Abstract
The clinical efficacy regarding bleaching sensitivity and tooth shade lightening using a standard hydrogen peroxide (H2O2) bleaching gel was compared with the additional use of ozone either before or after application of H2O2. Using computer-generated tables, 45 participants were randomly allocated into three groups (n = 15 each) in this investigator-driven, single-centre trial. In Group 1, upper anterior teeth were bleached using ozone (produced via a healOzone X4 device) for 60 seconds, then 38% H2O2 for 20 minutes; in Group 2, 38% H2O2 application (20 min) was followed by ozone (60 s); air produced by the healOzone machine (60 s) followed by 38% H2O2 (20 min) was used in Group 3 (control). Bleaching sensitivity was evaluated via visual analogue scales, and a treatment-blinded reader objectively recorded tooth shades using a colorimeter before and 24 hours after bleaching (at α = 0.05). The H2O2/ozone combination did not result in pain sensations, while both ozone/H2O2 and H2O2 alone increased bleaching sensitivity (p < 0.001). Teeth achieved lighter shades (higher L*/lower b* values) after bleaching in all groups (p < 0.001), while Ozone boosted lighter tooth shades, irrespective of its use before or after H2O2 (p < 0.05). Due to the complimentary effects, applying ozone after H2O2 seems preferable for bleaching.
Similar content being viewed by others
Introduction
Ozone (O3) is a strong oxidizing agent able to destroy bacteria, viruses, fungi, yeasts and protozoa as well as odours, and has been utilized for a long time in medicine and dentistry1,2,3,4,5. Beneath various other dental purposes, ozone has been applied for lightening of teeth in recent years6,7,8,9,10,11,12,13,14,15, but results from the available literature have been contradictory, at least to some extent. Some authors reported that using ozone did not potentiate the bleaching ability of 8% carbamide peroxide, and even reduced the bleaching efficacy if applied before 8% carbamide peroxide15, while others concluded that hydrogen peroxide (H2O2) had superior bleaching capacities if compared to ozone alone6, and that ozone did not improve the bleaching effectiveness of 35% hydrogen peroxide12.
In contrast, ozone alone has been reported to have similar bleaching capacities like some commercially available, highly concentrated carbamide peroxides (45%)13 or hydrogen peroxide (37.5%)11 bleaching agents. In addition, ozone was found to improve the shades of tetracycline stained rat teeth14. Moreover, 30% carbamide peroxide has been shown to have inferior bleaching outcomes if compared to ozonated gel when used to bleach stained resin composite discs16. Finally, our working group recently has revealed that 38% H2O2 offered superior bleaching results when used together with ozone7,9,10, and we found that ozone gas had similar bleaching results to 38% H2O28.
Notwithstanding, the available literature on bleaching action of ozone suffers from some limitations such as investigating low ozone or peroxide dosage and concentrations12,15, small study sample sizes12,15,16, using older models of ozone-generating machines13,15,17, inability to measure ozone concentrations produced by ozone machines12,14,15, following study designs not accurately imitating clinical conditions13,15, studying bleaching results on artificial extrinsic tea stains (but not the colour of dental structures)12,15, measuring hue component of tooth shade only14, or subjectively utilizing visual shade guides to measure tooth shades (without objective standardization)13,14. Therefore, concluding recommendations regarding bleaching efficacy are hardly educable from the current literature.
In medicine, locally applied ozone has been shown to alleviate painful conditions18,19, and to reduce inflammatory responses20,21. So far, however, no prior clinical trial has explored the results of utilizing ozone before H2O2 application in comparison to using ozone after H2O2 application for dental bleaching, and no information is retrievable with regards to prevailing pain sensations due to tooth whitening procedures, a phenomenon called bleaching sensitivity22. This inspired the current study to better understand the bleaching effects of ozone on discoloured teeth before or after the use of H2O2.
Hence, the aim of this investigation was to study tooth sensitivity and the clinical efficacy of tooth bleaching using both ozone/H2O2 gel and H2O2 gel/ozone treatment sequences, and to compare these with conventional bleaching using H2O2 gel only. The null hypotheses (H0) for this study were that applying ozone to teeth surfaces would not result in different tooth sensitivity or bleaching outcome if used before or after 38% H2O2, and that bleaching with ozone and H2O2 would produce similar effects compared to bleaching with H2O2 alone. These null hypotheses were tested against the alternative hypotheses of a difference (HA).
Methods
This investigation was organised in full ethical accordance with the Helsinki Declaration of 1964 (as revised and amended in its ninth version in 2013)23. Approval of the study protocol by the Deanship of Research, University of Jordan, Amman, Jordan (ethical vote number ARC-5-2015) was obtained, and all participants of this three-arm clinical trial gave their written informed consent for participation and the use of their respective data for research purposes. Blindness of the evaluator regarding the respective treatments in the assigned groups of the patients was assured. With the present report, we adhered to the CONSORT statement on reporting randomized trials24.
