Abstract
Neurogenesis from endogenous neural stem cells (NSCs) might contribute to functional recovery after stroke based on animal studies; however, the relationship between neurogenesis and post-stroke outcome has rarely been demonstrated in humans. We prospectively collected cerebrospinal fluid (CSF) from 36 patients with subarachnoid hemorrhage (SAH). The CSF was added to the culture medium of the rat NSCs to test the effects on proliferation (proliferation index [PI], percentage of Ki-67 immunoreactive cells). We correlated the PI with functional outcome based on the modified Rankin Scale at 3 months post-SAH. Treatment with the CSF samples collected from SAH patients showed a higher PI compared with those collected from patients with normal pressure hydrocephalus and untreated controls (20.3 ± 8.8 vs. 8.2 ± 5.1 and 7.8 ± 3.0, P < 0.001), indicating proliferation-promoting factors in CSF after SAH. The PI was positively correlated with SAH volume (p = 0.025). For patients with lower SAH volume, patients with favorable outcome had a higher PI than those with poor outcome (20.8 ± 6.9 vs. 14.6 ± 4.3, p = 0.047). Using multivariable logistic regression analysis, the PI was a positive determinant for favorable outcome (odds ratio, 1.17; 95% confidence interval, 1.00 to 1.36) that more proliferation-promoting factors in CSF was associated with better functional outcome in SAH patients.
Similar content being viewed by others
Introduction
Stroke is one of the major causes of death and disability all over the world1. Many recent studies have focused on regenerative therapies for stroke2,3. Endogenous neural stem cells (NSCs), which are located mainly in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus, may also provide another attractive source for cell therapy4. Recently, many studies have shown that various kinds of brain injury, including stroke, may enhance neurogenesis at the SVZ and SGZ regions5,6. Stroke-induced neurogenesis may play an important role in functional recovery after stroke7. This concept has been demonstrated by conditional ablation of NSCs in adult mice diminishing poststroke motor and cognitive functional improvement and reduced synaptic connectivity8,9. However, the beneficial effects of neurogenesis have not been demonstrated in human studies, primarily because there is no available method to evaluate the neurogensis capacity in the brain of stroke patients.
Subarachnoid hemorrhage (SAH) is a subtype of stroke, which carries significant morbidity and mortality, with one-third of survivors suffering long-term physical and neurocognitive impairments10. In rodent brain, increased proliferation capacity of NSCs has been reported after SAH11,12. In human brain, SAH also induced the expression of NSC proliferation markers, indicating enhancement of neurogenesis13. To investigate the relationship between neurogenesis and SAH provides a chance not only to delineate the pathogenesis of the disease, but also to develop a potential treatment strategy, especially since there is still no effective therapy for SAH patients14.
In pathological conditions, injured brain may release some factors that diffuse to the SVZ and SGZ regions and stimulate neurogenesis15. Since the SVZ and SGZ areas are close to cerebral ventricle without the blood–brain barrier, the above factors may also appear in cerebrospinal fluid (CSF). Investigating CSF is thus a potential way to understand the relationships between neurogenesis and features of SAH. We have demonstrated that the NSC proliferation capacity is increased at the SVZ regions in a rat model of SAH at post-SAH 5–7 days11. Notably, the CSF collected from SAH rats during post-SAH 5–7 days can enhance the proliferating potentials of cultured rodent NSCs11. These findings imply the existence of proliferation-promoting factors in CSF of SAH rats. In this study, we tried to obtain CSF from SAH patients, measure the proliferation-promoting capacity of the CSF samples, and correlate this capacity to clinical features of SAH patients. We showed that the proliferation capacity of cultured NSCs promoted by CSF collected from SAH patients correlates well with disease severity and functional outcome.
Results
Optimization of condition using CSF to promote the proliferation capacity of NSCs
We prospectively collected CSF samples from SAH patients (Fig. 1). The CSF was added to the culture medium of NSCs, which was obtained from rat embyronic day 15 (E15) fetal brain. The proliferation capacity of cultured NSCs with or without treatment was analyzed as the proliferation index (PI), which was defined by the percentage of NSCs having immunoreactivity to Ki-67, a proliferation marker16.
