Abstract
We recently advocated in favour of naming a novel H2-haplotype consisting of Kd, D/Ldm7, I-Ak and I-Ek in the atopic dermatitis (AD) mouse model NC/Nga as “H-2nc.” The role of the H2-haplotype in AD development was investigated in H2b-congenic NC/Nga mice (NC.h2b/b and NC.h2b/nc) established by backcrossing. A severe 2,4-dinitrofluorobenzene (DNFB)-induced dermatitis in NC/Nga was alleviated partially in NC.h2b/nc and significantly in NC.h2b/b. The AD phenotype was correlated with thymic stromal lymphopoietin (TSLP)-epidermal expression levels and serum levels of total IgE and IL-18/IL-33. Histologically, allergic contact dermatitis (ACD) was accompanied by lymphocytes and plasma cells-infiltrating perivasculitis in NC.h2b/nc and NC.h2b/b and clearly differed from AD accompanied by neutrophils, eosinophils and macrophages-infiltrating diffuse suppurative dermatitis in NC/Nga. Interestingly, IFN-γ/IL-17 production from autoreactive CD4+ T-cells remarkably increased in DNFB-sensitised NC.h2b/b but not in NC/Nga. Our findings suggest that AD or ACD may depend on haplotype H-2nc or H-2b, respectively, in addition to the NC/Nga genetic background.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease caused by the interaction between genetic and environmental factors. NC/Nga mice are used as an AD-like disease model by many researchers because these mice spontaneously develop an AD-like disease under conventional condition, but not under specific pathogen-free (SPF) condition1. However, the possibility of inducing sustained dermatitis following application of 2,4-dinitrofluorobenzene (DNFB) in NC/Nga mice2 is important for research purposes using laboratory mice under SPF condition. The sustained application of DNFB on skin can elicit allergic contact dermatitis (ACD)-like disease (type IV allergy) through its role as a hapten3, and while BALB/c (H-2d), A/J (H-2a) and CBA mice (H-2k) are susceptible to DNFB-induced dermatitis development, C57BL/6 (H-2b), C57BL/10 (H-2b) and AB.Y mice (H-2b) are not, depending on the H2-haplotype4. Interestingly, DNFB-induced dermatitis in NC/Nga mice under SPF condition is associated with the development of AD-like disease (type I allergy) rather than ACD-like disease (type IV allergy)2. Because AD-like disease can develop in DNFB-applied BALB/c mice by adaptive transfer of immunoglobulin E (IgE) recognising DNFB as a hapten5, the immunoglobulin class switching to IgE is possible key for the development of AD-like disease. Interestingly, Interleukin (IL)-4 expression is not induced after repeated DNFB challenge in NC/Nga mice2. Even though IL-4 is a major cytokine for IgE-class switching6, the mechanism underlying the hyperproduction of IgE is not dependent on IL-4 levels in DNFB-treated NC/Nga mice but could rely on IL-13 inducing IL-4-independent IgE synthesis7,8. AD and ACD are both common skin diseases with an immune pathogenesis9 and histologically, include spongiotic lesions10. During clinical diagnosis, sometimes it is difficult to distinguish between AD and ACD because they present as eczematous dermatitis and may co-exist10. The relevance between AD and ACD has not been fully elucidated. Therefore, the DNFB-induced dermatitis model using NC/Nga mice and H-2b-congenic NC/Nga mice may represent an appropriate model for investigating the potential association between AD and ACD.
