Abstract
The surgeon dissecting the base of the mesenterium, around the superior mesenteric vein (SMV) and artery, is facing a complex tridimensional vascular anatomy and should be aware of the anatomical variants in this area. The aim of this systematic review is to propose a standardized terminology of the superior mesenteric vessels, with impact in colon and pancreatic resections. We conducted a systematic search in PubMed/MEDLINE and Google Scholar databases up to March 2017. Forty-five studies, involving a total of 6090 specimens were included in the present meta-analysis. The pooled prevalence of the ileocolic, right colic and middle colic arteries was 99.8%, 60.1%, and 94.6%, respectively. The superior right colic vein and Henle trunk were present in 73.9%, and 89.7% of specimens, respectively. In conclusion, the infra-pancreatic anatomy of the superior mesenteric vessels is widely variable. We propose the term Henle trunk to be used for any venous confluence between gastric, pancreatic and colic veins, which drains between the inferior border of the pancreas and up to 20 mm downward on the right-anterior aspect of the SMV. The term gastrocolic trunk should not be synonymous, but a subgroup of the Henle trunk, together with to gastropancreatocolic, gastropancreatic, or colopancreatic trunk.
Similar content being viewed by others
Introduction
The global burden of colorectal cancer parallels the present human development levels, and by 2030 is expected to increase by 60%, to more than 2.2 million new cases and 1.1 million deaths1. For colon cancer patients, the surgical resection represents the mainstay of treatment, with a 5-year relative survival of 89.9% and 71.3% for localized and regional stages, respectively2. However, the location of the tumor in the right colon is emerging as a significant negative prognostic factor, with a 20% increased risk of death compared with the cancers arising on the left side3,4.
During the latest years, the western concept of complete mesocolic excision with central vascular ligation (CME-CVL)5 and the eastern D3 lymphadenectomy6 proved their oncological superiority over conventional colonic resections, with lower 5-year local recurrence rate and better overall survival7. The surgical safety, better perioperative results and non-inferior long-term oncological outcomes were proved for the laparoscopic CME-CVL8 or D3 lymphadenectomy (Supplementary Table I)9,10. However, these surgical procedures are technically difficult and associated with more intraoperative organ injuries and severe non-surgical complications11.
Understanding the complex tridimensional anatomy of the superior mesenteric vein (SMV) and artery (SMA) is of paramount importance to minimize the iatrogenic injuries during modern radical resections for right colon cancers or surgical resection of tumors located in the uncinate process of the pancreas12,13,14. Standard textbooks of surgery are schematic, often contradictory, and do not offer the required anatomical details for one who embark on refined techniques such as CME-CVL, D3 lymphadenectomy for right colon cancers or pancreatic resections for tumors located in the uncinate process. A comprehensive knowledge of the infra-pancreatic SMV and SMA surgical anatomy is required.
The objective of this systematic review is to propose a standardized terminology of the superior mesenteric vessels, resulted from meta-analysis of the existing evidence, with impact in colon and pancreatic resections.
Results
Description of studies
Results of the search
The initial electronic and printed literature research retrieved 2258 articles. 1905 papers were excluded after the title and abstract screening, and 353 full-text articles were further evaluated. 308 scientific articles were excluded, and 45 studies, involving a total of 6090 specimens, met the inclusion criteria and were included in the qualitative and quantitative (meta-analysis) synthesis (Fig. 1). 15 studies come from Europe15,16,17,18,19,20,21,22,23,24,25,26,27,28,29, 20 from Asia30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49, and ten from the United States of America50,51,52,53,54,55,56,57,58,59.
Included studies
The characteristics of the included studies are summarized in the Table 1. The studies were published between 1909–2017, with a sample size ranging from 9 to 610 specimens. The superior mesenteric vessels and their branches were investigated by dissections of cadaveric specimens in 21 studies15,16,22,23,24,25,26,27,29,38,39,44,45,49,52,54,55,56,57,58,59, by imaging methods in 16 studies (CT − 13 studies18,21,28,32,33,34,35,37,40,43,50,51,53, MRI – one study41, CT and surgery – one study20, Angiography – one study46), and by dissection during surgical procedures in eight studies (surgical dissection only – six studies17,30,31,36,47,48, surgical dissection and angiography – one study42, surgical and cadaveric dissection – one study19).
Quality assessment of the included studies
The risk of bias according to the authors of the present study was low for 24 studies, moderate for 15 studies, and high for six studies (Supplementary Table II).
The inter-observer agreement was 86.7% (k = 0.779, P < 0.001) for sample representativity for the target population, 93.3% (k = 0.641, P < 0.001) for participants recruitment, 91.1% (k = 0.830, P < 0.001) for the sample size adequacy, 91.1% (k = 0.463, P < 0.001) for the detail of description for subjects and setting, 93.3% (k = 0.536, P < 0.001) for the data analysis, 95.6% (k = 0.727, P < 0.001) for criteria used for measurement of the condition, 95.6% (could not be computed) for the reliability of measurement, 95.6% (k = 0.776, P < 0.001) for statistical analysis, 91.1% (k = 0.831, P < 0.001) for confounding factors and subgroups, and 88.9% (k = 0.845, P < 0.001) for subpopulations identification.
Pooled prevalence and morphometric data of superior mesenteric artery and vein
Ileocolic vessels
The ileocolic vessels were the most constant anatomical structures, with a pooled prevalence of 99.7% and 99.8% for ilecolic vein (ICV) and artery (ICA), respectively (Table 2 and Fig. 2). The ileocolic vein drainage was into the SMV in 97.6% of cases, into the Henle trunk in 1.9%, and into the jejunal trunk in 0.5% of cases. Related to the SMV, the ICA had a trajectory anterior to the vein in 42.6%, and posterior in 57.4% of cases (Fig. 2).