We previously demonstrated that CSF samples collected from SAH rats during post-SAH 5–7 days can enhance the proliferating capacity of cultured rodent NSCs in the neurosphere11. Therefore, we first tested the effects of human CSF samples collected from three SAH patients on post-SAH day 5 at different concentrations. The PI was higher in the SAH group than both normal pressure hydrocephalus (NPH) and untreated control groups in a dose-dependent manner (p < 0.05) (Supplementary Fig. S1). Since the proliferation promoting effects of CSF reached a plateau at the concentration of 0.5%, we applied this concentration in the experiments that follow.
We further examined the effects of CSF samples, which were collected at different post-SAH times (days 3, 5, and 7) in six SAH patients. Only NSCs treated with CSF samples collected at 5 days post-SAH showed a higher PI as compared to those treated with CSF samples obtained from NPH patients and those without treatment (18.5 ± 9.5 vs. 8.7 ± 3.8 and 7.6 ± 1.4, P < 0.05) (Fig. 2A and B). The PI was also higher in NSCs treated with CSF samples collected on post-SAH day 5 than on day 3 (18.5 ± 9.5 vs. 6.8 ± 3.8, P < 0.01). We thus collected the CSF samples on day 5 post-SAH in all other patients. We also used BrdU method and cell viability assay kit (Cell Counting Kit-8, CCK8) to analyze the NSC proliferation, and found similar results that NSCs treated with CSF samples (n = 6) collected at 5 days post-SAH showed higher proliferation capacity as compared to those treated with CSF samples obtained from NPH patients and those without treatment (BrdU: 12.1 ± 2.6 vs. 3.3 ± 1 and 4.8 ± 1.1, P < 0.05; CCK8: 0.85 ± 0.07 vs. 0.64 ± 0.09 and 0.53 ± 0.18, P < 0.05) (Supplementary Figs S2 and S3). Using immunocytochemical analysis, most of the cells (90–95%) in neurospheres were immunoreactive to nestin (Fig. 2C), indicating the cells we analyzed were mainly NSCs.
The effects of CSF samples collected at different post-SAH times on NSC proliferation. (A) The cultured NSCs without treatment (control) or treated with CSF samples collected from patients with NPH or SAH on day 3, 5, or 7 after onset were double immunostained with anti-Ki-67 (green) and anti-nestin (red) antibodies with Hoechst 33258 (blue) staining. Scale bar = 50 μm. (B) Comparison of the PI between different groups is shown. N = 6 for each group. (C) A high magnification image shows most Hoechst positive cells with or without Ki-67 immunoreactivity are also immunoreactive to nestin. Scale bar = 50 μm. *p < 0.05; **p < 0.01.
High proliferation index in treatment with CSF collected from SAH patients
The demographic data and clinical features of 36 SAH patients (age, 62 ± 13 years) are summarized in Supplementary Table S1. The SAH was caused by aneurysm rupture in all patients (two with dissecting aneurysm). Twenty patients (56%) had aneurysm located at anterior circulation and 16 (44%) at posterior circulation. The mean initial Glasgow Coma Scale (GCS) score was 11 ± 4 and the mean SAH volume was 19 ± 14 mL. Intraventricular hemorrhage (IVH) developed in 23 of 36 patients (63.9%) and vasospasm occurred in 21 patients (58.3%). All of the patients received aneurysmal clipping (n = 33) or endovascular embolization (n = 3) within 1 day after SAH onset except for 4 patients (2, 3, 4, and 6 days after onset without rebleeding before clipping or embolization). The median modified Rankin Scale (mRS) score accessed at post-SAH 3 months was 3 (0–6).
The NSCs treated with CSF samples collected from 36 SAH patients on day 5 post-SAH showed a higher PI as compared to those treated with CSF samples obtained from six NPH patients and those without treatment (20.3 ± 8.8 vs. 8.2 ± 5.1 and 7.8 ± 3.0, P < 0.001) (Fig. 3A and B). The PI was then correlated with clinical information such as patient age, initial SAH volume, initial GCS, the existence of IVH, the presence of post-SAH vasospasm, and the location of aneurysm (Fig. 1). The PI values were positively correlated with SAH volume (Pearson’s r = 0.145; p = 0.025) (Fig. 3A and C). On the other hand, the PI values were not significantly correlated with age, gender, initial GCS, presence of IVH or vasospasm, or location of aneurysm (Supplementary Fig. S4).
