Abstract
With their merits of precise dating and sensitivity to climate changes, laminated stalagmites are an important terrestrial archive for reconstructions of paleohydrological changes. In particular, the Ca isotope composition (δ44/42Ca) of the Heshang Cave stalagmite has been documented to record a precipitation decrease during the 8.2 ka event in central China. As an extension, this study directly compares near-annual resolution δ44/42Ca data with an instrumental precipitation record to evaluate the fidelity of δ44/42Ca as a paleohydrologic proxy on annual to decade timescales. Over the period 1881–2001 AD, the δ44/42Ca values correlate significantly with both precipitation from a nearby weather station and the dryness/wetness index in the middle Yangtze River, with a stronger correlation on decadal smoothed data. These results clearly show that the δ44/42Ca ratio from stalagmites is an effective proxy for paleohydrological changes on a decadal timescale. More study is encouraged to refine understanding of stalagmite Ca isotope ratios and hydrological conditions and their application in paleohydrologic reconstructions.
Similar content being viewed by others
Introduction
In the monsoon region of East Asia, drought events caused by decreased monsoon rain intensity often bring severe disasters1,2. According to China’s third assessment report on climate changes3, an increase appears both in the area influenced by droughts and in the frequency of severe droughts from 1951 to 2010. In order to effectively understand the mechanisms responsible for drought disasters, reconstruction of high resolution records of past extreme drought events is needed beyond the timescale of instrument records, which is usually ca. 50–100 yr.
Stalagmites with laminated layers present an excellent terrestrial archive to reconstruct paleohydrologic histories in the monsoon regions, and they can be easily dated by layer counting4,5,6. Over the last few decades, numerous proxies have been developed through study of stalagmites, for example, calcite oxygen isotope ratios6,7, calcite carbon isotope ratios4,8, trace elements9,10, and carbon isotope ratios of organic matter11. The calcite oxygen isotope ratios (δ18O) are widely applied to track monsoon intensity and associated hydrological changes in the late Quaternary6,7,12. In eastern China, the stalagmite δ18O ratio mainly reflects circulation changes or monsoon changes13. The ratios of trace elements such as Mg, Sr, Ba relative to Ca have also been widely used to qualitatively reflect changes of rainfall amounts10. However, these proxies, which are affected by several factors, have not been used to quantitatively reconstruct drought events and to estimate the extent of drought.
In a recent study, Owen et al.14 proposed that stalagmite Ca isotope compositions (δ44/42Ca) have the potential to be used as a proxy for aridity. In cave settings, calcium preserved in stalagmites mainly comes from bedrock carbonate and is transported by groundwater containing abundant HCO3−. When groundwater infiltrates through unsaturated zones, secondary carbonate will precipitate in aquifers, fractures, and cave ceilings in a process known as prior calcite precipitation (PCP)15. Such a process is caused by degassing in the unsaturated zone. Previous studies have proposed that PCP varies closely with water-residence time and associated drought16,17. Paralleling PCP, Ca isotopes fractionate between the secondary calcite and Ca ion remaining in solute14,18,19. Through the study of the δ44/42Ca values in modern dripwater, glass plate calcite, and the dolomite bedrock collected from Heshang Cave, central China, Owen et al.14 argued that PCP was the major process that controls the variations of stalagmite δ44/42Ca values. Enhanced PCP will result in a positive excursion of the Ca isotope composition ultimately preserved in stalagmites14. Aided by previous studies that proposed the extent of PCP correlates with local effective rainfall20,21 and the knowledge of Ca isotope fractionation in modern regimes, Owen et al.14 semi-quantitatively estimated that precipitation was reduced by one-third relative to the present during the onset of the 8.2 ka event.
In the study of Owen et al.14, only a one-point calibration between δ44/42Ca and precipitation was utilized. In order to confirm the reliability of stalagmite Ca isotope ratios as a paleohydrologic proxy, it is worth extending the previous work by comparing the near-annual resolution Ca isotope record with the local instrumental precipitation record and to test the potential of the Ca isotopes to record severe drought events on an annual timescale. Here we provided an annual resolution Ca isotope record from Heshang Cave, the same site studied by Owen et al.14 for the period 1881–2001 AD with the goal of evaluating its relationship with the local instrumental precipitation record.
