Abstract
A novel technique based on micro-CT scanning is developed to quantify coagulation in fibers of hemodialyzers. This objectivation is needed to allow accurate assessment of thrombogenicity of dialyzers used during hemodialysis, for example when comparing different strategies to avoid coagulation and/or fiber blocking. The protocol allowed imaging at a resolution of 25 µm, making it possible to count the open, non-coagulated fibers in a non-invasive way. In 3 fresh, non-used FX600 hemodialyzers, patent fiber counts were extremely consistent (10748 ± 2). To illustrate the potential of this technique, different dialysis parameters currently used as surrogates for fiber blocking were evaluated during 20 hemodialysis sessions. After dialysis, the FX600 dialyzers were visually scored for clotting, dried and subsequently weighed and scanned. The number of patent fibers (10003 [8763,10330], range 534–10692) did not correlate with any of the recorded surrogate parameters. Micro-CT scanning is a feasible, objective, non-invasive, accurate and reproducible tool for quantification of the degree of fiber blocking in a hemodialyzer after use, making it a potential gold standard for use in studies on fiber blocking during renal replacement therapies.
Similar content being viewed by others
Introduction
Over 2 million people worldwide require renal replacement therapy to sustain life. The vast majority of them are treated with hemodialysis, a technique in which patient’s blood is purified by circulating it through an extracorporeal circuit containing a hemodialyzer, consisting of around 10000 semi-permeable capillary fibers. The patient’s blood flows in the fiber lumen, while an electrolyte water solution (dialysate) flows counter-currently around the fibers to optimize the diffusive concentration gradient necessary to drive solute transport. Disordered blood flow and contact of the blood with the bio-incompatible material of the fibers activate the coagulation cascade leading to clotting and subsequent blocking of the capillary fibers1. This results in a reduction of the efficiency of the dialysis procedure, as less fibers remain available for solute exchange. When more pronounced, coagulation can even cause loss of the extracorporeal blood volume by complete clotting of the extracorporeal circuit, so blood can no longer be returned to the patient. To avoid clotting, appropriate anti-coagulant measures must be applied. Hemodialyzers can be constructed to minimally activate coagulation, either by their design or by using more biocompatible materials. In addition, substances with anticoagulant properties can be administered during the dialysis session. However, overdosing of these anticoagulants can cause bleeding and finding the right balance between under- and over-anticoagulation is challenging.
So far, assessment of the degree of fiber blocking during hemodialysis is measured with different surrogate parameters, but none of them is objective or validated against a gold standard. Some of these are available online during the treatment, such as the dialysis machine parameters venous pressure2,3, transmembrane pressure4 and online solute clearance5. Others are assessed offline, such as visual6,7,8,9,10 or software assisted7 dialyzer redness scoring. Also laboratory parameters representing coagulation activity in general6,7,8,11,12,13, measured solute clearance6,14, evaluation of rinse-back3 or fiber bundle volumes15,16,17 and electron microscopy9 have been used. None of these parameters is robust, objective or accurate, which limits the comparative study of different strategies to reduce coagulation and fiber blocking. There is thus a need for an objective, operator-independent and reproducible gold standard to determine the extent of fiber blocking during dialysis treatment18. The number of blocked capillary fibers most objectively represents the clinical impact of coagulation.
The present study examines the feasibility and accuracy of micro-computed tomography (micro-CT) to quantify the number of blocked fibers. As illustration, the number of blocked fibers as measured by micro-CT is compared to other surrogate parameters used to assess fiber blocking.
Results
Micro-CT scanning allowed to acquire high quality images of cross-sections of all scanned dialyzers. Individual capillary fibers could easily be distinguished and their total number quantified. Fiber counting in corresponding cross-sections of the 3 non-used dialyzers resulted in almost exactly the same number of fibers (e.g. 10748 ± 2 in the reference slice), as is expected taking into account the high consistency of the manufactural process of bundling fibers in individual dialyzer capsules.
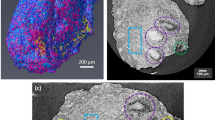
Figure 1 shows images representing different levels of coagulation in different used dialyzers. Complete circuit blocking because of dialyzer clotting did not occur during the sessions of the current experiments, but the 20 dialyzers under study represented a broad spectrum of coagulation activation, as reflected by the wide range (534 to 10692) of fibers remaining open after the dialysis session.