Sample size calculation
Sensitivity of teeth (24 h after beaching) was defined as the primary endpoint of the current study. Using the pooled variance based on our previous study9, we computed an effect size of 0.544 (G*Power: Statistical Power Analyses, version 3.1.9.3; Heinrich-Heine University)25 for an a-priori power analysis. A sample size of 12 per group was calculated as a function of the required power level (1 – β; 0.8), the pre-specified significance level α (0.05), and the population effect size to be detected with probability 1 − β25. In total, 15 participants were finally selected for each group to compensate for any unexpected (but sometimes inevitable) dropouts. The humane endpoint was defined as the point at which pain induced by the bleaching procedure was not bearable anymore26; in these cases, terminating the possibly painful procedure was scheduled.
Recruitment of patients
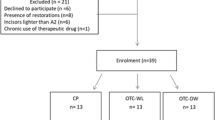
A total of 69 patients interested in this study were examined (Fig. 1). Forty-five participants (24 females and 21 males) were finally recruited into this study. All participants were regular patients visiting the dental clinics at the University of Jordan, and searching for bleaching treatment.
Each participant received a detailed explanation of the study and the involved procedures (along with the potential side effects)27 and was requested to provide written informed consent before being recruited into the study. Participants were included in this investigation if they had all their upper anterior teeth (from right canine to left canine) present and sound, if their teeth had never been bleached before, and if they had no previous prosthetic, endodontic or restorative treatment for their upper anterior teeth. Vitapan classical shades (Vita Zahnfabrik, Bad Säckingen, Germany) should be A3 or darker28, and lighter shades were not included. Participants who had missing upper anterior teeth or had their upper anterior teeth affected by carious lesions, periodontitis, recession, extensive tooth surface loss, or any other complicating medical history as well as pregnant/lactating women were excluded from the present study.
Randomisation
Subsequently, the participants were randomly allocated into three groups (n = 15 for each group), and the teeth were bleached as described below. A simple randomization process using computer-generated numbers was followed to distribute participants to the three treatment groups. To avoid any sex-based bias29, stratification according to gender was ensured.
Examination of patients
A comprehensive clinical examination was carried out on a dental chair equipped with a light unit (Diamond LED Dental Light; Daray Lighting, Derbyshire, England, UK). The upper anterior teeth received a prophylaxis using pumice and water, and were dried before being examined. An explorer probe (0700-9, anatomical handle single ended; ASA Dental, Bozzano, Italy) and a dental oral mirror (15/16 inch; Hahnenkratt, Königsbach-Stein, Germany) were used throughout all intra-oral examinations. If needed, the teeth were scaled and polished before commencing the investigation.
Then, tooth sensitivity was evaluated via a visual analogue scale (VAS) ranging from 0 (‘no tooth sensitivity at all’) to 10 (‘pain as bad as imaginable’). Following previous recommendations9,30, the tooth shades (from upper right canine to upper left canine) were evaluated objectively using a chroma meter (CR-400; Minolta, Osaka, Japan)28 from a standard distance (7 cm away from the measured tooth surface) while the participant was sitting upright in the dental unit. The colour-measuring device was placed on a movable metal tray connected to the vertical column of the dental chair light unit. A 7 cm long plastic rod was inserted between the centre of the colorimeter orifice and the centre of the assessed tooth surface to ensure that the colorimeter was repeatedly fixed at 7 cm from the tooth surface before starting the shade measurement. Standardization of lighting conditions was ensured by carrying out all shade measurements under natural daylight with the clinic room lights and the dental unit light on (but turned away from the study participant). All teeth to be examined were moistened with water from a 3-in-1 syringe. To standardize the measuring procedure, shade measurements were carried out following the same order for each participant, from the right canine to the left one. The colorimeter (CR-400; Minolta) recorded Vitapan classical shades (Vita Zahnfabrik) using descending values from light to dark as well as L*a*b* shade values by measuring the intensity of reflected visible light for red, green, yellow and blue wave lengths using the L*a*b* coordinates of colour arrangement in the CIELab colour scheme9,10.
Bleaching intervention
With all patients, a light-curing dental dam covering and protecting the gingival tissues (BMS white BM; BMS Dental, Capannoli, Italy) was used. Participants’ teeth in Group 1 were bleached by application of ozone for 60 seconds on the labial surface of each tooth; a 2,350 ppm ozone concentration at a 615 cc per minute flow rate was supplied by a well-known ozone producing machine (healOzone X4; Curozone, Wiesbaden, Germany)7,9,17. The ppm of ozone supplied was verified via an ozone detection device, and the ozone flow rate was verified by a flow meter directly before the start of the experiment. Ozone gas was distributed to the tooth surfaces through disposable silicone cups provided by the manufacturer and assured a perfect seal to prevent any ozone leakage. The healOzone X4 machine only supplies ozone once the cup provides an absolute seal; this element allows the machine to be safely employed to humans17,31. Subsequently, application of 38% H2O2 gel (BMS white 38%; BMS Dental) followed for 20 minutes. Then, the H2O2 gel was removed and the teeth were sprayed for 10 seconds with water from a 3-in-1 syringe.
In comparison to Group 1, participants’ teeth in Group 2 were first bleached by application of 38% H2O2 (BMS white 38%; BMS Dental) gel for 20 minutes, and then sprayed for 10 seconds with water from a 3-in-1 syringe. Subsequently, ozone was applied on each tooth surface for 60 seconds.
