format, ranging between 0 and 65,535. The produced 2D images were converted into CO2 saturations using the following equation: \({{\rm{S}}}_{{{\rm{CO}}}_{2}}=\,\frac{{{\rm{I}}}_{\exp }-{{\rm{I}}}_{{\rm{brinesat}}}}{{{\rm{I}}}_{{{\rm{CO}}}_{2}}-{{\rm{I}}}_{{\rm{brinesat}}}}\), where Ico2 and Ibrinesat are the values of grayscale intensity obtained from the background scans of the core, which were saturated with CO2 and brine in the core, respectively. Finally, Iexp was the value obtained for the multiphase condition. The obtained CO2 saturation map may include noise mainly due to the relatively small density contrast between the two fluid phases (CO2 and brine) compared with the contrast between any one of the fluids and the rock48. In the experiments, NaI was used as a dopant to enhance the X-ray attenuation so that the CO2 saturation could be distinguished from the brine in the scanned image.
Two multiphase flow tests were conducted: (i) CO2 injection into a brine-saturated core, simulating the CO2 injection period (drainage) conditions; and (ii) brine injection following the CO2 injection test, reproducing post-injection period (imbibition) conditions. Downstream pressure was maintained during these two experiments at 10 MPa, and the temperature was set at 50 °C to replicate the subsurface environment at Janggi pilot site. Two different injection rates (q = 1.0 and 0.1 ml/min) were applied. The capillary numbers, \({{\rm{N}}}_{{\rm{c}}}=\,\frac{{\rm{H}}}{|{\rm{\Delta }}{P}_{c}|}\frac{{\rm{\Delta }}P}{L}\) (where L and H represent the length and diameter of the core, respectively; \({\rm{\Delta }}P\) represents the pressure drop; and \(|{\rm{\Delta }}{P}_{c}|\) was calculated from the capillary pressure curves), were ~10−1 and ~10−2, for q = 1.0 and 0.1 ml/min, respectively.
Pore size distribution and capillary pressure
The pore size distributions and capillary pressure curves for the silty sand matrix and clasts in the conglomerate sample were determined using a mercury injection porosimeter (MICP). To perform MICP tests, two small subsamples (~1 cm3) were taken from the conglomerate core each representing either the silty sand matrix or clasts. These subsamples were dried in a vacuum oven at 70 °C for 24 hours prior to testing. The MICP and corresponding mercury saturation measured in the mercury/air system were transformed into a capillary pressure and corresponding CO2 saturation for a CO2/brine system using the following relationship: \(\frac{{P}_{c,C{O}_{2}}}{{P}_{c,Hg}}=\frac{{\sigma }_{C{O}_{2}}\,cos{\theta }_{C{O}_{2}}}{{\sigma }_{Hg}\,cos{\theta }_{Hg}}\), where \({P}_{c}\) (Pa) represents the capillary pressure, \(\sigma \) (mN m−1) represents the interfacial tension (IFT) between two fluids, and \(\theta \) (deg) represents the contact angle measured in the wetting phase. The IFT of \({\sigma }_{Hg}\) = 485 mN m−1 and \({\sigma }_{C{O}_{2}}\)=32.6 mN m−1 was used in the conversion for the geologic CO2 sequestration conditions. The contact angles for the two systems were assumed to be equal29.
References
IPCC. Intergovernmental panel on climate changes. Special report on CO2 capture and storage. (Cambridge University Press, 2005).
Schrag, D. P. Storage of carbon dioxide in offshore sediments. Science 325, 1658–1659 (2009).
Michael, K. et al. Geological storage of CO2 in saline aquifers-A review of the experience from existing storage operations. International Journal of Greenhouse Gas Control 4, 659–667 (2010).
Litynski, J. T., Plasynski, S., Mcllvried, H. G., Mahoney, C. & Srivastava, R. D. The United States Department of Energy’s regional carbon sequestration partnerships program validation phase. Environment International 34, 127–138 (2008).
Hosa, A., Esentia, M., Stewart, J. & Haszeldine, S. Injection of CO2 into saline formations: Benchmarking worldwide projects. Chemical Engineering Research and Design (2011).
Bacon, D. H., Sminchak, J. R., Gerst, J. L. & Gupta, N. Validation of CO2 injection simulations with monitoring well data. Energy Procedia 1, 1815–1822 (2009).
Gislason, S. R. & Oelkers, E. H. Carbon storage in basalt. Science 344, 373–374 (2014).
Matter, J. M. et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 352, 1312–1314 (2016).
Myer, L. Global status of geologic CO2 storage technology development. (United States carbon sequestration council report July 2011, 2011).
Sorai, M., Fujii, T., Kano, Y., Uehara, S. & Honda, K. Experimental study of sealing performance: Effects of particle size and particle-packing state on threshold pressure of sintered compacts. Journal of Geophysical Research: Solid Earth 119, 5482–5496 (2014).