Table 1 shows the number of counted open fibers in the non-used and used dialyzers and this in different cross-sections and for different thresholds of open fiber surface areas. Good similarity in counts of open fibers remained when images were assessed using different thresholds to define an ‘open fiber’ (i.e. difference ≤1.7%), showing the robustness and validity of the technique. Differences in the number of open fibers as counted in different cross-sections in the potting were within the clinical relevance (≤6.1%), endorsing the reliability of the technique.
Associations were found between the number of open fibers and the post-dialysis dry mass of the dialyzers (R² = 0.62), and the visual clotting scores of the dialyzers (R² = 0.41) and venous chambers (R² = 0.34). However, none of the online available parameters as indicated on the dialysis machine (i.e. pressures, blood volume monitoring and online clearance monitoring) as well as the post dialysis registered rinse-back volume were associated with the number of open fibers (Table 2 and Fig. 2).
Discussion
We demonstrate the feasibility to use a self-developed micro-CT technique to assess objectively and in an operator-independent manner the blocking of individual fibers in hemodialyzers with great accuracy and repeatability. Using this technique, we demonstrate that currently used parameters, based on data derived from the dialysis monitor, visual scoring or post-dialysis mass of the dialyzer are not representative to assess the degree of fiber blocking.
The circulation of blood through extracorporeal devices such as hemodialyzers activates the coagulation cascade, which might result in clotting and obstruction of the circuit. In hemodialysis, this progressive blocking of fibers within the dialyzer results in a progressive decrease of dialysis performance, and potential loss of blood when the circuit becomes completely obstructed. The degree of activation of the coagulation cascade depends on the biocompatibility of the dialyzer and the coagulability of the blood. Biocompatibility is in itself a combination of design, for example geometry to avoid turbulence, blood stagnation and shear stress, and the type of materials used19,20. Coagulability depends upon patient factors, for example presence of inflammation or coagulation disorders, and can be influenced by applying an anticoagulant strategy during the dialysis session. This should however be done with caution, as too much anticoagulation might induce bleeding and death of the patient.
Different designs of filters and anticoagulant strategies have been developed, but comparing the balance of efficacy and safety between different designs and strategies is hampered by the lack of a well-established and objective tool to evaluate the resulting degree of filter blocking in the hemodialyzer. There is thus need for a gold standard to assess and compare thrombogenicity of different dialyzers and/or anticoagulation strategies. Ideally, such technique quantifies the patency of the individual fibers in the complete dialyzer, to assess the impact of coagulation on the dialyzer performance. The technique should be repeatable, accurate and operator-independent (objective). The here presented micro-CT scanning technique fulfills these criteria. It allows consistent and reliable counting of open versus blocked fibers in an accurate and repeatable way, as demonstrated by the agreement of measurements at different cross-sections of the dialyzer. In addition, this high accuracy is independent of the threshold chosen to define patency of fibers.
The present technique offers multiple advantages over the currently available methods to assess the degree of fiber blocking. So far, visual assessment has been most frequently promoted to assess coagulation of dialyzers. In this technique, a visual assessment of the discoloration of the fibers from white to red by residual (clotted and blocking) blood is done to estimate the degree of fiber blocking within the dialyzer. Despite existence of tools to enhance standardization, such as calibrated reference pictures, this simple method remains operator-dependent. This operator-dependency can theoretically be decreased by using computer analysis of pictures of the dialyzer rather than visual assessment by the human eye. However, still then the technique allows only assessment of fibers at the outside of the dialyzer, as more internal fibers cannot be visualized. As coagulation is not homogenous, this can result in big discrepancies between estimated and true degree of coagulation. This is more of importance as coagulation can occur at specific areas exactly because of the design of the dialyzer, for example at places of high turbulence, or stagnation of flow. The lack of capacity to assess the complete cross-section by visual assessment can thus lead to misinterpretation of the advantage/disadvantage of certain designs or strategies. Micro-CT evaluation is performed on complete sections of the dialyzer, allowing assessment of all fibers, irrespective of their location. Last, visual scoring of clotting can be hampered by non-transparent fiber and housing design, and can thus not be used in all types of dialyzers while the presented micro-CT technique is independent of the type of housing. In our study, there was a relatively good association between the visual score of the hemodialyzer and the number of blocked fibers (R² = 0.36) (Table 2 and Fig. 2 panel B). However, the point prevalence power of the visual score to predict the number of open fibers in individual dialyzers was disappointing. For example, a visual score of 3 corresponded with 6000 in one and 10000 open fibers in another dialyzer, whereas 10000 open fibers resulted in a visual score of 1 in one and 3 in another dialyzer (Fig. 2 panel B), making that visual scoring is completely not reliable at the level of the individual patient.