The subgroup analysis of studies with more than 100 included specimens, the continent of origin (Europe, Asia, and the USA), and the method of vessel characterization (imagistic, surgical, and cadaveric) revealed no significant changes in the size of the effects (Supplementary Table III).
The pooled ICA crossing length was 15.2 mm.
Right colic vessels
The right colic vein (RCV) was present in 59.1% of cases (Table 2). The RCV’s drainage was into the Henle trunk, SMV, and ICV in 50.3%, 49.0%, and 0.8% of specimens. In 83.2% there was a single MCV, while in 13.4%, and 3.4% there were two, and three MCVs.
The right colic artery (RCA) was present in 60.1% of cases. The origin of the RCA was into the SMA, MCA, and ICA in 70.8%, 15.4%, and 13.8%. The trajectory of the RCA related to the SMV was anterior in 89.4%, and posterior in 10.6% of cases (Fig. 2).
The pooled ICA to RCA distance was 16.0 mm. The mean RCA crossing length was 20.7 mm.
Superior right colic vein (SRCV)
The SRCV was present in 73.9% of specimens (Table 2). The SRCV drained into the Henle trunk, SMV, MCV, and RCV in 94.1%, 3.6%, 1.5%, and 0.8% of cases, respectively.
Middle colic vessels
The middle colic vein (MCV) was present in 96.7% of cases (Table 2). There was one, two or three MCVs in 69.7%, 25.9%, and 4.4% of specimens. The MCV drained into the SMV in 83.2%, into the Henle trunk in 11.7%, into the inferior mesenteric vein in 1.9%, into the first jejunal trunk in 1.8%, or into the splenic vein in 1.5% of cases.
The middle colic artery (MCA) was present in 94.6% of cases. There was one MCA in 88.4% of cases, two in 10.6%, and three in 1.0%. The MCA origin was in the SMA in 78.7%, in the RCA in 17.8%, in the ICA in 0.8%, in the left colic artery in 0.8%, in the inferior pancreaticoduodenal artery in 0.6%, in the hepatic artery in 0.6%, in the splenic artery in 0.3%, and in the celiac artery in 0.3% of cases.
Henle trunk
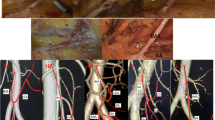
The Henle trunk, defined as confluence of the right gastroepiploic vein (RGEV) with one or more colic veins, and with or without a pancreatic vein, was present in 89.7% of specimens (Table 2 and Fig. 3). The Henle trunk was a gastro-pancreato-colic trunk (GPCT) in 60.5%, a gastro-pancreatic trunk (GPT) in 33.7%, a gastro-colic trunk (GCT) in 4.5%, and a colo-pancreatic trunk (CPT) in 1.3% of cases (Fig. 4).
The Henle trunk was a GCT made by the RGEV and SRCV in 5.4% of cases. It was a GPT made RGEV and anterosuperior pancreaticoduodenal vein (ASPDV) in 26.7%, and a CPT made by the ASPDV and SRCV in 1.1% of specimens.
The pooled prevalence of the Henle trunk as a GPCT trunk formed by RGEV, ASPDV and one colic vein was: 38.6% for RGEV + ASPDV + SRCV, 5.9% for RGEV + ASPDV + RCV, 2.1% for RGEV + ASPDV + MCV, and 0.9% RGEV + ASPDV + ICV.
The prevalence of the Henle trunk as a GPCT trunk formed by RGEV, ASPDV and 2 colic veins was: 9.5% for RGEV + ASPDV + RCV + SRCV, 2.6% for RGEV + ASPDV + RCV + MCV, 2.3% for RGEV + ASPDV + SRCV + MCV, 1.0% for RGEV + ASPDV + RCV + ICV.
The pooled prevalence of the Henle trunk as a GPCT trunk formed by RGEV, ASPDV and 3 colic veins was: 2.7% for RGEV + ASPDV + RCV + SRCV + MCV, and 1.2% for RGEV + ASPDV + RCV + SRCV + ICV.
The Henle trunk drained into the SMV, and right intestinal trunk of the SMV in 81.6% and 18.4% of cases, respectively.
The Henle trunk had a pooled mean diameter of 3.9 mm (Fig. 5), and a mean length of 14.2 mm. The pooled mean distance between the inferior border of the pancreas and the emergence of the Henle trunk was 7.5 mm.
Publication bias
Sensitivity analysis was conducted to assess statistical heterogeneity, through the exclusion of specific studies with high risk of bias (Supplementary Table IV). There were no relevant changes in the overall effects of the quantitative synthesis. Analysis of the LFK index revealed no asymmetries for 19 outcomes, minor asymmetries for 17 outcomes, and major asymmetries for 33 outcomes (Table 2, Supplementary Figures 1 and 2).
Discussions
The present systematic review and meta-analysis demonstrates anatomical variants of the superior mesenteric vessels with impact in surgical dissection during radical resections for right colon and pancreatic head cancer. The superiority of meta-analyzing the anatomical findings over simply pooling the results is that data of individual studies are weighted initially, then combined60.
Over the latest decade, the implementation of minimally invasive surgery has dramatically increased in the field of colorectal surgery, given is proven superior perioperative outocomes61,62. However, ongoing concerns were regarding the quality of the resected specimen and the long-term oncological outcomes, especially for the most refined techniques such as CME-CVL or D3 lymphadenectomy during right hemicolectomy63,64. The right hemicolectomy with CME-CVL has a long, and without a plateau learning curve, correlating with the complex anatomy and necessity for meticulous dissection around critical structures65. The reported conversion rate in laparoscopic colectomy is 10–20%, one of the most frequent reasons for that being bleeding66,67.