Materials and Methods
For this study, stalagmite samples were collected from the Heshang Cave (30°27′N, 110°25′E, 294 m altitude). This cave is located on the south bank of the Qingjiang River, central China. For more detailed information on the Heshang Cave, please refer to Hu et al.22. Generally, climate in this site has a distinct seasonality, with a warm-wet summer and a cool-dry winter. The mean annual temperature is 18 °C, while mean annual precipitation is 1144 mm.
Samples were obtained from the top of the HS6 stalagmite along the growth axis. HS6 is a laminated stalagmite that was cut from the cave in 200422. Its chronology is based on layer counting23, referring to the top as 2004. Close agreement between layer counting and U/Th dates confirmed the laminated layers to be annual layers6,23. As a consequence of the studies of He et al.23 and Liao and Hu24, samples from some layers were exhausted. In this study, we were able to select samples for the period from 1881–2001 AD for elemental and calcite isotope analyses.
Trace element analysis
The trace element analysis was conducted on a Thermo IRIS Intrepid II XSP Inductively coupled plasma atomic emission spectroscopy (ICP-OES) at the State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences. Mg/Ca, Sr/Ca, and Ba/Ca were analyzed using the “ratio” method. Samples were dissolved in 2% double distilled HNO3 that was also used as the blank solution and to dilute standards. The concentrations of these elements were computed from a standard curve and expressed as element/Ca ratios.
Calcium isotope analysis
The procedures for separation and analysis of Ca isotope compositions followed the approach used in Owen et al.14. In the prior study of Ca isotope composition in Heshang Cave, Owen et al.14 documented that no processing was necessary for speleothem samples owing to the quite low trace element contents. In the HS6 samples, the relative contents of Sr and Mg (Sr/Ca = 0.13 mmol/mol; Mg/Ca = 36.8 mmol/mol) were also quite low (Fig. 1). Thus, the column chemistry steps were omitted in this study.
Ca isotope analysis was performed on a Nu plasma II Instruments MC-ICP-MS with Aridus desolvating nebuliser at China University of Geosciences, following the approach used in Owen et al.14. Samples were standardized relative to NIST SRM 915a utilizing a sample-standard bracket method. Each sample was analyzed more than 12 times. A second standard (NIST SRM 915b) and a secondary internal standard (HPS Ca), identical with Owen et al.14, were used during the Ca isotope analysis. We used the method of Owen et al.14 to correct Sr interference.
The Ca isotope compositions were expressed as δ44/42Ca values. For NIST SRM 915b, the measured calcium isotope value was δ44/42Ca = 0.36 ± 0.08‰ (2σ, n = 42), and for HPS, the measured calcium isotope value was δ44/42Ca = 0.25 ± 0.07‰ (2σ, n = 38) in our laboratory. The above measured values agree well with those reported in Owen et al.14. In addition, a calcite sample from the HS4 stalagmite from Heshang Cave was used as our lab internal standard (named STM), with a mean value of δ44/42Ca = 0.31 ± 0.07‰ (2σ, n = 40).
Results
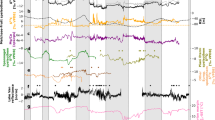
For the period 1881–2001 AD, the δ44/42Ca values varied between −0.14‰ and 0.21‰ and averaged 0.02‰ (Fig. 1). In this 121-yr period, three intervals have markedly larger δ44/42Ca values: 1896–1905 AD (t-test, p < 0.001), 1961–1975 AD (t-test, p < 0.01), and 1991–1999 AD (t-test, p < 0.05; Fig. 1). During the 1990s, the positive excursion of δ44/42Ca values in most data points is undistinguishable with the instrumental error (0.07‰; Fig. 2). The elemental ratios of Mg/Ca, Sr/Ca and Ba/Ca have higher values over a wide interval in the early 1900s (Fig. 1). Both Mg/Ca and Sr/Ca ratios clearly increase from 1961–1975 AD, while the Ba/Ca ratio has only a moderate increase. From 1991–2001 AD, only the Mg/Ca ratio parallels the increase of δ44/42Ca values. Fluctuations of the δ18O ratio show three distinctive stages, with less negative values in 1897–1906 AD, more negative values in 1903–1930 AD, and then less negative values upwards (Fig. 1).