The genotype of AD sensitivity has been investigated in the NC/Nga genetic background. Genome-wide expression analysis between NC/Nga and BALB/c mice revealed the differential expression of more than 1,000 genes in the skin of mite antigen-exposed NC/Nga mice11. These included cytokines, cytokine receptors, proteases, and adhesion molecules. In humans, the deficiency of the skin barrier molecule filaggrin leads to alkalisation of the skin, favouring bacterial infections and increased metal ion–protein hapten complexes12. In NC/Nga mice having developed AD, the expression of filaggrin is low, but increases with treatment of an opioid analgesic drug JTC-80113. Our group recently found that the clonal deletion of T cell repertoires with specific T cell receptor Vβ chains is induced by the expression of two endogenous superantigens (Mls-1a, MMTV(SHN)) in the genetic background of NC/Nga mice14. This finding was in line with a previous report showing that under conventional condition, the Th1 dominant state mediated by IL-12 or IL-18 after Staphylococcal enterotoxin B (SEB) exposure was inhibited by the absence of Vβ8+ T-cells in NC/Nga mice, resulting in the induction of the Th2 dominant state15.
Genome-wide expression analysis indicates that besides inflammatory factors or the skin barrier system, the human genotype of AD sensitivity is also associated with MHC regions16,17. We recently proposed the name “H-2nc” for the novel H2-haplotype consisting of Kd, Ddm7/Ldm7-hybrid mutant (D/Ldm7), I-Ak and I-Ek in NC/Nga mice18. Here, H-2b congenic NC/Nga mice (NC.h2b/b or NC.h2b/nc) established by backcrossing (8 generations) were investigated for their sensitivity to DNFB-induced dermatitis. The role of the H2-haplotype in AD development in the NC/Nga strain is discussed.
Results
Establishment of H-2 congenic NC/Nga mice (NC/Nga)
To investigate whether the H2-haplotype (H-2nc) in NC/Nga mice (NC/Nga) is a cause for high sensitivity to AD-like disease, we established H-2 congenic mice (NC.h2b/b, NC.h2b/nc and NC.h2nc/nc) by backcrossing (8 generations) the NC/Nga strain (Fig. 1A). Because the haplotype H-2nc in NC/Nga strain shows the phenotype (Kd, I-Ak, I-Ek)18, we tested peripheral blood mononuclear cells (PBMCs) from filial generation after backcrossing with NC/Nga strain by flow cytometry on the expression of Kd and Kb. Kd-negative and Kb-positive populations, Kd-positive and Kb-positive populations and Kd-positive and Kb-negative populations in PBMCs indicate the haplotypes H-2b, H-2b/nc and H-2nc, respectively (Fig. 1B). It was confirmed that the H2-phenotype of NC.h2b/b shows Kb, Db, and I-Ab (Fig. 1C).
Establishment of H-2 congenic mice with NC/Nga background. A. The process of establishing H-2 congenic mice (NC.h2b/b, NC.h2b/nc and NC.h2nc/nc) by NC/Nga backcross is indicated. B. H-2 congenic mice (NC.h2b/b, NC.h2b/nc and NC.h2nc/nc) were sorted according to the expression pattern of Kd and Kb of PBMCs by flow cytometry. C. The H2-haplotype of NC.h2b/b was checked based on the expression patterns of Kb, Db, and I-Ab of PBMCs using flow cytometry.
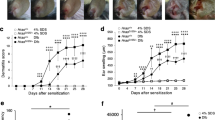
In DNFB-induced dermatitis model, the degrees of inflammation and dermatitis scores in NC.h2 b/nc and NC.h2 b/b are alleviated compared with that in NC/Nga
DNFB-induced dermatitis in NC/Nga (or NC.h2nc/nc), NC.h2b/b and NC.h2b/nc was evaluated by both a scoring index of AD19 and the myeloperoxidase (MPO) activity using in vivo Imaging System. The degree of dermatitis score in DNFB-applied NC.h2b/b was significantly alleviated compared with that in NC/Nga at days 12 and 14 (p < 0.05, ANOVA), whereas DNFB-applied NC.h2b/c was partially alleviated (Fig. 2A,C). Particularly, dryness and erosion were strongly reduced in NC.h2b/b and NC.h2b/c mice compared with the effects seen in NC/Nga (Fig. 2D). The result of in vivo Imaging System at day 14 were in line with the degree of dermatitis scores, reflecting the degree of inflammation with MPO production due to the accumulation of activated neutrophils and macrophages (Fig. 2B).