In contrast, participants’ teeth in Group 3 were exposed to 60 seconds of air only (no ozone) provided by the ozone machine (which was specifically modified, and was achieved by using a switch on the back). A 38% H2O2 gel (BMS white 38%; BMS Dental) was applied on the teeth for 20 minutes, and then washed for 10 seconds with water from a 3-in-1 syringe.
Follow-up
With the initial rebound of colour effect in mind, all participants were dismissed and requested to return 24 hours later to permit rehydration of tooth surfaces before shade and tooth sensitivity assessment. Tooth shades were then recorded using the already described chroma meter (CR-400; Minolta), and tooth sensitivities were evaluated using the VAS scale from 0 to 10 as explained above.
Statistical analysis
Assigning patients to interventions and tooth bleaching was accomplished by one investigator (M.K.A.-O.), while all shade measurements and tooth sensitivity assessments were carried out by another investigator (A.A.A.N.), who was blinded to the respective bleaching technique. Intra-examiner reliability was evaluated by recording 18 duplicate shade measurements by the same investigator and Kappa was considered adequate (κ = 0.91; almost perfect conformity), thus proving high intra-examiner agreement of the standardized assessment methods.
The SPSS computer software (Statistical Package for the Social Sciences, v19.0; IBM, Armonk, NY, USA) was utilized to carry out data analysis for the current study. Paired samples t-test was executed to compare shade values before and after bleaching within each group. Analysis of variance (ANOVA) was performed to compare shade values between groups. Post-hoc test was carried out for additional comparisons of shade values between groups at baseline and after bleaching. Statistically significant levels were set at p < 0.05, with a 95% confidence interval. A post-hoc power calculation analysis based on the sensitivity means and standard deviations was conducted to compute the actual power level (G*Power, version 3.1.9.3; Heinrich-Heine University, Düsseldorf, Germany)25.
Results
This investigation took place at the Department of Prosthodontics, University of Jordan (September 2016 till March 2017). From the initially 69 screened patients, 24 were excluded; reasons for exclusion of participants are given in Fig. 1. An overall of 270 upper anterior teeth in 45 participants were finally included and investigated in the current study. Participants’ age ranged between 19 and 33 years (mean ± SD = 25 ± 4 years). In each group, 8 participants were female.
Bleaching sensitivity
Table 1 demonstrates the means and standard deviations of the levels of tooth sensitivity and recorded Vita and L*a*b* shade values among the study groups at study baseline and following the respective bleaching sequences. None of the teeth in this clinical experiment was affected by sensitivity at baseline. However, Groups 1 (first ozone, then H2O2 bleaching) and 3 (H2O2 only controls) revealed some sensitivity after bleaching (p < 0.001) (Table 2). On the other hand, teeth in Group 2 (first H2O2, then ozone bleaching) did not show any bleaching sensitivity at all. Regarding bleaching sensitivity, the post-hoc power analysis resulted in a level considered adequate (>99.7%) to detect a clinically relevant difference between the outcomes of the three study arms.
Bleaching outcome
In addition, all bleaching techniques caused changes in L*a*b* shade values, and the investigated teeth acquired lighter shades (p < 0.001) (Table 2). Also, L* shade values were enhanced (leading to lighter shades) following bleaching with ozone then H2O2 in Group 1 (p < 0.001), following bleaching with H2O2 then ozone in Group 2 (p < 0.001), and following bleaching with H2O2 alone in Group 3 (p = 0.001) (Table 2). Moreover, b* shade values were reduced (leading to lighter shades) following bleaching with ozone then H2O2 in Group 1 (p < 0.001), following bleaching with H2O2 then ozone in Group 2 (p < 0.001), and following bleaching with H2O2 alone in Group 3 (p < 0.001) (Table 2). In contrast, a* shade values did not significantly change following bleaching with ozone then H2O2 in Group 1 (0 = 0.134). Notwithstanding, a* shade values were significantly decreased following bleaching with H2O2 then ozone in Group 2 (p = 0.029), and following bleaching with H2O2 alone in Group 3 (p = 0.028) (Table 2).
Comparisons between groups using ANOVA demonstrated that baseline tooth sensitivity, Vita shades, and L*a*b* shade values were comparable between groups (p > 0.05) (Table 3). In contrast, tooth sensitivity following bleaching was significantly different between groups (p < 0.001). In addition, final Vita shades acquired after bleaching were significantly different between groups (p = 0.03) (Table 3). On the other hand, final L* and b* shade values were not significantly different between groups (p > 0.05) (Table 3), whereas a* values were significantly different between groups (p = 0.001) (Table 3).
Additional comparisons between study groups by means of post-hoc statistics (Table 4) showed that baseline tooth sensitivity, Vita shades and L*a*b* shade values were not significantly different between any two groups (p > 0.05) (Table 4). Following bleaching, teeth in Group 2 revealed significantly less bleaching sensitivity than teeth in Groups 1 and 3 (p < 0.001), while teeth in Group 3 had less sensitivity than teeth in Group 1 (p < 0.001). Consequently, application of ozone after H2O2 was associated with less sensitivity following bleaching.