Dialyzer mass after post dialysis rinsing could be used as a proxy of blocked fibers. This method is based on the postulate that it is impossible to remove debris and blood from blocked fibers, so that more mass corresponds with more blocked fibers. In our experiments, post dialysis dialyzer mass corresponded best of all parameters with the number of open fibers as assessed by micro-CT, with an R² of 0.62 (Table 2 and Fig. 2 panel A). However, also here, the point prevalence predictive power was disappointing and insufficient to accept post dialysis dialyzer mass as an acceptable surrogate for degree of fiber blocking.
Laboratory markers for coagulation activation have been used to assess degree of coagulation during a hemodialysis session. However, there is a lack of standardization of how these tests should be performed and how results should be interpreted. More important, the results of these tests are representing coagulation in the complete system, including the patient. As such, it is unclear from these tests where exactly the coagulation cascade has been activated, either in the patient, for example due to inflammation, or in the dialyzer. As a consequence, these tests are less informative about the impact of dialyzer design or anticoagulation strategy on the blocking of fibers.
In many centers, parameters that are routinely available from the dialysis machine, such as venous pressure and estimated transmembrane pressure are being used to assess the degree of fiber blocking. From the regression analyses, we found that none of these monitor derived parameters accurately reflects degree of coagulation. Pre-dialyzer pressure and measured transmembrane pressure potentially would perform better, but none of these parameters is provided on the presently commercially available dialysis machines.
Also the volume needed to achieve clean rinse-back, as determined by a sensor on the venous blood line or chamber, seems not to be able to predict the number of blocked fibers. This corresponds with the observation that visual inspection of blood lines and chambers does not necessarily reflect coagulation within the dialyzer.
This study also showed for the first time that online clearance monitoring (OCM), claimed to be an indicator of dialysis performance, does not correspond with the degree of fiber blocking during dialysis. For example, in a dialyzer ending up with only 534 patent fibers out of more than 10000 at start, (as shown in Fig. 1, lower right image), OCM values were only decreased by 28% after the 4 hour dialysis session. Although dialyzer clotting is an important issue in clinical practice, dialysis machines do thus not provide any parameter to accurately predict progressive coagulation in the extracorporeal circuit.
The micro-CT technique does not allow mechanistic assessment of underlying causes of fiber blocking at the molecular level. This would require much higher resolutions than what is achieved in the current set-up. Such higher resolution would be at the expense of the fact that the current set-up of the micro-CT scan allows to visualize the complete cross-section of the dialyzer, as it would necessitate analysis of processes at the level of membrane pores. As the main goal of the current micro-CT set-up was to evaluate the impact of different dialyzer designs and/or anticoagulation strategies on the clinical performance of the dialyzer, the current resolution allowing to determine blocking at the level of individual fibers is sufficient. However, the current set-up could also be used to indirectly explore underlying pathophysiology of coagulation, by comparing different strategies, for example with or without addition of antibodies21 blocking specific coagulation pathways, and assess their impact on filter blocking.
In conclusion, there is a need for a gold standard to assess blocking of hemodialyzers at the level of individual fibers if we want to evaluate progress in the development of new strategies to reduce coagulation during hemodialysis. Advantage of strategies or designs claiming to improve anticoagulation over existing practice can be demonstrated or refuted by using this gold standard. The micro-CT approach also offers new perspectives for research on coagulation regimens in renal replacement therapies. Much needed online surrogate markers of coagulation can be validated against this method.
Patients and Methods
This single center, open-label, non-controlled prospective trial included twenty adult patients (11 male) undergoing 245 ± 20 min maintenance hemodialysis (pressure controlled on-line postdilution hemodiafiltration, replacement fluid 20 ± 4 L). The median age was 75 [69–79]. Median weight was 65 [58–72] kg. Diabetic and hypertensive nephropathy were the most frequent underlying diseases. Sixty percent of the patients received antiplatelet therapy. Table 3 lists the patient’s clinical characteristics and baseline laboratory findings.