Comparisons of the δ44/42Ca values (a) with the yearly precipitation (b), net precipitation (c), yearly air temperature (d) in Yichang, and the averaged dryness/wetness index from Wuhan and Jiangling27 (e). The dryness/wetness index in a year is classified into 5 grades: grade 1- very wet, grade 2- wet, grade 3- normal, grade 4-dry, and grade 5-very dry. The dashed horizontal lines in (a) refer the upper limit and lower limit of deviation from the averaged δ44/42Ca value (bold line) during 1881–2001.
Discussion
Comparing δ44/42Ca in HS6 with instrumental precipitation
In the previous work on the cave Ca isotope ratios, Owen et al.14 provided robust evidence to support the hypothesis that cave δ44/42Ca ratio was a novel proxy for drought. This study can further support this hypothesis by comparing the near annual resolution Ca isotope records directly with an instrumental precipitation record (Fig. 2). The annual precipitation data were obtained from the Yichang Meteorological Station. The data between 1938–1946 were absent because of the Japan invasion into China during the Second World War. The calculation of net precipitation followed Willmott et al.25 and Tremaine and Froelich26. Historical documentary data can also be cited to evaluate the potential of the speleothem δ44/42Ca ratio as an index of drought on the annual timescale. Here we use the mean dryness/wetness index (D/W) from nearby Jiangling and Wuhan from the benchmark publication of “Atlas of droughts and floods for the last 500 years”27. All these cities are located in the middle reaches of the Yangtze River Basin and have a similar climate context as the Heshang Cave. The δ44/42Ca values correlate negatively with the annual rainfall, the summer precipitation, and positively with the averaged D/W index (Table 1). The seemingly positive relation between δ44/42Ca and yearly air temperature probably results from the close relation between air temperature and net precipitation. Therefore, the results of this study clearly support the hypothesis that the Ca isotope ratio can indicate aridity. The not very strong correlation between δ44/42Ca values and rainfall indicates that other factors can mediate the isotope fractionation between solute and speleothem, such as calcite growth rate18.
We tested the sensitivity of the HS6 δ44/42Ca values to severe drought events. The instrumental precipitation record in Yichang shows a sharp reduction of precipitation from 1896 to 1905 AD (Fig. 2). This severe drought event was also recorded in the 71-station averaged rainfall across China since 1880 AD28 and the dryness/wetness index27. Paralleling the severe drought recorded by the Yichang meteorological station, the HS6 δ44/42Ca values shows a positive excursion of near 0.2‰ (Fig. 2). Using the same one-box model as Owen et al.14, we obtain a mean precipitation of 980 mm during the severe drought event of 1896–1905 AD, which is higher than the 772 mm recorded in the instrumental record from the Yichang Station (Fig. 2). The overestimation of precipitation probably results from the accuracy of the simple one-box model. In addition, in years with quite low precipitation (e.g. 1896–1905 AD), the δ44/42Ca values in HS6 stalagmite are only a little higher than the mean values in HS4 from 8.5–7.9 ka. Such a difference may result from the quite different duration time of drought events; the 8.2-ka event lasted as long as 150 yr as recorded by the HS4 stalagmite10.
In the other stage with δ44/42Ca deviation different from the instrumental error (1961–1975 AD), the annual precipitation measured in Yichang does not show a persistent decrease, but it records droughts in single years. The changes of annual precipitation in Yichang City are in accord with the drought index smoothed over the two cities and the yearly precipitation anomaly in China (Fig. 2). However, the increase of δ44/42Ca values is a continuous pattern. In this case, we should be cautious with the different sensitivities between the δ44/42Ca values and the annual rainfall. The δ44/42Ca ratios may be more sensitive to drought events with duration time >10 yrs. This deduction is further supported by the correlation analysis based on 10-yr smoothed data, with improved correlation coefficients between δ44/42Ca and rainfall (Table 2). Such a pattern may result from the residence time of percolating water in Heshang Cave. Previous studies in this cave proposed that the retention time of percolating water is >1 yr14,24,29. Thus, under conditions when drought occurs in a single year or over less than 10 yr, the positive excursion of annual δ44/42Ca signals will be only moderate or even absent.
It is noteworthy that some data in HS6 stalagmite are very negative (<−0.10‰; Fig. 3). With the δ44/42Ca values of dolomite bedrock and the fractionation factor from Owen et al.14, if the PCP is completely absent, the deposited stalagmite δ44/42Ca values will be close to −0.2‰. Interestingly, the years with relatively negative δ44/42Ca values correspond with higher precipitation. It seems that enhanced precipitation may cause groundwater to flush quickly through the percolating layers until producing stalagmites.