Finally, Group 1 was not significantly different from Groups 2 and 3 regarding final Vita Classic shades and final L* and b* shade values (p > 0.05) (Table 4). In contrast, Group 1 revealed higher final a* shade values (darker shades) if compared to Groups 2 (p = 0.002) and 3 (p = 0.005). Also, Group 2 had lighter final Vita Classic shades (p = 0.029) and higher final L* shade values (lighter shades) (p = 0.04) than Group 3. Therefore, bleaching with H2O2 then ozone produced lighter shades than bleaching with H2O2 alone. Groups 2 and 3 were not significantly different regarding final a* and b* shade values (p > 0.05) (Table 4).
Harms and unintended side effects
Apart from the bleaching sensitivities observed in Groups 1 and 3 (this was regarded as a predictable and common side effect), we did not observe any harms or unintended effects, neither with one of the used materials nor in any of the study groups in the present study. None of the observed bleaching sensitivities was considered unbearable.
Discussion
The current investigation showed that application of ozone after H2O2 was associated with no tooth sensitivity at all, and this was in line with a recent study9. Bleaching via 38% H2O2 followed by ozone resulted in effects comparable to bleaching with ozone followed by 38% H2O2. In addition, bleaching with ozone and H2O2 (in any application sequence) proved to be superior compared to H2O2 alone. Consequently, the null hypothesis of this study (speculating that no variation in efficacy would be observed between the three bleaching procedures) was rejected.
In the present study, shade evaluation was standardized through recording the shade from a fixed distance around mid-day within the same clinical settings for all participants, and the results were adequately reproducible. The healOzone appliance was used to provide ozone (or air only in the control group) since it has been shown to be safe as its ozone releasing system can be effectively sealed before the appliance supplies ozone17,31.
Regarding the baseline colours, most included teeth were of dark A shades, and most patients showed more than one shade with their anterior teeth (even after a thorough prophylaxis). In the present trial, we dealt with the original colours as numbers according to the Vita shade arrangement from lighter to darker shades (From B1 to C4) and numbers from 16 to 1 were given to the shades (B1 = 16, A1 = 15 to C4 = 1), according to their sequence in the Vita shade arrangement28. The analysis was done accordingly after assessing how many degrees the respective tooth had advanced on the Vita shade arrangement following bleaching; this was in accordance with previous investigations using computer-aided shade evaluations28,32.
Bleaching procedures were performed within clinical settings. Whitening by means of hydrogen peroxide was used as a control; this was not accompanied by another control subgroup (i. e. air after bleaching), since the latter was not considered to comply with clinical practice. The whitening gel used to treat the dental dyschromia (BMS White 38%; BMS Dental) contained 38% hydrogen peroxide, and thus was comparable to other in-office bleaching gels marketed worldwide. It is known that H2O2 can cause enamel etching due to release of protons (H+), thus opening tiny pores26.
From the present outcome it seems clear, that ozone and H2O2 successfully acted in concert to boost tooth shades. Ozone is a provider of superoxide (O˙), and could contribute additional hydroxyl radicals (OH˙) when combined with peroxides; this would suggest more effective bleaching capacities. Besides, ozone is classified as one of the most powerful oxidants (after fluorine and persulfate)4. Moreover, the synergistic dental bleaching actions of combined peroxides and ozone (a process called peroxonation) would seem in accordance with superior oxidation actions reported in areas other than dentistry33,34. Such advanced oxidative processes have been reported to promote oxidative degradation of endotoxins (induced by in situ generation of a more powerful oxidizing agent, such as hydroxyl radicals)33,35, thus decreasing the induction of cell signalling proteins involved in inflammation (i. e. tumour necrosis factors α), and reducing the inflammatory activities1,20,35.
Pain perception was measured by means of visual analogue scales (VAS). This valuable tool is generally accepted and has been widely used to evaluate pain sensations (or particular characteristics or attitudes) believed to represent a continuum of subjective data not assessable by objective measurements. In the present study, VAS pain scores as documented by the participants were significantly increased in both the ozone/H2O2 and the H2O2 alone groups. Regarding these observations, the post-hoc power analysis indicated that the present investigation had adequate power to meet the statistical requirement of a power level of at least 0.8.
Bleaching sensitivity is a well-known side effect of tooth whitening; however, this adverse reaction has not been fully understood up to now22. It is known that bleaching with high-concentrated hydrogen peroxides results in an increased expression of inflammatory mediators such as Substance P36, which in turn interacts with a great variety of cells, thus inducing the release of inflammatory mediators such as prostaglandins and cyclooxygenases37, which both have a recognized role in triggering nociceptive impulses for the perception of pain. Subsequently, both the concomitant increase in vascular permeability and the tissue pressure rise will result in pain, and this local inflammatory response of the dental pulp may be intense38,39.
In contrast, the findings of the current study revealed that bleaching sensitivity was not observed with the participants of the H2O2/ozone group. This observation might be attributed to the documented analgesic properties of ozone18,19,40. Topically applied ozone has been shown to exert ameliorative effects on lumbar disc herniation patients21, and low-concentrated (non-toxic) ozone concentrations have revealed potent anti-oxidant and anti-inflammatory effects on oxidative stress-induced tissue injuries41. Interestingly enough, exposure of human tracheal epithelial cells to ozone obviously results in a prolonged decrease in prostaglandin production42 and inactivates cyclooxygenase43, thus suggesting that the inflammatory pathways will be suppressed by ozone1.