The CME-CVL or D3 lymphadenectomy require ligation of the ICV, RCV, Henle trunk, and MCV on their emergence from the SMV, and of the ICA, RCA, and MCA on their emergence from the SMA. In our meta-analysis, we found a wide range of anatomical variability of the major vascular structures, which suggests that surgical dissection during right hemicolectomy with CME-CVL is not straightforward, and should be done carefully, following the embryological planes. The D3 area has the following anatomical boundaries: (a) cranially – five mm proximal to the horizontal line through the Henle trunk and MCA origins; (b) caudally – five mm distal to the horizontal line through the origin of the ICA; (c) medially – the left edge of the SMA; (d) laterally – one cm from the right edge of the SMV25. Should be noted the difficulty of the CME-CVL surgical technique, which requires reflection of SMV to centrally ligate the colic arteries68. A recent concept included the ICA and RCA crossing lengths, which are the length of these arteries which traverse the anterior or posterior aspects of the SMV22. We found a pooled mean ICA, and RCA crossing lengths of 15.2 mm, and 20.7 mm, respectively (Fig. 6). The reported incidence rate of metastasis in central ileocolic lymph nodes was up to 11.1%, which justifies the surgeon struggling to centrally ligate the vessels42,52,69.
In the present study, the ICA and ICV were the most constant anatomical structures and should be used as landmarks for starting dissection along the SMV axis. The RCA and RCV were the most inconstant anatomical structures. The middle colic vessels were constantly present. Should be noted that right and middle colic arteries were also multiple, two or even three, in a significant number of cases. The reported rate of intraoperative bleeding during minimally invasive colectomies range from 3% to 9.2%70. We found that ICA and RCA had a trajectory posterior to the SMV in 57.4% and 10.6%, which suggests the high risk of vein injury when the operating surgeon try to control bleeding from one of these pedicles, retracted posteriorly to the SMV.
The SRCV was a common anatomical structure in our study, being present in almost 74% of specimens. Should be noted the anatomical difference between the RCV, which drains the blood from the marginal veins of the ascending colon and the SRCV which drains the hepatic flexure of the colon. We consider inappropriate the terminology of accessory RCV or MCV be used for the anatomical structure that drains the hepatic flexure of the colon. We propose a common terminology which should include the SRCV terminology.
The Henle trunk had a very complex and highly variable tridimensional anatomical structure. In 1868, Henle described a venous confluence formed by the RGEV and the superior right colic vein71, and Descomps and De Lalaubie added in 1912 the third element, the ASPDV56,72. We are proposing a standardized terminology, with impact in the right colon, pancreatic, and gastric oncological resections (Table 3 and Fig. 7). We propose the term Henle trunk to be used for any venous confluence between gastric, pancreatic and colic veins, which drains between the inferior border of the pancreas and up to 20 mm downward on the right-anterior aspect of the SMV. We propose that term ‘gastrocolic trunk’ should not be synonymous, but a subgroup of the Henle trunk, together with to ‘gastropancreatocolic, gastropancreatic, or colopancreatic trunk’. To propose a common terminology, easy to be implemented in clinical practice, we grouped all the anatomical variants with a pooled prevalence less than 5.0% in the ‘Type VI’ (Table 3). The Type I has the highest pool prevalence, and the Type V the lowest, but higher than 5%. Usually, intraoperative bleeding occurs through inadvertent traction by the surgical assistant, with tearing of these fragile veins.
Our proposed standardized terminology for Henle trunk surgical anatomy. ASPDV – anterosuperior pancreaticoduodenal vein; RGEV – right gastroepiploic vein; RCV – right colic vein; SRCV – superior right colic vein. To proposed a common terminology for Henle trunk, we grouped all the anatomical variants with a pooled prevalence less than 5.0% in the ‘other’ group of ‘Type VI’. Should be noted that Type I has the highest pooled prevalence, and the Type V the lowest.
Bertelsen et al. showed that CME-CVL technique is associated with higher rate of intraoperative organ injuries (9.1% vs. 3.6%, P < 0.001), including SMV lesions (1.7% vs. 0.2%, P < 0.001)11. The CME-CVL group had a higher rate of sepsis requiring vasopressors (6.6% vs. 3.2%, P = 0.001) and respiratory failure (8.1% vs. 3.4%, P < 0.001)11. Freund et al. described five cases (1.6%) of SMV injuries from a total of 304 radical right colectomies73. Only two of these injuries were observed during the initial surgery, and three patients required saphenous graft reconstruction, with one postoperative death73.
Preoperative planning of the right hemicolectomy and pancreatic resection, based on high-quality imaging, is expecting to decrease the rate of adverse intraoperative events while improving the quality of the resected specimen33,74,75. Mari et al. showed that patient’s vascular mapping using CT angiography, before right hemicolectomy (38 patients), significantly reduced the operating time (130 16.3 vs. 147 28.2 minutes, P = 0.027), decreased the difficult identification of the mesenteric vessels intraoperative identification of the SMV (1 vs. 7 cases, P = 0.053), and decreased the intraoperative bleeding (P = 0.006)76.
Laparoscopic pancreaticoduodenectomy is a very complex procedure, which expands its indications and clinical implementation worldwide. The current evidence proposed a hospital threshold of 22 cases per year to minimize the associated postoperative complications77. However, in experienced centers, the long-term oncological outcomes of minimally invasive approach are non-inferior to the open surgery78.