δ44/42Ca values comparisons among the HS6 stalagmite (red circle) over 1881–2001 AD with dripwater (black inverted triangle) and glass plate calcite (green triangle), and the HS4 stalagmite over 8.5–7.9 ka (blue square)14. For dripwater and glass plate calcite, the Mg/Ca values were set as 30 mmol/mol, which is the averaged value in HS6 stalagmite during 1881–2001 AD.
PCP reconstruction over 1881–2001 AD
The mean δ44/42Ca value (0.02‰) in HS6 from 1881–2001 AD is close to that of the mean values of glass plate calcite collected in the HS4 drip-site from 2005–2007 (0.05‰) and is negative relative to those in HS4 covering the 8.2 ka event (ca. 0.35‰) and in dripwater over a two-year monitoring experiment (0.68‰)14. These comparisons are consistent with the assumptions that dolomite bedrock (δ44/42Ca ≈ 0.40‰) is the primary Ca source for the HS4 and HS6 stalagmites and that PCP is an important process in controlling the Ca isotope compositions preserved in the Heshang Cave speleothems14.
In their study of Ca isotope compositions in Heshang Cave, Owen et al.14 established a simple model to estimate the fraction of Ca remaining in the solution:
where rs is the Ca isotope ratio of the instantaneous precipitation from dripwater (rs = δCaCO3/1000 + 1), r0 is the Ca isotope ratio of the initial dripwater, α is the Ca isotope fractionation factor between dripwater and calcite precipitation, and f is the proportion of Ca left in the solution. The fraction f is inversely closely related to PCP. When no PCP occurred, the f value is 1. In contrast, if almost all Ca is precipitated before reaching the cave ceiling, the f value is close to zero.
As done in Owen et al.14, the Ca isotope composition of dolomite bedrock was used as r0 (r0 = 1.0004 ± 0.00007), whereas the Ca isotope fractionation factor (α = 0.99937 ± 0.00003) between dripwater and glass plate calcite in modern conditions was used as the mean α for the period of 1881–2001 AD. In this short period, we can omit the influence of changes in the source compositions and in the Ca isotope fractionation factor. Thus, the f values can refer to the variations of PCP in the period 1881–2001 AD (Fig. 1).
The calculated f values vary between 0.50 and 0.86 (Fig. 1). The mean f value (0.68) in HS6 during 1881–2001 AD is a little higher than that over the 2005–2007 monitoring period (f = 0.64 ± 0.03; n = 15) and is relatively higher than the values covering the 8.2 ka event in the HS4 stalagmite (average 0.40)14. In the annual resolution PCP record from HS6 over the period 1881–2001 AD, three stages show a major decrease of f values (Fig. 1). All three stages are characterized by a decrease of ca. 0.10, which is half of the amplitude during the onset of the 8.2 ka event (0.20)14.
The influence of PCP on calcite precipitation is further supported by the elemental ratios of Mg/Ca, Sr/Ca and Ba/Ca, which also have the potential to record severe drought events and the associated PCP proportions30. In this study, the δ44/42Ca values correlate positively with Mg/Ca, but not with the other two elemental ratios (Table 3). During the interval with severe drought from 1896 to 1905 AD, both the Ca isotope composition and the Mg/Ca ratio show marked increases. The difference is that the increase of δ44/42Ca values happens in a brief period, whereas higher Mg/Ca ratios persist over a much longer time (Fig. 1). This comparison highlights that the δ44/42Ca values may be more sensitive to the changes of PCP proportions.
The elemental ratios of Mg/Ca, Ba/Ca, Sr/Ca change differently than the instrumental climate records (Table 3). The Mg/Ca ratio displays a negative relation with both the rainfall amount and air temperature, while the other two ratios have a very poor correlation with the climatic parameters. These comparisons with instrumental records highlight that these trace elemental ratios may be complicated by hydrochemical processes in the epikarst in addition to the water residence time. For example, in a recent study on the modern dripwater chemistry in the Hollow Ridge Cave, USA, Tremaine and Froelich26 concluded that dripwater Mg and Sr were controlled by mixing of sourced calcites and PCP. In a study of an annually layered stalagmite collected also in central China, the Sr/Ca and Ba/Ca ratio varied closely with the growth rate31.