The protocol was approved by the ethics committee of the Ghent University Hospital, and written informed consent was obtained from all included patients or their legal representatives (EC 2016/0908–B670201629247).
Dialysis and anticoagulation
All dialysis treatments were performed in accordance with the relevant guidelines and regulations. Patients received their standard Low-Molecular-Weight Heparin (Enoxaparin, Sanofi Belgium or Tinzaparin, Leo Pharma, Belgium) anticoagulation at the beginning of the dialysis session. All sessions were performed with single-use high flux FX600 Cordiax® polysulfone dialyzers with a surface area of 1.6 m² on 5008 dialysis machines (Both Fresenius Medical Care, Bad Homburg, Germany). Blood flow was maintained between 200 and 350 ml/min (297 ± 43), while ultrafiltration was set according to the patient’s interdialytic weight gain and clinical status (509 ± 194 ml/h). In all dialysis sessions, double-needle vascular access was achieved through a native arteriovenous fistula or a well-functioning double lumen tunneled central venous catheter.
Online dialysis machine parameters
Different dialysis parameters as reported by the dialysis machine were registered every 30 min: i.e. venous and arterial pressure, transmembrane pressure (TMP), blood volume monitoring (BVM) and online clearance monitoring (OCM). For further regression analyses, absolute changes, calculated as post versus predialysis values, were used. Rinse-back volume, representing the volume of dialysate needed to restore all extracorporeal blood back to the patient at the end of the session was registered from the dialysis monitor.
Dialyzer and venous chamber scoring
Hemodialyzers and blood lines were removed immediately once the post-dialysis standard rinsing procedure had been completed. Subsequently, a member of the nursing staff and a clinician independently scored the extent of clotting in the dialyzer and the venous chamber in a semi-quantitative way using a reference scoring system10. Dialyzers were graded having no signs of clotting (1), few dark red fibers (2), less than 50% dark red fibers (3) or overall redness with >50% dark red fibers (4). Thrombus formation in the air bubble catcher (venous chamber) was quoted using a four graded scale: no detectable clotting (1), minimal clot formation (2), clots up to 5 cm but dialysis still possible (3), and complete occlusion of the air bubble catcher (4) (Table 4).
Next, in an in vitro setting, continuous subtle positive pressure ventilation was applied to the dialyzer both through the blood inlet as through the dialysate outlet for 24 hours, to dry the dialyzer membrane. Finally, dialyzer dry mass was measured using a precise scale (CT 6000, Ohaus®, USA - calibration by Eldon Enterprises France).
Micro-CT scanning
Dialyzer fiber clotting was visualized using a 3D CT scanning technique on micrometer resolution. HECTOR is a High Energy CT scanner Optimized for Research22, built by the Ghent University Centre for X-ray Tomography (UGCT) in collaboration with the UGCT spin-off company XRE (Gent, Belgium). In front of the X-ray source, the dialyzer was mounted vertically on a precision rotation stage, and radiographies were recorded over 360° with an angular interval of 0.15°. Scan conditions were optimized to maximize the signal-to-noise ratio based on the sample size and structure, and the scanner properties. The tube voltage was set at 80 kV, at a power of 20 Watts, the maximal power that allowed imaging at a resolution of 25 µm. A total of 2401 projections were recorded with 500 ms exposure each, resulting in a total exposure time of 20 minutes. Acquired images at 0 (projection 1) and 360° (projection 2401) were compared to exclude movement of the hemodialyzer during the scanning process. Reconstruction of the raw projection data is performed with the Octopus Reconstruction software package, licensed by XRE23. This resulted in two types of images as presented in Fig. 3: fibers are visible as rings for cross-sections in the free fiber bundle (left panel), and as black dots for cross-sections in the dialyzer potting (right panel), a polyurethane sealing near the blood inlet and outlet, fixing the ends of the capillary fibers.
CT images of cross-sections in the same used dialyzer halfway the dialyzer (left panel) and at the central cross-section in the outlet potting (right panel). Central fibers are open and peripheral fibers are blocked. Sections in the potting offer optimal visualization of air inflated open fibers, appearing as black dots.