In DNFB-induced dermatitis, degrees of inflammation and dermatitis scores in NC.h2b/nc and NC.h2b/b are alleviated compared with NC/Nga. A. Gross lesion of DNFB-induced dermatitis shown for each H-2 congenic mice on day 14. B. MPO activity by in vivo Imaging System shown for each H-2 congenic mice on day 14. C. The gross lesion at day 14 of DNFB-induced dermatitis in each H-2 congenic mice was evaluated by a scoring index of atopic dermatitis. *NC/Nga vs. NC.h2b/b or NC.h2b/nc, p < 0.05 ANOVA. D. The scoring index of DNFB-induced dermatitis in NC/Nga, NC.h2b/b and NC.h2b/nc are indicated. E. The concentration of serum IL-18, IL-33 and IgE in each mouse on day 14 were measured by ELISA method. *DNFB vs. control, p < 0.05, ANOVA. †NC.h2b/b vs. NC/Nga, p < 0.05, ANOVA.
Serum IL-18, IL-33 and IgE produced by DNFB-induced dermatitis in NC.h2 b/nc and NC.h2 b/b are decreased compared with NC/Nga
Serum levels of IL-18 and IL-33 are generally known as mast cell activators, and when binding to Fc epsilon receptors (FcεRs) on the surface of mast cells, IgE stimulate antigen-specific degranulation. Serum levels of IL-18, IL-33 and IgE were significantly increased in NC/Nga, NC.h2b/nc or NC.h2b/b by DNFB-application against each control (p < 0.05, ANOVA). Serum levels of IL-18, IL-33 and IgE increased by DNFB-application were lower in NC.h2b/b than in NC/Nga (p < 0.05, ANOVA) (Fig. 2E). Although there were no statistically significant differences, serum levels of IL-18 (p = 0.14, ANOVA), IL-33 (p = 0.16, ANOVA) and IgE (p = 0.064, ANOVA) in NC.h2b/c tended to be lower than those in NC/Nga.
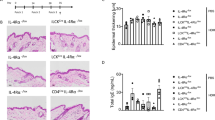
Histologically, DNFB induces ACD-like lesions in NC.h2 b/nc and NC.h2 b/b, whereas AD-like lesions are induced in NC/Nga
Histologically, DNFB application induced dermatitis lesions in all mice including NC/Nga, NC.h2b/nc and NC.h2b/b (Fig. 3A). Thickening of the epidermis and dermis in the skin or ear were strongest developed in the following order: NC/Nga < NC.h2b/nc < NC.h2b/b (Fig. 3A). A sign of AD-like lesion accompanied by band-like infiltration of neutrophils, eosinophils and macrophages in the dermis was observed in NC/Nga (Fig. 3B,C). Additionally, signs of ACD-like lesions20 accompanied by fibroblast proliferation in dermis (Fig. 3E) and distinctive perivasculitis with infiltrating lymphocytes and plasma cells (Fig. 3F) were mainly observed in both NC.h2b/nc and NC.h2b/b. Although high expression levels of thymic stromal lymphopoietin (TSLP) was remarkably detected in the NC/Nga epidermis (Fig. 3D), TSLP expression was observed in perivascular tissues rather than in the epidermis in NC.h2b/nc and NC.h2b/b (Fig. 3G). Although it was at a low level, serum TSLP levels also significantly increased in DNFB-induced dermatitis of NC/Nga than in that of NC.h2b/b (p < 0.05, ANOVA; Supplemental Figure S1A,B). Furthermore, serum TNF-α and CCL2 levels in DNFB-applied NC/Nga also partially increased compared with those in DNFB-applied NC.h2b/b. However, there is no difference in serum IL-1β levels in both mice (Supplemental Figure S1A, B).