Conclusions
This study provides a near-annual resolution record of Ca isotope compositions from the HS6 stalagmite in central China with the aim to evaluate the potential to indicate aridity by directly comparing Ca isotope ratios with instrumental precipitation data. During the period from 1881 to 2001 AD, the δ44/42Ca values of the HS6 stalagmite ranged from −0.14‰ to 0.21‰. The correlation analysis clearly supports the hypothesis in Owen et al.14 that the stalagmite Ca isotope ratio is a powerful tool to infer past paleohydrological changes.
Using the model in Owen et al.14, the calculated PCP from Ca isotope compositions varied between 0.50 and 0.86, averaging 0.67. Two stages of time had with a markedly high PCP proportions: 1896–1905 AD and 1961–1975 AD. The first stage parallels a multi-year severe drought event recorded by the Yichang meteorological station. However, the latter stage of enhanced PCP proportions occurred in periods with drought only in single years. This inconsistency may result from the residence time of percolating water that is more than 1 yr in Heshang Cave and/or the influence of factors other than PCP. Thus, this study clearly shows that the δ44/42Ca values in the HS-6 stalagmite correlate more effectively with precipitation on decadal timescales than on annual timescales. More study is needed to fill the knowledge gap between stalagmite Ca isotope ratios and hydrological conditions.
References
Cook, E. R. et al. Asian monsoon failure and megadrought during the last millennium. Science 5977, 486–489 (2010).
Stager, J. C., Ryves, D. B., Chase, B. M. & Pausata, F. S. R. Catastrophic drought in the Afro-Asian monsoon region during Heinrich Event 1. Science 6022, 1299–1302 (2011).
Committee of the China’s third assessment report on climate changes. China’s third assessment report on climate changes. Science Press, Beijing (in Chinese) (2015).
Genty, D. et al. Precise dating of Dansgaard-Oeschger climate oscillations in western Europe from stalagmite data. Nature 42, 833–837 (2003).
Tan, M. et al. Cyclic rapid warming on centennial-scale revealed by a 2650-year stalagmite record of warm season temperature. Geophys. Res. Lett. 30, 1617–1620 (2003).
Hu, C. Y. et al. Quantification of Holocene Asian monsoon rainfall from spatially separated cave records. Earth Planet. Sci. Lett. 266, 221–232 (2008b).
Wang, Y. J. et al. A high-resolution absolute-dated Late Pleistocene monsoon record from Hulu Cave, China. Science 294, 2345–2348 (2001).
McDermott, F. Palaeo-climate reconstruction from stable isotope variations in speleothems: a review. Quaternary Sci. Rev. 23, 901–918 (2004).
Borsato, A., Frisia, S., Fairchild, I. J., Somogyi, A. & Susini, J. Trace element distribution in annual stalagmite laminae mapped by micrometer-resolution X-ray fluorescence: Implications for incorporation of environmentally significant species. Geochim. Cosmochim. Acta 71, 1494–1512 (2007).
Liu, Y. et al. Links between the East Asian monsoon and North Atlantic climate during the 8,200 year event. Nat. Geosci. 6, 117–120 (2013).
Li, X. L., Hu, C. Y., Huang, J. H., Xie, S. C. & Baker, A. A 9000-year carbon isotopic record of acid-soluble organic matter in a stalagmite from Heshang Cave, Central China: Paleoclimate implications. Chem. Geol. 388, 71–77 (2014).
Cheng, H. et al. Ice age terminations. Science 326, 248–252 (2009).
Tan, M. Circulation effect: response of precipitation δ18O to the ENSO cycle in monsoon regions of China. Clim. Dyn. 42, 1067–1077 (2014).
Owen, R. A. et al. Calcium isotopes in caves as a proxy for aridity: Modern calibration and application to the 8.2 kyr event. Earth Planet. Sci. Lett. 443, 129–138 (2016).
Fairchild, I. et al. Controls on trace element (Sr–Mg) compositions of carbonate cave waters: implications for speleothem climatic records. Chem. Geol. 166, 255–269 (2000).
McDonald, J., Drysdale, R. & Hill, D. The 2002-2003 El Niño recorded in Australian cave drip waters: Implications for reconstructing rainfall histories using stalagmites. Geophys. Res. Lett. 31, L22202 (2004).