Moreover, concentrations of vitality protector enzymes such as superoxide dismutase (an enzyme catalysing the conversion of the superoxide radical [O2−] into oxygen or hydrogen peroxide) have been reported to be low in healthy dental pulp tissue; with the proceeding of inflammatory responses, the pulp tissues showed a considerable adaptation to this situation44. Consequently, to find a large increase in catalase activity in inflamed pulp tissues would not seem surprising45,46. With a controlled application, ozone increases the activity of anti-oxidant enzymes (including catalase, glutathione peroxidase and superoxide dismutase), thus preparing the host to face pathophysiological and damaging conditions mediated by reactive hydrogen peroxide4,41. At present, these considerations undoubtedly are translational in nature, but confirming the anti-oxidative, anti-inflammatory and analgesic effects of ozone20 for dental pulp tissues would constitute a novel and momentous approach to combat bleaching sensitivity, and clearly merits further research.
Other possible explanations for the ozone-based reduced sensitivity have been presented in the available literature; these include decrease of number and diameter of open dentinal tubules47,48 and collagen degradation38,49, with potentially reduced sensitivities by mechanical blocking of the dentinal tubules. Moreover, some remineralisation of tooth surfaces in teeth bleached with H2O2/ozone might contribute to decreased pain perception, too. However, the aspects provided above are considered to take some time, and, therefore, would seem speculative at present.
In contrast, our present findings showed that using ozone before H2O2 was associated with the highest levels of sensitivity following bleaching. This effect might be in accordance with the synergistic function of ozone and peroxides for bleaching and handling of water pollutants or industrial wastes including in the textile industry; the latter procedure has been recognized as an advanced oxidative process33,35. A quick and potent oxidative consumption of coloured substances incorporated into enamel and/or dentin might have facilitated deeper penetration of hydrogen peroxide. It would seem conceivable that residual ozone remaining on the tooth surface has resulted in more advanced oxidative processes which in turn may have led to higher amounts of more free radicals reacting with the pulpal complex in a shorter time.
The secondary endpoint with respect to efficacy was the whitening effect after 24 hours (including the initial rebound after water sorption), and this set-up was conforming with a previous study9. It should be emphasised that secondary endpoints usually are lacking the same statistical authority if compared to the primary endpoint. Thus, positive effects with regard to secondary endpoints frequently are due to chance, should be interpreted with caution, and require α level correction for multiplicity; however, secondary endpoints would seem suitable to construe the primary result of a trial, and to demonstrate additional effects. Notwithstanding, it may be argued for the present outcome that efficacy with regard to bleaching outcome is strongly interlinked with pain perception; in other words, using hydrogen peroxide for in-office bleaching is a well-established clinical procedure commonly leading to bleaching sensitivity22,26, and any treatment option should strive for painless whitening. Hence, both endpoints (termed co‐primary endpoints) should achieve statistical significance to be considered clinically efficacious, and there is broad agreement that no multiplicity correction of the type I error is required in such situations50.
The outcomes of the current investigation revealed that lighter tooth shades (>4 Vita shades) were obtained following bleaching with both H2O2 and O3, irrespective of using ozone before or following hydrogen peroxide, and the teeth obtained significantly lighter shades in contrast to teeth bleached using H2O2 alone. This could be due to an additional and rapid production of free radicals (due to the ozone application) showing potent bleaching capacities and being capable of influencing tooth shades. Furthermore, this concurs with the results of previous studies concluding that ozone enhanced the shades of tetracycline stained rats’ incisor teeth14, and revealing bleaching outcomes comparable to high carbamide peroxide concentrations13. While a recent paper has elaborated that ozone (if used alone) does not outmatch the bleaching capacity of hydrogen peroxide6, the present outcomes harmonize with our previous investigations7,8,9,10, thus deducing that ozone boosted H2O2 dental bleaching. It would seem probable that some residual H2O2 or O3 may have remained in the porous system of the teeth prior to the following application of ozone or hydrogen peroxide, respectively, thus leading to advanced oxidative processes.
Notwithstanding, the outcomes of the current research contrast with another study having shown that 8% carbamide peroxide bleaching capacities would not be enhanced by ozone application and that using ozone before application of 8% carbamide peroxide would result in inferior bleaching outcomes15. This difference could be due to variations in sample size and study settings (as the respective study employed another ozone-producing device, supplying lower concentrations) which used ozone for 40 seconds, bleached external tea stains instead of internal tooth colour, and tested 8% carbamide peroxide. The latter is known to need a longer duration to effectively finish the bleaching process because of its low concentration providing 12 times less peroxides than the peroxide applied in the present study, and due to its mode of action first requiring a dissociation process to H2O2 and urea. Additionally, neither the supplied ozone concentration nor its flow rate had been reported15, thus not allowing for any further comparisons. Moreover, the findings of the present study disagree with the results of a previous investigation that has not uncovered any synergistic actions for ozone on H2O2 bleaching12. Again, this variation could be explained with teeth stained extrinsically by black tea; thus, the authors did not evaluate actual colour change of dental tissues, and used a minimal ozone concentration (140 ppm) for four minutes12.