Histologically on DNFB-induced dermatitis, contact dermatitis was induced in NC.h2b/nc and NC.h2b/b while AD-like lesions were induced in NC/Nga. (A) Thickening of the epidermis (black arrow) and the perivasculitis of the dermis (blue arrow) in upper panel (skin) are indicated. Thickening of the epidermis (blue arrow) and dermis (black arrow) in lower panel (ear) are indicated. Partially extended thickening of the dermis is highlighted (red arrow). Scale bar corresponds to 100 µm in all images of (A). Against the infiltration of eosinophils in the dermis (B) and in perivascular (C), and the TSLP expression in epidermis (D) in NC/Nga, the infiltration of lymphocytes and plasma cells in the dermis (E) and in perivascular (F), and the TSLP expression in perivascular (G) in NC.h2b/b is indicated. Scale bar in (B), (C), (E), (F) represents 25 µm. Scale bar in (D) and (G) is 50 µm.
In axillary lymphadenopathy with DNFB-induced dermatitis, the production of IFN-γ and IL-17 from autoreactive CD4 + T-cells in NC.h2 b/b were enhanced compared with that in NC/Nga
Mice with DNFB-induced dermatitis exhibit an axillary lymphadenopathy phenotype (data not shown) that we further investigated in this study. As IL-4, IFN-γ and IL-17 are hardly detected in serum, we assessed their productions from CD4+ T-cells in axillary lymph nodes (LNs) by co-culture of these CD4+ T-cells with naïve CD11b+ myeloid cells (as antigen-presenting cells) whose growth response was stopped by X-ray irradiation. The productions of IFN-γ and IL-17, but not of IL-4, from proliferating autoreactive CD4+ T-cells in NC.h2b/b were remarkably detected compared with NC/Nga (p < 0.05, ANOVA) (Fig. 4A–C). This result indicates that the total IgE production in DNFB-induced dermatitis of the NC/Nga strain is not dependent on IL-4-based mechanisms because IL-4 was hardly detected.
In axillary lymph nodes (LNs) with DNFB-induced dermatitis, the production of IFN-γ and IL-17 from autoreactive CD4+ T-cells in NC.h2b/b is increased compared with that in NC/Nga. The autoreactive proliferation of CD4+ T-cells purified from axillary LNs was analyzed by co-culturing with naïve CD11b+ myeloid cells from the same strain. Growth picture (A) and graph of MTT assay (B) are indicated. *DNFB vs. control, p < 0.05, ANOVA. †NC.h2b/b vs. NC/Nga, p < 0.05, ANOVA. (C) The levels of IL-17, IFN-γ and IL-4 in culture supernatant was analyzed by the ELISA method. P/I; PMA+ Ionomycin. *DNFB vs. control, p < 0.05, ANOVA. †P/I vs. Nil, p < 0.05, ANOVA. The bar graphs (n = 4) are indicated as mean ± SD.
Discussion
Here, we investigated the role of the H2-haplotype in the NC/Nga genomic background using H2-congenic mice (NC.h2b/b, NC.h2b/nc and NC.h2nc/nc) established by backcrossing the NC/Nga strain. Severe dermatitis induced by repeated DNFB-application under SPF condition in NC/Nga was alleviated partially in NC.h2b/nc and significantly in NC.h2b/b as shown by decreased epidermal expression levels of TSLP and serum levels of total IgE, TSLP, IL-18 and IL-33. Histologically, ACD-like lesion of NC.h2b/nc and NC.h2b/b differed from AD-like lesions observed in NC/Nga. With the poor expression of IL4, the total IgE production in DNFB-induced dermatitis of NC/Nga is likely stimulated via an IL-4-independent mechanism.