Reynolds, C. A., Menke, H., Andrew, M., Blunt, M. J. & Krevor, S. Dynamic fluid connectivity during steady-state multiphase flow in a sandstone. Proceedings of the National Academy of Sciences, https://doi.org/10.1073/pnas.1702834114 (2017).
Kweon, H. & Deo, M. The impact of reactive surface area on brine-rock-carbon dioxide reactions in CO2 sequestration. Fuel 188, 39–49 (2017).
Carroll, S. A., McNab, W. W., Dai, Z. & Torres, S. C. Reactivity of Mount Simon Sandstone and the Eau Claire Shale under CO2 storage conditions. Environmental Science and Technology 47, 252–261 (2013).
Iglauer, S., Wulling, W., Pentland, C. H., Al-Mansoori, S. K. & Blunt, M. J. Capillary-trapping capacity of sandstones and sandpacks. SPE Journal 16, 778–783 (2011).
Xu, R., Li, R., Ma, J., He, D. & Jiang, P. Effect of mineral dissolution/precipitation and CO2 exsolution on CO2 transport in geological carbon storage. Accounts of Chemical Research 50, 2056–2066 (2017).
Bourg, I. C., Beckingham, L. E. & DePaolo, D. J. The nano basis of CO2 trapping for geologic storage. Envrionmental Science & Technology 49, 10265–10284 (2015).
Yang, C., Dai, Z., Romanak, K. D., Hovorka, S. D. & Trevino, R. H. Inverse modeling of water-rock-CO2 batch experiments: Potential impacts on groundwater resources at carbon sequestration sites. Environmental Science and Technology 48, 2798–2806 (2014).
Smith, M. M., Hao, Y. & Carroll, S. A. Development and calibration of a reactive trasnport model for carbonate reservoir porosity and permeability changes based on CO2 core-flood experiments. International Journal of Greenhouse Gas Control 57, 73–88 (2017).
Sun, Y., Li, Q., Yang, D. & Liu, X. Laboratory core flooding experimental systems for CO2 geosequestration: An updated review over the past decade. Journal of Rock Mechanics and Geotechnical Engineering 8, 113–126 (2016).
Chaouche, M., Rakotomalala, N., Salin, D., Xu, B. & Yortsos, Y. C. Capillary effects in drainage in heterogeneous porous media: continuum modelling, experiments and pore network simulations. Chemical Engineering Science 49, 2447–2466 (1994).
Krause, M., Perrin, J.-C. & Benson, S. Modeling permeability distribution in a sandstone core for history matching coreflood experiments. SPE Journal 16, 768–777 (2011).
Pini, R. & Benson, S. Characterization and scaling of mesoscale heterogeneities in sandstones. Geophysical Research Letters 40, 3903–3908 (2013).
Akbarabadi, M. & Piri, M. Relative permeability hysteresis and capillary trapping characteristics of supercritical CO2/brine systmes: An experimental study at reservoir conditions. Advances in Water Resources 52, 190–206 (2013).
Bachu, S. & Bennion, B. Effects of in-situ conditions on relative permeability characteristics of CO2-brine systems. Environmental Geology 54, 1707–1722 (2008).
Krevor, S. C., Pini, R., Zuo, L. & Benson, S. M. Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resources Research 48, W02532 (2012).
Aggelopolos, C. A., Robin, M. & Vizika, O. Interfacial tension between CO2 and brine (NaCl + CaCl2) at elevated pressure and temperature: The additive effect of different salts. Advances in Water Resources 34, 505–511 (2011).
Chalbaud, C. et al. Interfacial tension measurements and wettability evaluation for geological CO2 storage. Advances in Water Resources 32, 98–109 (2009).
Pini, R. & Benson, S. Simultaneous determination of capillary pressure and relative permeability curves from core-flooding experiments with various fluid pairs. Water Resources Research 49, 3516–3530 (2013).
Pentland, C. H., El-Maghraby, R., Iglauer, S. & Blunt, M. J. Measurements of the capillary trapping of super-critical carbon dioxide in Berea sandstone. Geophysical Research Letter 38, https://doi.org/10.1029/2011GL046683 (2011).
Plug, W.-J. & Bruining, J. Capillary pressure for the sand-CO2-water system under various pressure conditions. Application to CO2 sequestration. Advances in Water Resources 30, 2339–2353 (2007).
Oh, J., Kim, K.-Y., Han, W. S., Park, E. & Kim, J.-C. Migration behavior of supercritical and liquid CO2 in a stratified system: Experiments and numerical simulations. Water Resources Research 51, https://doi.org/10.1002/2015WR017022 (2015).
Oh, J., Kim, K.-Y., Han, W. S. & Park, E. Transport of CO2 in heterogeneous porous media: Sptio-temporal variation of trapping mechanisms. International Journal of Greenhouse Gas Control 57, 52–62 (2017).