In view of to the present outcome, it might be useful to apply ozone after H2O2 as has been utilised for dental bleaching because this might decrease both retention time and concentration of H2O2, thus possibly obtaining better bleaching effects. Additionally, this should minimize the chance for tissue irritation and could lead to less post bleaching sensitivity, reduce treatment costs and duration, and enhance patients’ compliance with treatment. The delivered ozone is more controlled by the care provider since the supplying device permits adequate control of delivery site, volume, flow rate and concentration of ozone. Furthermore, the application of ozone does not involve light activation, is quick, less costly, convenient, less irritant to soft tissues, and does not induce tooth sensitivity.
A limitation of the present investigation might be that this research was carried out within clinical settings that are more difficult to monitor if compared to laboratory investigations. Nevertheless, the investigation settings were thoroughly standardized to ensure maximum control of the implemented methodologies and shade assessments. Moreover, the tested sample size was equivalent to or larger than earlier studies in this area6,9,28.
Future clinical studies are advocated on larger samples within clinical settings to verify the long-term bleaching outcomes of ozone on natural teeth. Moreover, further research is required to investigate the potentials of ozone for bleaching difficult dental stains like tetracycline or fluorosis staining. Additionally, it would seem appealing to establish the minimum peroxide concentration, which can be applied together with ozone to achieve bleaching outcomes similar to 38% hydrogen peroxide in the same time intervals. Decreasing hydrogen peroxide concentrations to satisfyingly bleach teeth would be advantageous because of the possible clinical benefits by avoiding the side effects of bleaching using higher levels of peroxide26,27.
Conclusion
Within the limitations of the current study, it can be concluded that bleaching efficacy of H2O2 (20 minutes) will be boosted by a 60-second application of ozone, thus leading to lighter tooth shades, and this is considered irrespective of implementing ozone before or following H2O2. Using ozone after H2O2 does not result in increased bleaching sensitivity, while the latter will be observed when applying ozone before H2O2 or with conventional bleaching alone. Thus, the efficacy of the H2O2/ozone combination is regarded as advantageous and clinically meaningful when striving for satisfying and rapid bleaching effects. Additional clinical research assessing the acknowledged efficiency of the peroxide/ozone combination is suggested.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
AL-Omiri, M. K., Alhijawi, M., AlZarea, B. K., Abul Hassan, R. S. & Lynch, E. Ozone treatment of recurrent aphthous stomatitis: a double blinded study. Sci. Rep. 6, 27772, https://doi.org/10.1038/srep27772 (2016).
Domb, W. C. Ozone therapy in dentistry. A brief review for physicians. Interv. Neuroradiol. 20, 632–636, https://doi.org/10.15274/INR-2014-10083 (2014).
Elvis, A. M. & Ekta, J. S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2, 66–70, https://doi.org/10.4103/0976-9668.82319 (2011).
Bocci, V. A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 37, 425–435, https://doi.org/10.1016/j.arcmed.2005.08.006 (2006).
Baysan, A. & Lynch, E. The use of ozone in dentistry and medicine. Prim. Dent. Care 12, 47–52, https://doi.org/10.1308/1355761053695158 (2005).
Aykut-Yetkiner, A. et al. Color assessment after bleaching with hydrogen peroxide versus ozone: A randomized controlled clinical trial. Gen. Dent. 65, e12–e17 (2017).
AL-Omiri, M. K., Abul Hassan, R. S., Kielbassa, A. M. & Lynch, E. Bleaching efficacy of ozone/hydrogen peroxide versus hydrogen peroxide/ozone application. Quintessence Int. 48, 783–791, https://doi.org/10.3290/j.qi.a39044 (2017).
AL-Omiri, M. K., Abul Hassan, R. S., AlZarea, B. K. & Lynch, E. Comparison of dental bleaching effects of ozone and hydrogen peroxide: An ex vivo study. Am. J. Dent. 29, 251–254 (2016).
AL-Omiri, M. K., Abul Hassan, R. S., AlZarea, B. K. & Lynch, E. Effects of combining ozone and hydrogren peroxide on tooth bleaching: A clinical study. J. Dent. 53, 88–93, https://doi.org/10.1016/j.jdent.2016.08.002 (2016).
AL-Omiri, M. K., Abul Hassan, R. S., AlZarea, B. K. & Lynch, E. Improved tooth bleaching combining ozone and hydrogen peroxide - a blinded study. J. Dent. 46, 30–35, https://doi.org/10.1016/j.jdent.2016.01.010 (2016).
Santana, M. S. et al. Dental bleaching with ozone: Effects on color and enamel microhardness. Acta Odontol. Latinoam. 29, 68–75 (2016).
Zanjani, V. A. et al. Bleaching effect of ozone on pigmented teeth. Dent. Res. J. (Isfahan) 12, 20–24 (2015).