The IL-1 family members IL-18 and IL-33 are highly inflammatory cytokines constitutively expressed in barrier cell types21, acting as regulators of innate and acquired immune responses by amplifying both Th1 and Th2 responses with or without TCR activation22. IL-18 and IL-33 signal their biologically activities through the heterodimeric receptors IL-18R and IL-33R in mast cells. IL-18 serum levels are elevated in AD patients or AD-induced NC/Nga mice23,24. IL-33 is an important mediator of allergy through its ability to induce Th2 cytokines and has been associated with development of severe AD25. IL-33 initiates allergic inflammation by activating innate lymphoid cells type 2 (ILC2s) to produce large amounts of Th2 cytokines IL-5 and IL-13 responsible for eosinophilia and IgE-class switching, respectively7,26,27,28.
Our results indicate that autoreactive CD4+ T-cells in axillary lymphadenopathy of DNFB-sensitised mice produce increased levels of IFN-γ and IL-17 in NC.h2b/b compared with NC/Nga. Autoreactive CD4+ T-cells were previously reported to be induced in the DNFB-induced ACD model29. We confirm in this study that autoreactive CD4+ T-cells lead to ACD-like lesions in both DNFB-induced NC.h2b/b and BALB/c mice. Although there are no reports on autoallergens specific to autoreactive CD4+ T-cells in ACD-developed mice, it is conceivable that autoreactive CD4+ T cells recognizing a carrier-protein of DNFB (a hapten) as self-antigens may be generated. In humans, the autoallergens Hom s 1–5 had been identified by screening of a human epithelial complementary DNA library for IgE binding of sera from AD patients30,31,32. Hom s 2 corresponds to the alpha-chain of the nascent polypeptide-associated complex (α-NAC) and α-NAC-specific autoreactive CD8+ T-cells were identified as secreting both IL-4 and IFN-γ in AD patients33. However, autoreactive CD4+ T-cells from PBMCs are not generated by α-NAC peptides in AD patients34. Recently, we detected IFN-γ-producing autoreactive CD8+ T-cells from NC/Nga mice with DNFB-induced AD that were expanded by co-culturing with dendritic cells treated with proteasome inhibitor MG132 (data not shown). Although an MHC class I mutant D/Ldm7 has been downregulated by degradation with proteasome activation, the increase in D/Ldm7 by MG132 addition was associated with the generation of autoreactive CD8+ T-cells, but not of autoreactive CD4+ T-cells (data not shown). Thus, autoreactive CD8+ T-cells or CD4+ T-cells are induced in AD-like lesions or in ACD-like lesions, respectively. Although it is a matter of speculation, the autoreactive Th1/Th17-like cells detected in ACD-like lesions may be inhibited in vivo by immunosuppressive cells other than CD4+ T-cells as these lesions display a weak inflammation phenotype compared with AD-like lesions and no sign of autoimmune disease and may play a role of inhibiting the generation of autoreactive CD8+ T-cells in changing AD-like lesions into ACD-like lesions.
Materials and Methods
Mice
NC/Nga (H-2nc) (male and female) and C57BL/6 (H-2b) mice (male) (Japan SLC, Hamamatsu, Japan) were maintained in SPF conditions, and used at 18–20 weeks of age. H2-congenic NC/Nga strain (NC.h2nc/nc, NC.h2b/nc and NC.h2b/b) were generated by backcrossing (8 generations) of NC/Nga mice. Mice were maintained by breeding in SPF air conditions with microbiological monitoring tests (twice/year). All mice were maintained in our full-barrier animal facility under controlled temperature, humidity and 12 hour light/dark regimen. All experiments were approved by the Institutional Review Board for animal studies of NVLU, and performed following the guidelines provided by the Committee.
Flowcytometry
Peripheral blood mononucleated cells (PBMCs) were isolated from mouse blood using Lymphoprep (ProGen, Heidelberg, Germany). For analysis, PBMCs suspended in PBS were stained with a mixture of FITC-conjugated Kd (SF1-1.1), I-Ab (KH74), I-Ak (11-5.2), I-Ek (14-4-45), Db (27-11-13S)-specific mAbs (BioLegend, St. Louis, MO) and PE-conjugated Kb (AF6-88.5), Ld (28-14-8)-specific mAbs (BioLegend). Two-color or one-color analysis was conducted by FACS (FACSCalibur, Nippon Becton Dickinson, Japan).