Fiber counting and coagulation quantification
Fibers were counted in the central cross-section of the dialyzer outlet potting (reference slice) since this region offers optimal visualization of air inflated open fibers, appearing as black dots (Fig. 3–right panel). These dots can easily be counted in a computer-based way, in contrast to the use of cross-sectional images taken out of the potting showing fibers as white cylinders (Fig. 3–left panel).
The non-coagulated fibers (i.e. black dots) were counted using the Fiji image processing toolkit of ImageJ analysis software24,25 (ImageJ 1.51 H, NIH, Bethesda, USA), an open-source platform for biological-image analysis26 (Fig. 4). To ameliorate selection and count of the patent fibers, the brightness of each individual image was optimized using image thresholding27 (Fig. 4–panel B). The region of interest (ROI) was selected bordering the entire fiber bundle to avoid count of any disturbances outside the fiber bundle (Fig. 4–panel B). Watershed segmentation was employed to distinguish sticky fibers28. Image particles with a circularity of 0.5–1 were selected (Fig. 4–panel C).
Based on the reference images of the non-used dialyzers, only fibers with a surface area of >70% (threshold) of the cross section of a non-used fiber were considered to be open. This correlates with a surface area of 0.0204 mm². Validity and reliability of the method were checked, the former by comparing the impact of different arbitrary thresholds (60 and 80% open fiber) to define open fibers, the latter by performing counts in a cross-section 100 slices (i.e. 2.5 mm) proximal of the reference slice.
Statistical analysis
Statistical analyses were performed using SPSS (version 24, SPSS Inc, Chicago, USA). Continuous variables were summarized as mean ± SD if normally distributed (normality was checked with Shapiro-Wilk test); otherwise median value with interquartile range [IQR] were reported. Categorical variables were expressed as frequencies and percentages. Regression analyses were performed (R²) with the number of open fibers as independent variable and the dialysis parameters as dependent variables.
References
Unger, J. K. et al. Relation of haemofilter type to venous catheter resistance is crucial for filtration performance and haemocompatibility in CVVH–an in vitro study. Nephrol Dial Transplant 21, 2191–2201, https://doi.org/10.1093/ndt/gfl068 (2006).
Guery, B. et al. Hemodialysis without systemic anticoagulation: a prospective randomized trial to evaluate 3 strategies in patients at risk of bleeding. PLoS One 9, e97187, https://doi.org/10.1371/journal.pone.0097187 (2014).
Kessler, M. et al. Heparin-grafted dialysis membrane allows minimal systemic anticoagulation in regular hemodialysis patients: a prospective proof-of-concept study. Hemodial Int 17, 282–293, https://doi.org/10.1111/j.1542-4758.2012.00733.x (2013).
Schetz, M., Van Cromphaut, S., Dubois, J. & Van den Berghe, G. Does the surface-treated AN69 membrane prolong filter survival in CRRT without anticoagulation? Intensive Care Med 38, 1818–1825, https://doi.org/10.1007/s00134-012-2633-x (2012).
Rocha, A. D. et al. Effects of citrate-enriched bicarbonate based dialysate on anticoagulation and dialyzer reuse in maintenance hemodialysis patients. Hemodial Int 18, 467–472, https://doi.org/10.1111/hdi.12113 (2014).
Evenepoel, P. et al. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: a prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am J Kidney Dis 49, 642–649, https://doi.org/10.1053/j.ajkd.2007.02.001 (2007).
Dorsch, O. et al. A multi-center, prospective, open-label, 8-week study of certoparin for anticoagulation during maintenance hemodialysis–the membrane study. BMC Nephrol 13, 50, https://doi.org/10.1186/1471-2369-13-50 (2012).
Ziai, F. et al. The effect of oral anticoagulation on clotting during hemodialysis. Kidney Int 68, 862–866, https://doi.org/10.1111/j.1523-1755.2005.00468.x (2005).
Hofbauer, R. et al. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney Int 56, 1578–1583, https://doi.org/10.1046/j.1523-1755.1999.00671.x (1999).