The uncinate process pancreatic cancers (UPPC) have been regarded as tumors associated with an ominous prognosis and even lower resection rate compared with similar tumors located in the pancreatic head14. This is attributed mainly to their very intimate relationships with the superior mesenteric vessels79. A study comparing 161 patients with UPPC with 292 non-UPPC patients showed that uncinate tumors had a higher rate of SMA invasion (P < 0.001), lower resectability (P = 0.003), and lower R0 resection rate (22.3% vs. 35.6%, P = 0.003)80. After R0 resection, the UPPC patients had a poorer overall survival (median 21 vs. 26 months, P = 0.018), with a higher local recurrence rate (P = 0.038) and early occurrence of the local relapse (median 13 vs. 52 months, P < 0.001)81. We consider that careful preoperative planning of the surgical technique, and understanding of the complex vascular anatomy from the base of the mesenterium is especially important in patients with tumors located in the uncinate process of the pancreas. Miyazawa et al. used the tridimensional CT to map the Henle trunk vascular anatomy in 120 patients before pancreaticoduodenectomy33. The authors concluded that understanding of the vascular anatomy might prevent bleeding in the separation of the pancreas and transverse colon during pancreaticoduodenectomy, especially in obese patients33. For invasive pancreatic cancers in the uncinate process, venous resection including spleno-mesenteric junction is often required to achieve R0 resection. In such cases, a marginal vein in the hepatic flexure later becomes a thick collateral drainage of the splenic venous flow82. SRCV often forms a part of this marginal way, and should be ligated as central as possible to preserve the passway. Careless sacrifice of SRCV at peripheral part causes defect of marginal passway, leading to intraluminal varices at the hepatic flexure or bleeding of varicose veins. If preservation of SRCV nor right colic vessels were not possible due to cancer invasion, concomitant right colectomy is needed. In such a case, reconstruction of the splenic vein would be an option to prevent postoperative sinistral portal hypertension.
Although the most comprehensive study in the literature about the topic according to our knowledge, should be acknowledged that this meta-analysis has several limitations, especially due to the heterogeneity of the terminology used in the included studies. Another important limitation of the current study is related to the inherent differences between imagistic, surgical and imagistic methods of vessel characterization. Third, between the included studies there was a significant variability of the patients’ geographical origin, number of specimens, and pre-existing morbidities. However, by using the random effects model for pooled data and a large number of specimen analyzed we minimized the effects of heterogeneity.
Conclusions
The infra-pancreatic anatomy of the superior mesenteric vessels is widely variable. The surgical dissection during right hemicolectomy with CME-CVL is not straightforward and should be done carefully, following the embryological planes. We propose the term Henle trunk to be used for any venous confluence between gastric, pancreatic and colic veins, which drains between the inferior border of the pancreas and up to 20 mm downward on the right-anterior aspect of the SMV. The term gastrocolic trunk should not be synonymous, but a subgroup of the Henle trunk, together with to gastropancreatocolic, gastropancreatic, or colopancreatic trunk.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)83 and Meta-Analysis of Observational Studies in Epidemiology (MOOSE)84 guidelines in conducting and reporting the results of this systematic review and meta-analysis.
Data sources and search strategy
We searched the PubMed/MEDLINE and Google Scholar databases, up to March 31, 2017. The search strategy combined key words related to the superior mesenteric vein and artery surgical anatomy. We used no language restrictions. We screened the reference list of the full-text articles to identify additional relevant studies. The search strategy used in PubMed/Medline database was detailed in Supplementary Table V.
Study selection
Study eligibility criteria: We included all the studies detailing the branching pattern and morphometric data of the SMV and SMA. Exclusion criteria: (1) conference proceedings; (2) sample in a specific subset of the general population (e.g. portal hypertension patients); (3) animal studies; (4) case reports, review articles, editorials and letters to the editor; (5) overlapping or duplicate reports.
Outcome measures
Primary outcomes: branching pattern of the SMV and SMA. Secondary outcomes: anatomical relationships between the venous and arterial branches, anatomical relationships between arterial branches and SMV, morphometric data of the blood vessels with impact in right colon and pancreatic surgical oncology. The clinical questions to be addressed are: (a) Which is an adequate nomenclature for the Henle trunk; (b) Which type of dissection is recommended during right colectomy according to vascular variability; (c) How should central vascular ligation during right hemicolectomy be performed; (d) What is “risky” anatomy of the gastrocolic trunk or mesenteric-portal venous systems during right colectomy or pancreatiododenectomy; (e) Which are the surgical technique options to manage pancreatic tumor located in the uncinate process.
Data extraction
Data from individual studies were extracted independently by two authors (IN, SH). We used a predefined electronic protocol; the disagreements being resolved by discussion. We extracted from full texts and supplemental materials the following data: year of publication, first author, title, journal, contact address, country of the study, inclusion and exclusion criteria, sample size, demographic data, subgroup of patients, method of vessels investigation, branching pattern of the SMV and SMA, diameter of vessels, anatomical relationships between the venous and arterial branches, distance between the origins of these vessels.
Quality assessment
We used the JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data85 to assess the methodological quality of the included studies. This grades sample representativity for the target population, participants recruitment in an appropriate way, if the sample size is adequate, the detail of description for subjects and setting, if the data analysis was conducted with sufficient coverage of the identified sample, if objective, standard criteria were used for measurement of the condition, the reliability of measurement, if statistical analysis was appropriate, if all confounding factors and subgroups were identified and accounted for, and if subpopulations were identified using objective criteria85. For each of the ten domains, we have attributed 2 points for Yes, 1 point for Unclear, and 0 points for No. According to the total score, studies were considered to present a low, moderate or high risk of bias if this was 17, 13–16, 12 points, respectively. Two authors (IN, SH) independently performed the quality assessment. The inter-observer agreement of the quality assessment was calculated using percent agreement and Cohen’s kappa coefficient86,87. The disagreements were resolved by a consensus process.