Treble, P. et al. Impacts of cave air ventilation and in-cave prior calcite precipitation on Golgotha Cave dripwater chemistry, southwest Australia. Quaternary Sci. Rev. 127, 61–72 (2015).
Tang, J., Dietzel, M., Böhm, F., Köhler, S. J. & Eisenhauer, A. Sr2+/Ca2+ and 44Ca/40Ca fractionation during inorganic calcite formation: II. Ca isotopes. Geochim. Cosmochim. Acta 72, 3733–3745 (2008).
Reynard, L., Day, C. & Henderson, G. Large fractionation of calcium isotopes during cave-analogue calcium carbonate growth. Geochim. Cosmochim. Acta 75, 3726–3740 (2011).
Tooth, A. F. & Fairchild, I. J. Soil and karst aquifer hydrological controls on the geochemical evolution of speleothem-forming drip waters, Crag Cave, southwest Ireland. J. Hydrol. 273, 51–68 (2003).
Cruz, F. W. et al. Evidence of rainfall variations in Southern Brazil from trace element ratios (Mg/Ca and Sr/Ca) in a Late Pleistocene stalagmite. Geochim. Cosmochim. Acta 71, 2250–2263 (2007).
Hu, C. Y., Henderson, G. M., Huang, J. H., Chen, Z. & Johnson, K. R. Report of a three-year monitoring programme at Heshang Cave, Central China. Int. J. Speleol. 37, 143–151 (2008a).
He, L. Y., Hu, C. Y., Huang, J. H., Xie, S. C. & Wang, Y. X. Characteristics of large-scale circulation of East Asian monsoon indicated by oxygen isotope of stalagmite. Quaternary Sci. 29, 950–956 (in Chinese with English abstract) (2009).
Liao, J. & Hu, C. Y. Thermoluminescence based thermometer from stalagmites. Quaternary Sci. 33, 1123–1129 (in Chinese with English abstract) (2013).
Willmot, C., Rowe, C. & Mintz, Y. Climatology of the terrestrial seasonal water cycle. J. Clim. 5, 589–606 (1985).
Tremaine, D. M. & Froelich, P. N. Speleothem trace element signatures: a hydrologic geochemical study of modern cave dripwaters and farmed calcite. Geochim. Cosmochim. Acta 121, 522–545 (2013).
Chinese Meteorological Science Institute. Atlas of drought/flood distribution for the last 500 years (in Chinese). SinoMaps Press, Beijing (in Chinese) (1981).
Pu, B., Wang, S. & Zhu, J. Spatial pattern of seasonal precipitation over the eastern part of China. Acta Sci. Nat. Univ. Pek. 43, 620–629 (in Chinese with English abstract) (2007).
Noronha, A. L. et al. Assessing influences on speleothem dead carbon variability over the Holocene: implications for speleothem-based radiocarbon calibration. Earth Planet. Sci. Lett. 394, 20–29 (2014).
Fairchild, I. J. & Treble, P. C. Trace elements in speleothems as recorders of environmental change. Quaternary Sci. Rev. 28, 449–468 (2009).
Tan, L. et al. Trace-element variations in an annually layered stalagmite as recorders of climatic changes and anthropogenic pollution in Central China. Quaternary Res. 81, 181–188 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants Nos 41672172 and 41731177). We thank Robert Owen of the University of Oxford and the annonymous reviewers for their helpful comments to improve the quality of the manuscript. Prof. Philip A. Meyers from the University of Michigan is thanked for language editing.
Author information
Authors and Affiliations
Contributions
X.L. and C.H. organized the sample collection and designed the research. X.L., X.C. and D.H. conducted Ca isotope analysis. The manuscript was written by X.L., L.J. and C.H. with contributions from all authors. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Cui, X., He, D. et al. Evaluation of the Heshang Cave stalagmite calcium isotope composition as a paleohydrologic proxy by comparison with the instrumental precipitation record. Sci Rep 8, 2615 (2018). https://doi.org/10.1038/s41598-018-20776-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20776-5
This article is cited by
-
Summer Monsoon Rainfall Variability in Central China over the Past 4700 Years and Its Possible Link to Solar Activity
Journal of Meteorological Research (2021)
-
Chinese stalagmite paleoclimate researches: A review and perspective
Science China Earth Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.