Al-Menhali, A. & Krevor, S. Capillary trapping of CO2 in oil reservoirs: Observations in a mixed-wet carbonate rock. Environmental Science & Technology 50, 2727–2734 (2016).
Huo, D., Li, B. & Benson, S. M. Investigating aperture-based stress-dependent permeability and capillary pressure in rock fractures. Society of Petroleum Engineers https://doi.org/10.2118/170819-MS (2014).
Oh, J. et al. Experimental and numerical study on supercritical CO2/brine transport in a fractured rock: Implications of mass transfer, capillary pressure and storage capacity. Advances in Water Resources 62, 442–453 (2013).
Peysson, Y., Laurent, A. & Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers-Part 1: Experimental investigation of drying effects, salt precipitation and capillary forces. International Journal of Greenhouse Gas Control 22, 291–300 (2014).
Miri, R., van Noort, R., Aagaard, P. & Hellevang, H. New insights on the physics of salt precipitation during injection of CO2 into saline aquifers. International Journal of Greenhouse Gas Control 43, 10–21 (2015).
Chang, C., Zhou, Q., Xia, L., Li, X. & Yu, Q. Dynamic displacement and non-equilibrium dissolution of supercritical CO2 in low-permeability sandstone: An experimental study. International Journal of Greenhouse Gas Control 14, 1–14 (2013).
Pruess, K., Oldenburg, C. & Moridis, G. TOUGH2 user’s guide, version 2. (Lawrence Berkeley National Laboratory, Berkeley, California, 2012).
Juanes, R., Spiteri, E. J., Orr, F. M. Jr. & Blunt, M. J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resources Research 42, W12418 (2006).
Krevor, S. C., Pini, R., Li, B. & Benson, S. M. Capillary heterogeneity trapping of CO2 in a sandstone rock at reservoir conditions. Geophysical Research Letter 38, L15401, https://doi.org/10.1029/2011GL048239 (2011).
Land, C. S. Calculation of imbibition relative permeability for two and three-phase flow from rock properties. SPE Journal 8, 149–156 (1968).
Iglauer, S., Wulling, W., Pentland, C. H., Al Mansoori, S. K. & Blunt, M. J. Capillary trapping capacity of sandstones and sandpacks. Paper SPE 120960 presented at the EUROPE/EAGE conference and exhibition, Amsterdam, 8–11 June (2009).
Pentland, C. H. et al. Measurement of nonwetting-phase trapping in sandpacks. SPE Journal 15, 274–281 (2010).
Harrison, W. B., Grammer, G. M. & Barnes, D. A. Reservoir characteristics of the Bass Islands Dolomite in Otsego County, Michigan: results for a saline reservoir CO2 sequestration demonstration. 2. Environmental Geosciences 16, 139–151 (2009).
Gu, H.-C. & Hwang, I. G. Depositional history of the Janggi Conglomerate controlled by tectonic subsidence, during the early stage of Janggi Basin evolution. Journal of the Geological Society of Korea 53, 221–240 (2017).
KIGAM. Selection and characterization of pilot-scale geological storage site. 281 (Korea Institute of Geoscience and Mineral Resources, Daejeon, South Korea, 2017).
Pini, R., Krevor, S. C. M. & Benson, S. M. Capillary pressure and heterogeneity for the CO2/water system in sandstone rocks at reservoir conditions. Advances in Water Resources 38, 48–59 (2012).
Acknowledgements
This research was supported by the Korea CCS R&D Center (Korea CCS 2020 Project) grant funded by the Ministry of Science and ICT (KCRC-2014M1A8A1049287). The authors also appreciate partial financial support by the Basic Research Project of the Korea Institute of Geoscience and Mineral Resources (KIGAM) also funded by the Ministry of Science and ICT; and the Yonsei University Future-leading Research Initiative of 2017 (2017-22-0044).
Author information
Authors and Affiliations
Contributions
K.K. conceived and designed the research; K.K. and J.O. performed the experiments. K.K., J.O. and H.W. interpreted the results and wrote the manuscript. K.P., Y.S. and E.P. analyzed the data. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, KY., Oh, J., Han, W.S. et al. Two-phase flow visualization under reservoir conditions for highly heterogeneous conglomerate rock: A core-scale study for geologic carbon storage. Sci Rep 8, 4869 (2018). https://doi.org/10.1038/s41598-018-23224-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23224-6
This article is cited by
-
Analysis of the Pressure-Pulse Propagation in Rock: a New Approach to Simultaneously Determine Permeability, Porosity, and Adsorption Capacity
Rock Mechanics and Rock Engineering (2019)
-
An experimental method to visualize shear-induced channelization of fluid flow in a rough-walled fracture
Hydrogeology Journal (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.