Statistical analysis
For statistical analysis, we used as statistical software the MetaXL version 5.3 (EpiGear International Pty Ltd, Queensland, Australia)88, and openMeta[Analyst]TM89 version 12.11.14. The venous and arterial branching pattern was defined by calculating the multi-categorical pooled prevalence. When the estimate for a specific study tends toward 0% or 100%, the variance moves toward zero, and in consequence, its weight is overestimated in a meta-analysis of prevalence88. Therefore, we preferred to use the double arcsine transformation over the logit when calculated multiple category prevalences, as this stabilizes the variance and makes it dependent only on the population size88. For the continuous data, we calculated the pooled mean of the superior mesenteric vein, artery, or of their branches. We used Cochran’s Q test (2) and I2 statistics to evaluate the studies’ heterogeneity90. The P < 0.1 and a 50% were considered the cut-off value between low and high heterogeneity91. To allow the between-study variation, we used the random-effect model meta-analyses85. To assess the publication bias we used the Begg’s funnel plot92, Doi plot, and Luis Furuya-Kanamori (LFK) index93. An LFK index within 1 was interpreted as no asymmetry, exceeding 1 but within 2 as minor asymmetry, and exceeding 2 as major asymmetry. The subgroup analysis and meta-regression considered the influence on the size of the effect of the method of vessels characterization (surgical, imagistic Computed Tomography, Magnetic Resonance Imaging, Angiography or cadaveric dissection or corrosion casts), the continent origin of the study, year of publication, the number of included patients. Reasons for statistical heterogeneity were explored using sensitivity analyses, through the exclusion of specific studies one by one and compared the results.
Data availability
All the data are available at the corresponding authors and can be offered on request.
References
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66, 683–691, https://doi.org/10.1136/gutjnl-2015-310912 (2017).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2014, https://seer.cancer.gov/csr/1975_2014/ (2017).
Petrelli, F. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncology 3, 211–219, https://doi.org/10.1001/jamaoncol.2016.4227 (2017).
Wang, B. et al. Tumor location as a novel high risk parameter for stage II colorectal cancers. PLOS ONE 12, e0179910, https://doi.org/10.1371/journal.pone.0179910 (2017).
Hohenberger, W., Weber, K., Matzel, K., Papadopoulos, T. & Merkel, S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 11, 354–364, discussion 364–355, https://doi.org/10.1111/j.1463-1318.2008.01735.x (2009).
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. International journal of clinical oncology, https://doi.org/10.1007/s10147-017-1101-6 (2017).
West, N. P. et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 30, 1763–1769, https://doi.org/10.1200/jco.2011.38.3992 (2012).
Siani, L. M., Lucchi, A., Berti, P. & Garulli, G. Laparoscopic complete mesocolic excision with central vascular ligation in 600 right total mesocolectomies: Safety, prognostic factors and oncologic outcome. American journal of surgery 214, 222–227, https://doi.org/10.1016/j.amjsurg.2016.10.005 (2017).
Yamamoto, S. et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Annals of surgery 260, 23–30, https://doi.org/10.1097/sla.0000000000000499 (2014).
Kitano, S. et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. The lancet. Gastroenterology & hepatology 2, 261–268, https://doi.org/10.1016/s2468-1253(16)30207-2 (2017).
Bertelsen, C. A. et al. Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. The British journal of surgery 103, 581–589, https://doi.org/10.1002/bjs.10083 (2016).
Rotellar, F. et al. Laparoscopic resection of the uncinate process of the pancreas: the inframesocolic approach and hanging maneuver of the mesenteric root. Surgical endoscopy 25, 3426–3427, https://doi.org/10.1007/s00464-011-1740-z (2011).
Machado, M. A., Makdissi, F. F., Surjan, R. C. & Machado, M. C. Laparoscopic resection of uncinate process of the pancreas. Surgical endoscopy 23, 1391–1392, https://doi.org/10.1007/s00464-009-0390-x (2009).
O’Sullivan, A. W., Heaton, N. & Rela, M. Cancer of the uncinate process of the pancreas: surgical anatomy and clinicopathological features. Hepatobiliary & pancreatic diseases international: HBPD INT 8, 569–574 (2009).
Zhang, J. et al. Radioanatomic study of the gastrocolic venous trunk. Surgical and radiologic anatomy: SRA 16, 413–418 (1994).
Birtwisle, Y. et al. Venous drainage of the pancreas and its relations to pancreatic phlebography. Anatomia Clinica 5, 103–113 (1983).
VanDamme, J. & Bonte, J. Vascular anatomy in abdominal surgery. (Thieme Medical Publisher, 1990).
Ferrari, R. et al. Anatomical variations of the coeliac trunk and the mesenteric arteries evaluated with 64-row CT angiography. La Radiologia medica 112, 988–998, https://doi.org/10.1007/s11547-007-0200-2 (2007).
Lange, J. F. et al. The gastrocolic trunk of Henle in pancreatic surgery: an anatomo-clinical study. Journal of hepato-biliary-pancreatic surgery 7, 401–403, https://doi.org/10.1007/s005340050208 (2000).
Nesgaard, J. M., Stimec, B. V., Bakka, A. O., Edwin, B. & Ignjatovic, D. Navigating the mesentery: a comparative pre- and per-operative visualization of the vascular anatomy. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 17, 810–818, https://doi.org/10.1111/codi.13003 (2015).
Spasojevic, M., Stimec, B., Fasel, J., Terraz, S. & Ignjatovic, D. 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surgical endoscopy 25, 1883–1886 (2011).
Ignjatovic, D., Sund, S., Stimec, B. & Bergamaschi, R. Vascular relationships in right colectomy for cancer: clinical implications. Techniques in coloproctology 11, 247–250, https://doi.org/10.1007/s10151-007-0359-5 (2007).