2,4-dinitrofluorobenzene (DNFB)-induced dermatitis model
The methods in this study have been described previously2. AD-like skin lesions were induced by the repeated application of 25 µl of 0.15% DNFB (Wako Pure Chemicals, Tokyo, Japan) in acetone/olive oil (3:1) to the skin of the ears, calva, and neck on days 0, 3, 5, 7, 9, 11, and 13. The control mice were applied the acetone/olive oil (3:1) alone as the vehicle of DNFB. Dermatitis was evaluated by assigning an inflammation score19. Briefly, inflammation of the face, ears, and the anterior part of the body was scored as follows: 0, none; 1, mild; 2, moderate; and 3, severe. This scoring was based on the severity of erythema/hemorrhage (e/h), edema (ed), excoriation/erosion (e/e), and scaling/dryness (s/d), and total points were evaluated as the severity of dermatitis.
In vivo Imaging for MPO activity
A chemiluminescent in vivo reagent for monitoring inflammation (XenoLight RediJect Inflammation probe, ParkinElmer) was administrated by intraperitoneal (i.p.) injection at 120 μL /mouse. MPO activity was analyzed by using IVIS in vivo imaging (IVIS Lumina II, ParkinElmer) at 10 minutes post i.p. injection of the probe under Pentobarbital anesthesia (Somnopentyl, Kyoritsu seiyaku).
ELISA
Blood samples were collected from the orbital sinus in mice by using hematocrit tube, and serum samples were obtained by centrifugation. The total serum IgE was measured by a mouse IgE ELISA kit (Shibayagi Co., Ltd., Gunma, Japan). Serum IL-18 was measured by mouse IL-18 ELISA kit (MBL). Serum IL-33 was measured by mouse IL-33 Quantikine ELISA kit (R&D system). Culture supernatants were collected from each assay. The IFN-γ and IL-4 were measured by mouse Th1/Th2 ELISA Ready-SET-Go! (eBioscience). The IL-17 was measured by mouse IL-17 Quantikine ELISA kit (R&D system). All assays were following the manufacturer’s instructions.
Histopathology and immunohistochemistry
Histopathological analyses were performed on a minimum of forth animals per experimental group. Tissues were immersion-fixed in 10% buffered formalin and processed by routine methods for paraffin sectioning. Paraffin sections, 5 μm thickness, were stained with hematoxylin and eosin (H&E), and examined by light microscopy.
In immunohistochemistry, rabbit polyclonal TSLP antibody (1:500, Abcam Inc., Cambridge, MA) was added and mixed with biotin goat anti-rabbit secondary antibody (1:1500, Dako A/S, Glostrup, Denmark), followed by peroxidase-conjugated streptavidin (Dako A/S). Finally, the reaction to antigen was visualized by addition of 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) and counterstained with hematoxylin.
MTT assay
The culture medium was RPMI 1640 containing 10% heat-inactivated FCS, L-glutamine, non-essential amino acids, sodium pyruvate, 2-ME, and penicillin-streptomycin (Wako, Tokyo, Japan). CD4+ T-cells and CD11b+ myeloid cells (3 × 105/well) were enriched from LNs by using anti-mouse CD4 or anti-mouse CD11b Magnetic Particles-DM (BD Biosciences). CD4+ T-cells were co-cultured for 72 h at 37 °C in 5% CO2 with CD11b + myeloid cells (3 × 105/well) irradiated 30 Gy X-ray (CP160, Faxitron, Wheeling, IL). The cell proliferation was analyzed by MTT assay based on WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl) -5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] uptake (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan). 10 μl CCK-8 was added for 4 h prior to the end of culture, and absorbance was measured at 450 nm with a microplate reader (Bio-RAD Labs, Hercules, CA, USA). The average of auto-response was calculated as 100%.