Rossignol, P. et al. Rationale and design of the HepZero study: a prospective, multicenter, international, open, randomized, controlled clinical study with parallel groups comparing heparin-free dialysis with heparin-coated dialysis membrane (Evodial) versus standard care: study protocol for a randomized controlled trial. Trials 14, 163, https://doi.org/10.1186/1745-6215-14-163 (2013).
Lavaud, S. et al. Assessment of the heparin-binding AN69 ST hemodialysis membrane: II. Clinical studies without heparin administration. ASAIO J 51, 348–351 (2005).
Klingel, R. et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant 19, 164–170 (2004).
Salvade, I., Del Giorno, R., Gaetano, D. & Gabutti, L. Assessing the contact-activation of coagulation during hemodialysis with three different polysulfone filters: A prospective randomized cross-over trial. Hemodial Int 21, 375–384, https://doi.org/10.1111/hdi.12509 (2017).
Claure-Del Granado, R. et al. Anticoagulation, delivered dose and outcomes in CRRT: The program to improve care in acute renal disease (PICARD). Hemodial Int 18, 641–649, https://doi.org/10.1111/hdi.12157 (2014).
Meijers, B. et al. A noninferiority trial comparing a heparin-grafted membrane plus citrate-containing dialysate versus regional citrate anticoagulation: results of the CiTED study. Nephrol Dial Transplant 32, 707–714, https://doi.org/10.1093/ndt/gfw461 (2017).
Narsipur, S. S. Measurement of fiber bundle volume in reprocessed dialyzers. Clin Nephrol 61, 130–133 (2004).
Krivitski, N. M. et al. In vivo measurement of hemodialyzer fiber bundle volume: theory and validation. Kidney Int 54, 1751–1758, https://doi.org/10.1046/j.1523-1755.1998.00146.x (1998).
Shen, J. I. & Winkelmayer, W. C. Use and safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis 60, 473–486, https://doi.org/10.1053/j.ajkd.2012.03.017 (2012).
Daugirdas, J. T. & Bernardo, A. A. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int 82, 147–157, https://doi.org/10.1038/ki.2012.130 (2012).
Vienken, J., Diamantoglou, M., Hahn, C., Kamusewitz, H. & Paul, D. Considerations on developmental aspects of biocompatible dialysis membranes. Artif Organs 19, 398–406 (1995).
Frontera, J. A. et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care 24, 6–46, https://doi.org/10.1007/s12028-015-0222-x (2016).
Dierick, M., Van Loo, D., Masschaele, B., Boone, M. & Van Hoorebeke, L. A LabVIEW(R) based generic CT scanner control software platform. J Xray Sci Technol 18, 451–461, https://doi.org/10.3233/xst-2010-0268 (2010).
Vlassenbroeck, J. et al. Software tools for quantification of X-ray microtomography. Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment 580, 442–445, https://doi.org/10.1016/j.nima.2007.05.073 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682, https://doi.org/10.1038/nmeth.2019 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675 (2012).
Eliceiri, K. W. et al. Biological imaging software tools. Nature Methods 9, 697–710, https://doi.org/10.1038/Nmeth.2084 (2012).
Ridler, T. W. & Calvard, S. Picture Thresholding Using an Iterative Selection Method. Ieee Transactions on Systems Man and Cybernetics 8, 630–632 (1978).
Soille, P. & Vincent, L. Determining Watersheds in Digital Pictures Via Flooding Simulations. Visual Communications and Image Processing 90, Pts 1–3 1360, 240–250, https://doi.org/10.1117/12.24211 (1990).
Author information
Authors and Affiliations
Contributions
F.V.O., W.V.B., A.D. and S.E. developed the study design. F.V.O. and S.E. performed the clinical part of the study. M.D. and M.B. performed the micro C.T. scanning and the image reconstruction. F.V.O. and S.E. performed the fiber counting and statistical analysis. F.V.O., S.E. and W.V.B. wrote the main manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vanommeslaeghe, F., Van Biesen, W., Dierick, M. et al. Micro-computed tomography for the quantification of blocked fibers in hemodialyzers. Sci Rep 8, 2677 (2018). https://doi.org/10.1038/s41598-018-20898-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20898-w
This article is cited by
-
Association between anticoagulation strategy and quality of life in chronic hemodialysis patients
Scientific Reports (2023)
-
Impact of intradialytic fiber clotting on dialyzer extraction and solute removal: a randomized cross-over study
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.