Ignjatovic, D., Stimec, B., Finjord, T. & Bergamaschi, R. Venous anatomy of the right colon: three-dimensional topographic mapping of the gastrocolic trunk of Henle. Techniques in coloproctology 8, 19–21, https://doi.org/10.1007/s10151-004-0045-9 (2004). discussion 21-12.
Ignjatovic, D., Spasojevic, M. & Stimec, B. Can the gastrocolic trunk of Henle serve as an anatomical landmark in laparoscopic right colectomy? A postmortem anatomical study. The American Journal of Surgery 199, 249–254 (2010).
Spasojevic, M. et al. Lymph node distribution in the d3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Diseases of the colon and rectum 56, 1381–1387, https://doi.org/10.1097/01.dcr.0000436279.18577.d3 (2013).
Gamo, E. et al. The superior mesenteric artery and the variations of the colic patterns. A new anatomical and radiological classification of the colic arteries. Surgical and radiologic anatomy: SRA 38, 519–527, https://doi.org/10.1007/s00276-015-1608-3 (2016).
Haywood, M., Molyneux, C., Mahadevan, V., Lloyd, J. & Srinivasaiah, N. The right colic artery: An anatomical demonstration and its relevance in the laparoscopic era. Annals of the Royal College of Surgeons of England 98, 560–563, https://doi.org/10.1308/rcsann.2016.0257 (2016).
Kaye, T. L., West, N. P., Jayne, D. G. & Tolan, D. J. CT assessment of right colonic arterial anatomy pre and post cancer resection - a potential marker for quality and extent of surgery? Acta radiologica (Stockholm, Sweden: 1987) 57, 394–400, https://doi.org/10.1177/0284185115583033 (2016).
Jamieson, J. K. & Dobson, J. F. VII. Lymphatics of the Colon: With Special Reference to the Operative Treatment of Cancer of the Colon. Annals of surgery 50, 1077–1090 (1909).
Cao, L. L. et al. The Impact of Confluence Types of the Right Gastroepiploic Vein on No. 6 Lymphadenectomy During Laparoscopic Radical Gastrectomy. Medicine 94, e1383, https://doi.org/10.1097/md.0000000000001383 (2015).
Cheng, B. C. et al. [Surgical anatomy of the colic vessels in Chinese and its influence on the operation of esophageal replacement with colon]. Zhonghua yi xue za zhi 86, 1453–1456 (2006).
Murono, K. et al. Evaluation of the vascular anatomy of the right-sided colon using three-dimensional computed tomography angiography: a single-center study of 536 patients and a review of the literature. International journal of colorectal disease 31, 1633–1638, https://doi.org/10.1007/s00384-016-2627-1 (2016).
Miyazawa, M. et al. Preoperative evaluation of the confluent drainage veins to the gastrocolic trunk of Henle: understanding the surgical vascular anatomy during pancreaticoduodenectomy. Journal of hepato-biliary-pancreatic sciences 22, 386–391, https://doi.org/10.1002/jhbp.205 (2015).
Ogino, T. et al. Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Annals of surgical oncology 21(Suppl 3), S429–435, https://doi.org/10.1245/s10434-014-3572-2 (2014).
Hirai, K. et al. Three-dimensional computed tomography for analyzing the vascular anatomy in laparoscopic surgery for right-sided colon cancer. Surgical laparoscopy, endoscopy & percutaneous techniques 23, 536–539, https://doi.org/10.1097/SLE.0b013e31828f66fb (2013).
Tajima, Y. et al. Three-dimensional vascular anatomy relevant to oncologic resection of right colon cancer. International surgery 96, 300–304 (2011).
Sakaguchi, T. et al. Analysis of anatomic variants of mesenteric veins by 3-dimensional portography using multidetector-row computed tomography. American journal of surgery 200, 15–22, https://doi.org/10.1016/j.amjsurg.2009.05.017 (2010).
Jin, G. et al. Anatomic study of the superior right colic vein: its relevance to pancreatic and colonic surgery. American journal of surgery 191, 100–103, https://doi.org/10.1016/j.amjsurg.2005.10.009 (2006).
Shatari, T. et al. Vascular anatomy for right colon lymphadenectomy. Surgical and radiologic anatomy: SRA 25, 86–88, https://doi.org/10.1007/s00276-003-0100-7 (2003).
Yamada, Y. et al. CT assessment of the inferior peripancreatic veins: clinical significance. American Journal of Roentgenology 174, 677–684 (2000).
Ito, K., Blasbalg, R., Hussain, S. M. & Mitchell, D. G. Portal vein and its tributaries: evaluation with thin-section three-dimensional contrast-enhanced dynamic fat-suppressed MR imaging. Radiology 215, 381–386, https://doi.org/10.1148/radiology.215.2.r00ap04381 (2000).
Yada, H. et al. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World journal of surgery 21, 109–115 (1997).
Mori, H., McGrath, F. P., Malone, D. E. & Stevenson, G. W. The gastrocolic trunk and its tributaries: CT evaluation. Radiology 182, 871–877, https://doi.org/10.1148/radiology.182.3.1535911 (1992).
Adachi, B. Das Arteriensystem der Japaner. 18–64 (Kyoto, Kaiserlich-japanische Universität zu Kyoto, in kommission bei “Maruzen Co.”, Kyoto and Tokyo, 1928).
Yamaguchi, S., Kuroyanagi, H., Milsom, J. W., Sim, R. & Shimada, H. Venous anatomy of the right colon: precise structure of the major veins and gastrocolic trunk in 58 cadavers. Diseases of the colon and rectum 45, 1337–1340, https://doi.org/10.1097/01.dcr.0000027284.76452.84 (2002).
Chung, W. & Jun, S. Anatomical Variations of the Right Colic Artery. J Korean Surg Soc. 54, 991–995 (1998).