Statistical analysis
Statistical analysis was performed with ANOVA using Excel (Microsoft) and StatPlus (AnalystSoft, Alexandria, VA). A p-value < 0.05 was considered significant. ANOVA test was performed after normal distribution test (Shapiro-Wilk test).
References
Matsuda, H. et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9, 461–466, https://doi.org/10.1093/intimm/9.3.461 (1997).
Tomimori, Y., Tanaka, Y., Goto, M. & Fukuda, Y. Repeated topical challenge with chemical antigen elicits sustained dermatitis in NC/Nga mice in specific-pathogen-free condition. J Invest Dermatol 124, 119–124, https://doi.org/10.1111/j.0022-202X.2004.23516.x (2005).
Miller, J. F., Vadas, M. A., Whitelaw, A., & Gamble, J. H-2 gene complex restricts transfer of delayed-type hypersensitivity in mice. Proc Natl Acad Sci USA 72, 5095–5098. PMC388882 (1975)
Sauder, D. N. & Katz, S. I. Strain variation in the induction of tolerance by epicutaneous application of trinitrochlorobenzene. J Invest Dermatol 80, 383–386, https://doi.org/10.1111/1523-1747.ep12551991 (1983).
Katayama, I., Tanei, R., Yokozeki, H., Nishioka, K. & Dohi, Y. Induction of eczematous skin reaction in experimentally induced hyperplastic skin of Balb/C mice by monoclonal anti-DNP IgE antibody: possible implications for skin lesion formation in atopic dermatitis. Int Arch Allergy Appl Immunol. 93, 148–154, https://doi.org/10.1159/000235294 (1990).
Kühn, R., Rajewsky, K. & Müller, W. Generation and analysis of interleukin-4 deficient mice. Science. 254, 707–710, https://doi.org/10.1126/science.1948049 (1991).
Punnonen, J. et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA 90, 3730–3734, https://doi.org/10.1073/pnas.90.8.3730 (1993).
Emson, C. L., Bell, S. E., Jones, A., Wisden, W. & McKenzie, A. N. Interleukin (IL)-4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 188, 399–404, https://doi.org/10.1084/jem.188.2.399 (1998).
Thyssen, J. P., McFadden, J. P. & Kimber, I. Themultiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 69, 28–36, https://doi.org/10.1111/all.12358 (2014).
Rundle, C. W., Bergman, D., Goldenberg, A. & Jacob, S. E. Contact dermatitis considerations in atopic dermatitis. Clin Dermatol. 35, 367–374, https://doi.org/10.1016/j.clindermatol.2017.03.009 (2017).
Heishi, M. et al. Gene expression analysis of atopic dermatitis-like skin lesions induced in NC/Nga mice by mite antigen stimulation under specific pathogen-free conditions. Int Arch Allergy Immunol 132, 355–363, https://doi.org/10.1159/000074903 (2003).
Cabanillas, B. & Novak, N. Atopic dermatitis and filaggrin. Curr Opin Immunol. 42, 1–8, https://doi.org/10.1016/j.coi.2016.05.002 (2016).
Otsuka, A. et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J Allergy Clin Immunol. 133(139–146), e1–10, https://doi.org/10.1016/j.jaci.2013.07.027 (2014).
Ohkusu-Tsukada, K., Tsukada, T. & Takahashi, K. Clonal deletion of T cell repertoires with specific T cell receptor Vβ chains by two endogenous superantigens in NC/Nga mice. Biosci Biotechnol Biochem. 22, 1–4, https://doi.org/10.1080/09168451.2017.1374829 (2017).
Habu, Y. et al. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J. Immunol. 166, 5439–5447, https://doi.org/10.4049/jimmunol.166.9.5439 (2001).
Hirota, T. et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 44, 1222–1226, https://doi.org/10.1038/ng.887 (2012).
Weidinger, S. et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum Mol Genet. 22, 4841–4856, https://doi.org/10.1093/hmg/ddt317 (2013).