Grundlingh, A. A., Grossman, E. S. & Witcomb, M. J. Tooth colour change with Ozicure Oxygen Activator: A comparative in vitro tooth bleaching study. SADJ 67, 332–337 (2012).
Tessier, J., Rodriguez, P. N., Lifshitz, F., Friedman, S. M. & Lanata, E. J. The use of ozone to lighten teeth. An experimental study. Acta Odontol. Latinoam. 23, 84–89 (2010).
Manton, D. J., Bhide, R., Hopcraft, M. S. & Reynolds, E. C. Effect of ozone and Tooth Mousse on the efficacy of peroxide bleaching. Aust. Dent. J. 53, 128–132, https://doi.org/10.1111/j.1834-7819.2008.00021.x (2008).
Abd Elhamid, M. & Mosallam, R. Effect of bleaching versus repolishing on colour and surface topography of stained resin composite. Aust. Dent. J. 55, 390–398, https://doi.org/10.1111/j.1834-7819.2010.01259.x (2010).
Millar, B. J. & Hodson, N. Assessment of the safety of two ozone delivery devices. J. Dent. 35, 195–200, https://doi.org/10.1016/j.jdent.2006.07.010 (2007).
Clavo, B. et al. Long-term improvement in refractory headache following ozone therapy. J. Altern. Complement. Med. 19, 453–458, https://doi.org/10.1089/acm.2012.0273 (2013).
Bocci, V., Borrelli, E., Zanardi, I. & Travagli, V. The usefulness of ozone treatment in spinal pain. Drug Des. Devel. Ther. 9, 2677–2685, https://doi.org/10.2147/DDDT.S74518 (2015).
Azuma, K. et al. Anti-inflammatory effects of ozonated water in an experimental mouse model. Biomed. Rep. 2, 671–674, https://doi.org/10.3892/br.2014.290 (2014).
Fuccio, C. et al. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharmacol. 603, 42–49, https://doi.org/10.1016/j.ejphar.2008.11.060 (2009).
Kielbassa, A. M., Maier, M., Gieren, A. K. & Eliav, E. Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem. Quintessence Int. 46, 881–897, https://doi.org/10.3290/j.qi.a34700 (2015).
WMA. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194, https://doi.org/10.1001/jama.2013.281053 (2013).
Schulz, K. F., Altman, D. G. & Moher, D., Consort Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 7, e1000251, https://doi.org/10.1371/journal.pmed.1000251 (2010).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Dahl, J. E. & Pallesen, U. Tooth bleaching - a critical review of the biological aspects. Crit. Rev. Oral Biol. Med. 14, 292–304 (2003).
Goldberg, M., Grootveld, M. & Lynch, E. Undesirable and adverse effects of tooth-whitening products: a review. Clin. Oral Investig. 14, 1–10, https://doi.org/10.1007/s00784-009-0302-4 (2010).
Matis, B. A., Cochran, M. A., Wang, G. & Eckert, G. J. A clinical evaluation of two in-office bleaching regimens with and without tray bleaching. Oper. Dent. 34, 142–149, https://doi.org/10.2341/08-64 (2009).
Paller, C. J., Campbell, C. M., Edwards, R. R. & Dobs, A. S. Sex-based differences in pain perception and treatment. Pain Med. 10, 289–299, https://doi.org/10.1111/j.1526-4637.2008.00558.x (2009).
Derdilopoulou, F. V., Zantner, C., Neumann, K. & Kielbassa, A. M. Evaluation of visual and spectrophotometric shade analyses: A clinical comparison of 3758 teeth. Int. J. Prosthodont. 20, 414–416 (2007).
Johansson, E., Andersson-Wenckert, I., Hagenbjörk-Gustafsson, A. & Van Dijken, J. W. Ozone air levels adjacent to a dental ozone gas delivery system. Acta Odontol. Scand. 65, 324–330, https://doi.org/10.1080/00016350701687247 (2007).
Kielbassa, A. M., Beheim-Schwarzbach, N. J., Neumann, K. & Zantner, C. In vitro comparison of visual and computer-aided pre- and post-tooth shade determination using various home bleaching procedures. J. Prosthet. Dent. 101, 92–100, https://doi.org/10.1016/S0022-3913(09)60001-9 (2009).
Oturan, M. A. & Aaron, J. J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A Review. Crit. Rev. Environment. Sci. Technol. 44, 2577–2641, https://doi.org/10.1080/10643389.2013.829765 (2014).
Matilainen, A. & Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 80, 351–365, https://doi.org/10.1016/j.chemosphere.2010.04.067 (2010).
Oh, B. T. et al. Oxidative degradation of endotoxin by advanced oxidation process (O3/H2O2 & UV/H2O2). J. Hazard. Mater. 279, 105–110, https://doi.org/10.1016/j.jhazmat.2014.06.065 (2014).
Caviedes-Bucheli, J. et al. The effect of tooth bleaching on substance P expression in human dental pulp. J. Endod. 34, 1462–1465, https://doi.org/10.1016/j.joen.2008.09.013 (2008).
Caviedes-Bucheli, J. et al. Substance P receptor expression in healthy and inflamed human pulp tissue. Int. Endod. J. 40, 106–111, https://doi.org/10.1111/j.1365-2591.2006.01189.x (2007).