Alsabilah, J. F., Razvi, S. A., Albandar, M. H. & Kim, N. K. Intraoperative Archive of Right Colonic Vascular Variability Aids Central Vascular Ligation and Redefines Gastrocolic Trunk of Henle Variants. Diseases of the colon and rectum 60, 22–29, https://doi.org/10.1097/dcr.0000000000000720 (2017).
Lee, S. J., Park, S. C., Kim, M. J., Sohn, D. K. & Oh, J. H. Vascular Anatomy in Laparoscopic Colectomy for Right Colon Cancer. Diseases of the colon and rectum 59, 718–724, https://doi.org/10.1097/dcr.0000000000000636 (2016).
Kuzu, M. A. et al. Variations in the Vascular Anatomy of the Right Colon and Implications for Right-Sided Colon Surgery. Diseases of the colon and rectum 60, 290–298, https://doi.org/10.1097/dcr.0000000000000777 (2017).
Vedantham, S., Lu, D. S., Reber, H. A. & Kadell, B. Small peripancreatic veins: improved assessment in pancreatic cancer patients using thin-section pancreatic phase helical CT. AJR. American journal of roentgenology 170, 377–383, https://doi.org/10.2214/ajr.170.2.9456949 (1998).
Graf, O. et al. Anatomic variants of mesenteric veins: depiction with helical CT venography. AJR. American journal of roentgenology 168, 1209–1213, https://doi.org/10.2214/ajr.168.5.9129413 (1997).
Garcia-Ruiz, A., Milsom, J. W., Ludwig, K. A. & Marchesa, P. Right colonic arterial anatomy. Implications for laparoscopic surgery. Diseases of the colon and rectum 39, 906–911 (1996).
Crabo, L. G., Conley, D. M., Graney, D. O. & Freeny, P. C. Venous anatomy of the pancreatic head: normal CT appearance in cadavers and patients. AJR. American journal of roentgenology 160, 1039–1045, https://doi.org/10.2214/ajr.160.5.8385877 (1993).
Nelson, T. M., Pollak, R., Jonasson, O. & Abcarian, H. Anatomic variants of the celiac, superior mesenteric, and inferior mesenteric arteries and their clinical relevance. Clinical Anatomy 1, 75–91, https://doi.org/10.1002/ca.980010202 (1988).
Michels, N. A., Siddharth, P., Kornblith, P. L. & Parke, W. W. The variant blood supply to the descending colon, rectosigmoid and rectum based On 400 dissections. its importance in regional resections: A review of medical literature. Diseases of the colon and rectum 8, 251–278 (1965).
Gillot, C. et al. The superior mesenteric vein, an anatomic and surgical study of eighty-one subjects. The Journal of the International College of Surgeons 41, 339–369 (1964).
Sonneland, J., Anson, B. J. & Beaton, L. E. Surgical anatomy of the arterial supply to the colon from the superior mesenteric artery based upon a study of 600 specimens. Surgery, gynecology & obstetrics 106, 385–398 (1958).
Basmajian, J. V. The main arteries of the large intestine. Surgery, gynecology & obstetrics 101, 585–591 (1955).
Steward, J. A. & Rankin, F. W. Blood supply of the large intestine: Its surgical considerations. Archives of Surgery 26, 843–891, https://doi.org/10.1001/archsurg.1933.01170050113008 (1933).
Bravata, D. M. & Olkin, I. Simple pooling versus combining in meta-analysis. Evaluation & the health professions 24, 218–230, https://doi.org/10.1177/01632780122034885 (2001).
Veldkamp, R. et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. The Lancet. Oncology 6, 477–484, https://doi.org/10.1016/s1470-2045(05)70221-7 (2005).
Nelson, H. et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. The New England journal of medicine 350, 2050–2059, https://doi.org/10.1056/NEJMoa032651 (2004).
West, N. P. et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 272–278, https://doi.org/10.1200/jco.2009.24.1448 (2010).
Lu, J. Y. et al. The Radical Extent of lymphadenectomy — D2 dissection versus complete mesocolic excision of LAparoscopic Right Colectomy for right-sided colon cancer (RELARC) trial: study protocol for a randomized controlled trial. Trials 17, https://doi.org/10.1186/s13063-016-1710-9 (2016).
Melich, G. et al. Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy–analysis of learning curves for a novice minimally invasive surgeon. Canadian journal of surgery. Journal canadien de chirurgie 57, 331–336 (2014).
Tekkis, P., Senagore, A. & Delaney, C. Conversion rates in laparoscopic colorectal surgery: a predictive model with, 1253 patients. Surgical Endoscopy and Other Interventional Techniques 19, 47–54 (2005).
Belizon, A., Sardinha, C. & Sher, M. Converted laparoscopic colectomy: what are the consequences? Surgical endoscopy 20, 947 (2006).
Alsabilah, J., Kim, W. R. & Kim, N. K. Vascular Structures of the Right Colon: Incidence and Variations with their Clinical Implications. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society, https://doi.org/10.1177/1457496916650999 (2016).
Park, I. J., Choi, G.-S., Kang, B. M., Lim, K. H. & Jun, S. H. Lymph node metastasis patterns in right-sided colon cancers: is segmental resection of these tumors oncologically safe? Annals of surgical oncology 16, 1501–1506 (2009).
Marcello, P. W., Roberts, P. L., Rusin, L. C., Holubkov, R. & Schoetz, D. J. Vascular pedicle ligation techniques during laparoscopic colectomy. A prospective randomized trial. Surgical endoscopy 20, 263–269, https://doi.org/10.1007/s00464-005-0258-7 (2006).
Henle, J. Handbuch der systematschen anatomise des menschen. III. Handbuch der gefaesslehre des Menschen note 1. 371 (Friedrich Vieweg und Sohn Braunschweig, 1868).