Ohkusu-Tsukada, K., Yamashita, T., Tsukada, T, & Takahashi, K. Low expression of a Ddm7/Ldm7-hybrid mutant (D/Ldm7) in the novel haplotype H-2nc identified in atopic dermatitis model NC/Nga mice. Gene Immun. In press
Leung, D. Y. et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 85, 927–933, https://doi.org/10.1016/0091-6749(90)90079-J (1990).
Rowe, A., Farrell, A. M. & Bunker, C. B. Constitutive endothelial and inducible nitric oxide synthase in inflammatory dermatoses. Br J Dermatol. 136, 18–23, https://doi.org/10.1046/j.1365-2133.1997.d01-1136.x (1997).
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA 106, 13463–13468, https://doi.org/10.1073/pnas.0906988106 (2009).
Smithgall, M. D. et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030, https://doi.org/10.1093/intimm/dxn060 (2008).
Tanaka, H. et al. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J. Allergy Clin. Immunol. 107, 331–336, https://doi.org/10.1067/mai.2001.112275 (2001).
Tanaka, T. et al. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int. Arch. Allergy Immunol. 125, 236–240. 53821 (2001)
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490, https://doi.org/10.1016/j.immuni.2005.09.015 (2005).
Yasuda, K. et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA 109, 3451–3456, https://doi.org/10.1073/pnas.1201042109 (2012).
Lopez, A. F. et al. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 167, 219–224, https://doi.org/10.1084/jem.167.1.219 (1998).
McKenzie, G. J. et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 9, 423–432, https://doi.org/10.1016/S1074-7613(00)80625-1 (1998).
Fairchild, R. L. & Moorhead, J. W. Soluble factors in tolerance and contact sensitivity to DNFB in mice. VIII. Regulation of T suppressor cell function by autoreactive T helper cells. Cell Immunol. 117, 35–44, https://doi.org/10.1016/0.008-8749(88)90074-3 (1998).
Valenta, R. et al. Molecular characterization of an autoallergen, Hom s 1, identified by serum IgE from atopic dermatitis patients. J Invest Dermatol 111, 1178–1183, https://doi.org/10.1046/j.1523-1747.1998.00413.x (1998).
Natter, S. et al. Isolation of cDNA clones coding for IgE autoantigens with serum IgE from atopic dermatitis patients. FASEB J 12, 1559–1569 (1998).
Hradetzky, S., Werfel, T. & Rösner, L. M. Autoallergy in atopic dermatitis. Allergo J Int. 24, 16–22, https://doi.org/10.1007/s40629-015-0037-5 (2015).
Roesner, L. M. et al. α-NAC-Specific autoreactive CD8+ T cells in atopic dermatitis are of an effector memory type and secrete IL-4 and IFN-γ. J Immunol. 196, 3245–3252, https://doi.org/10.4049/jimmunol.1500351 (2016).
Hradetzky, S. et al. Cytokine effects induced by the human autoallergen α-NAC. J Invest Dermatol. 134, 1570–1578, https://doi.org/10.1038/jid.2014.25 (2014).
Acknowledgements
We thank Dr. Tohei for microbiological monitoring tests of mice, Dr. Hakamata for management of full-barrier animal facility, and Dr. Yokosuka for welfare of laboratory animal research. The authors would like to thank Enago for the English language review.
Author information
Authors and Affiliations
Contributions
K.O.-T. designed the study and the study was supervised by T.T. Most of the experiments and the data analysis were performed by K.O.-T., D.I. and Y.O. assisted it. K.O.-T. wrote most of the manuscript, K.T. advised it.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohkusu-Tsukada, K., Ito, D., Okuno, Y. et al. Signs of atopic dermatitis and contact dermatitis affected by distinct H2-haplotype in the NC/Nga genetic background. Sci Rep 8, 2586 (2018). https://doi.org/10.1038/s41598-018-21049-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21049-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.