Vaz, M. M. et al. Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J. Appl. Oral Sci. 24, 509–517, https://doi.org/10.1590/1678-775720160137 (2016).
Costa, C. A., Riehl, H., Kina, J. F., Sacono, N. T. & Hebling, J. Human pulp responses to in-office tooth bleaching. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, e59–64, https://doi.org/10.1016/j.tripleo.2009.12.002 (2010).
Bocci, V. A., Zanardi, I. & Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 9, 66, https://doi.org/10.1186/1479-5876-9-66 (2011).
Kucukgul, A., Erdogan, S., Gonenci, R. & Ozan, G. Beneficial effects of nontoxic ozone on H2O2-induced stress and inflammation. Biochem. Cell Biol. 94, 577–583, https://doi.org/10.1139/bcb-2016-0033 (2016).
Alpert, S. E. & Walenga, R. W. Ozone exposure of human tracheal epithelial cells inactivates cyclooxygenase and increases 15-HETE production. Am. J. Physiol. 269, L734–743 (1995).
Alpert, S. E., Walenga, R. W., Jaspers, I., Qu, Q. & Chen, L. C. Ozone inactivates cyclooxygenase in human tracheal epithelial cells without altering PGHS-2 mRNA or protein. Am. J. Physiol. 272, L879–887 (1997).
Tulunoglu, O., Alacam, A., Bastug, M. & Yavuzer, S. Superoxide dismutase activity in healthy and inflamed pulp tissues of permanent teeth in children. J. Clin. Pediatr. Dent. 22, 341–345 (1998).
Esposito, P., Varvara, G., Murmura, G., Terlizzi, A. & Caputi, S. Ability of healthy and inflamed human dental pulp to reduce hydrogen peroxide. Eur. J. Oral Sci. 111, 454–456 (2003).
Esposito, P., Varvara, G., Caputi, S. & Perinetti, G. Catalase activity in human healthy and inflamed dental pulps. Int. Endod. J. 36, 599–603 (2003).
Gürsoy, H., Çakar, G., İpçi, Ş. D., Kuru, B. & Yilmaz, S. In vitro evaluation of the effects of different treatment procedures on dentine tubules. Photomed. Laser Surg. 30, 695–698, https://doi.org/10.1089/pho.2012.3336 (2012).
Raafat Abdelaziz, R., Mosallam, R. S. & Yousry, M. M. Tubular occlusion of simulated hypersensitive dentin by the combined use of ozone and desensitizing agents. Acta Odontol. Scand. 69, 395–400, https://doi.org/10.3109/00016357.2011.572290 (2011).
Atabek, D., Bodur, H., Yalcin, G. & Kalayci, S. Effects of oxidative irrigants on root dentin structure: Attenuated Total Reflection Fourier Transform Infrared Spectroscopy study. Oral Health Dent. Manag. 13, 753–756 (2014).
Snapinn, S. Some remaining challenges regarding multiple endpoints in clinical trials. Stat. Med. 36, 4441–4445, https://doi.org/10.1002/sim.7390 (2017).
Acknowledgements
The authors would like to thank Mrs AbdelAziz for her help throughout the preparation of this investigation. The University of Jordan has permitted this study project, and this is greatly acknowledged. Queens University Belfast has sold and assigned the rights for healOzone patents in Edward Lynch’s name to Curozone; Edward Lynch has been the principal investigator for various healOzone grants to Universities, but does not receive any royalties. Regardless of the support from the authors and their respective institutions, no external funding was available for this investigator-driven trial.
Author information
Authors and Affiliations
Contributions
M.K.A.-O. and E.L. made substantial contributions to the design of the study. M.K.A.-O. and A.A.A.N. conducted the experiments, and M.K.A.-O., A.M.K. and E.L. were responsible for analysis and interpretation of data. M.K.A.-O. drafted the work, and A.M.K. and E.L. revised the draft critically for important intellectual content. Final approval of the version to be published was given by M.K.A.-O., A.A.A.N., A.M.K. and E.L.; all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AL-Omiri, M.K., Al Nazeh, A.A., Kielbassa, A.M. et al. Randomized controlled clinical trial on bleaching sensitivity and whitening efficacy of hydrogen peroxide versus combinations of hydrogen peroxide and ozone. Sci Rep 8, 2407 (2018). https://doi.org/10.1038/s41598-018-20878-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20878-0
This article is cited by
-
Amoxicillin Degradation by Reactive Oxygen Species on H2O2-Alone Process
Brazilian Journal of Chemical Engineering (2024)
-
Ex vivo study of molecular changes of stained teeth following hydrogen peroxide and peroxymonosulfate treatments
Scientific Reports (2023)
-
Investigation of the bleaching efficiencies of different office type bleaching techniques and the changes caused on the enamel surface
Lasers in Medical Science (2023)
-
Fluoride varnish, ozone and octenidine reduce the incidence of white spot lesions and caries during orthodontic treatment: randomized controlled trial
Scientific Reports (2022)
-
In-office tooth bleaching with chitosan-enriched hydrogen peroxide gels: in vitro results
Clinical Oral Investigations (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.