Descomps, P. & De Lalaubie, G. Les veines mésentériques. J Anat Physio Norm Pathol Homme Anim 48, 337–376 (1912).
Freund, M. R., Edden, Y., Reissman, P. & Dagan, A. Iatrogenic superior mesenteric vein injury: the perils of high ligation. International journal of colorectal disease 31, 1649–1651, https://doi.org/10.1007/s00384-016-2624-4 (2016).
Ignjatovic, D. Safe D3 Right Hemicolectomy for Cancer Through 3D MDCT Angiography Reconstruction, https://clinicaltrials.gov/ct2/show/NCT01351714 (2017).
Matsuki, M. et al. Dual-phase 3D CT angiography during a single breath-hold using 16-MDCT: assessment of vascular anatomy before laparoscopic gastrectomy. AJR. American journal of roentgenology 186, 1079–1085, https://doi.org/10.2214/ajr.04.0733 (2006).
Mari, F. S. et al. Role of CT angiography with three-dimensional reconstruction of mesenteric vessels in laparoscopic colorectal resections: a randomized controlled trial. Surgical endoscopy 27, 2058–2067 (2013).
Adam, M. A. et al. Defining a Hospital Volume Threshold for Minimally Invasive Pancreaticoduodenectomy in the United States. JAMA surgery 152, 336–342, https://doi.org/10.1001/jamasurg.2016.4753 (2017).
Conrad, C. et al. Comparable long-term oncologic outcomes of laparoscopic versus open pancreaticoduodenectomy for adenocarcinoma: a propensity score weighting analysis. Surgical endoscopy, https://doi.org/10.1007/s00464-017-5430-3 (2017).
Liu, C. et al. Comparison of Uncinate Process Cancer and Non-Uncinate Process Pancreatic Head Cancer. Journal of Cancer 7, 1242–1249, https://doi.org/10.7150/jca.15062 (2016).
Kang, M. J. et al. Comparison of the long-term outcomes of uncinate process cancer and non-uncinate process pancreas head cancer: poor prognosis accompanied by early locoregional recurrence. Langenbeck’s archives of surgery 395, 697–706, https://doi.org/10.1007/s00423-010-0593-6 (2010).
Kang, C. M. et al. Pancreatoduodenectomy following neoadjuvant chemoradiation therapy in uncinate process pancreatic cancer. Pancreas 41, 467–473, https://doi.org/10.1097/MPA.0b013e31822a68bc (2012).
Ono, Y. et al. Sinistral portal hypertension after pancreaticoduodenectomy with splenic vein ligation. The British journal of surgery 102, 219–228, https://doi.org/10.1002/bjs.9707 (2015).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, https://doi.org/10.1136/bmj.b2700 (2009).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000).
Munn, Z., Moola, S., Lisy, K., Riitano, D. & Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. International journal of evidence-based healthcare 13, 147–153, https://doi.org/10.1097/xeb.0000000000000054 (2015).
Cohen, J. A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement 20, 37–46, https://doi.org/10.1177/001316446002000104 (1960).
McHugh, M. L. Interrater reliability: the kappa statistic. Biochemia Medica 22, 276–282 (2012).
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E. & Vos, T. Meta-analysis of prevalence. Journal of epidemiology and community health 67, 974–978, https://doi.org/10.1136/jech-2013-203104 (2013).
Henry, B. M., Tomaszewski, K. A. & Walocha, J. A. Methods of Evidence-Based Anatomy: a guide to conducting systematic reviews and meta-analysis of anatomical studies. Annals of anatomy=Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft 205, 16–21, https://doi.org/10.1016/j.aanat.2015.12.002 (2016).
Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. 2010 36, 48, https://doi.org/10.18637/jss.v036.i03 (2010).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560, https://doi.org/10.1136/bmj.327.7414.557 (2003).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634, https://doi.org/10.1136/bmj.315.7109.629 (1997).
Barendregt, J. J. & Doi, S. A. MetaXL User Guide version 5.3. (EpiGear International Pty Ltd, 2016).
Author information
Authors and Affiliations
Contributions
Conception and design: I.N. and M.B. Analysis and interpretation of data: I.N., M.B., S.H., R.I.N., and Y.I. Contribution to discussion: I.N., M.B., S.H., R.I.N., and Y.I. Writing of the manuscript: I.N. Study supervision: M.B. and Y.I. All authors have equally contributed to this scientific paper and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Negoi, I., Beuran, M., Hostiuc, S. et al. Surgical Anatomy of the Superior Mesenteric Vessels Related to Colon and Pancreatic Surgery: A Systematic Review and Meta-Analysis. Sci Rep 8, 4184 (2018). https://doi.org/10.1038/s41598-018-22641-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22641-x
This article is cited by
-
A SICE (Società Italiana di Chirurgia Endoscopica e Nuove Tecnologie) observational prospective multicenter study on anatomical variants of the superior mesenteric artery: intraoperative analysis during laparoscopic right hemicolectomy—CoDIG 2 database (ColonDx Italian Group)
Updates in Surgery (2024)
-
Usefulness of intraoperative ultrasound examination for laparoscopic right-side colon cancer surgery: a propensity score-matched study
Scientific Reports (2023)
-
The usefulness of the endoscopic surgical skill qualification system in laparoscopic right hemicolectomy: a single-center, retrospective analysis with propensity score matching
Langenbeck's Archives of Surgery (2023)
-
Safe oncological and standardised (“SOS”) right hemicolectomy for colon cancer
Techniques in Coloproctology (2023)
-
Use of individualized 3D-printed models of pancreatic cancer to improve surgeons’ anatomic understanding and surgical planning
European Radiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.