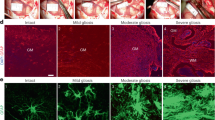
The effects of CSF samples collected at post-SAH day 5 on NSC proliferation. (A) The cultured NSCs without treatment (control) or treated with CSF samples collected from patients with NPH or SAH were double immunostained with anti-Ki-67 (green) and anti-nestin (red) antibodies with Hoechst 33258 (blue) staining. Initial head CT scan shows SAH in three patients (Patients 33, 1, and 26) with different severity (SAH volume of 9.9, 24.5, and 43.8 mL, respectively). The PI values in these three patients were 9.0, 22.6, and 45.9, respectively. Scale bar = 50 μm. (B) Comparison of the PI between different groups is shown, including control (n = 6), NPH (n = 6), and SAH (n = 36) groups. ***p < 0.001. (C) Correlation between the SAH volume and PI in 36 patients with SAH. p = 0.025 using linear regression analysis. The red solid line is the regression line and dashed lines are 95% confidence limits
High PI associated with good post-SAH outcome
The overall correlation between the PI and mRS at post-SAH 3 months was not significant (Pearson’s r = 0.002; p = 0.75) (Supplementary Fig. S4). However, in patients with lower SAH volume (less than 15 mL in the subarachnoid cistern), a higher PI seemed to be associated with favorable outcome (mRS < 3) (Fig. 4A and B). Since the PI was correlated with SAH volume, which was potentially associated with outcome, we stratified SAH patients into two groups according the SAH volume in the subarachnoid cistern: low SAH volume (<15 mL) and high SAH volume (>15 mL). The median PI value of 36 patients was 18.6; therefore, we set the cutoff value for high and low PI at 18. In the low SAH volume group (n = 18), six of eight patients (75%) with high PI had favorable outcome while only three of ten patients (30%) with low PI had favorable outcome (Fig. 4C). On the other hand, in the high SAH volume group (n = 18), there was no obvious association between PI and outcome.
The relationship between the PI and post-SAH outcome. (A) The cultured NSCs treated with CSF samples collected from SAH patients were double immunostained with anti-Ki-67 (green) and anti-nestin (red) antibodies with Hoechst 33258 (blue) staining. Initial head CT scan shows SAH in two patients (Patients 7 and 18) with similar severity (SAH volume of 9.7 and 9.9 mL, respectively). The PI values in these two patients were 12.7 and 31.0, respectively; the mRS scores at post-SAH 3 months were 3 and 1, respectively. Scale bar = 50 μm. (B) The outcome of SAH patients according to the SAH volume and PI. White spots indicate the patients with favorable outcome (mRS < 3) and black spots indicate poor outcome. The red solid line is the regression line, blue dashed lines are 95% confidence limits, and the red dashed line marks the SAH volume at 15 mL. N = 36. (C) The percentage of patients with mRS of 0-2, 3-4, and 5-6 at post-SAH 3 months according to PI values in low and high SAH volume groups. The low or high SAH volume is defined as the SAH volume at the subarachnoid cistern less or more than 15 mL, respectively.
We further compared the features of patients with favorable and poor outcome (Table 1). In the low SAH volume group, patients with favorable outcome had a higher initial GCS score (13.3 ± 3.0 vs. 8.9 ± 3.9; p = 0.007) and a higher PI (20.8 ± 6.9 vs. 14.6 ± 4.3; p = 0.047) than those with poor outcome. Age, gender, SAH volume, aneurysm location, and presence of IVH or vasospasm were not significantly different between the groups. In the high SAH volume group, patients with favorable outcome had a higher proportion of males (80% vs. 15.4%; p = 0.022) than those with poor outcome, while other parameters including the PI were not significantly different between the groups.
Using univariate logistic regression analysis, only initial GCS (odds ratio [OR], 1.46; 95% confidence intervals [CI], 1.12 to 1.90) was a positive determinant for favorable outcome (Table 2). Using multivariable logistic regression analysis, the GCS (OR, 1.56; CI, 1.13 to 2.17) and PI (OR, 1.17; CI, 1.00 to 1.36) were positive determinants for favorable outcome. The SAH volume showed a trend to be a negative determinant (OR, 0.91; CI, 0.81 to 1.01) for favorable outcome. Adding other variables such as age, presence of vasospasm or IVH in the model showed similar results (Supplementary Table S2).
Discussion
Endogenous neurogenesis might contribute to the repair process following brain damage after stroke based on studies in mice which show ablation of neurogenesis impedes motor function recovery after cerebral ischemia9,17. However, the relationship between neurogenesis and post-stroke outcome has rarely been demonstrated in humans. After stroke, injured brain may release some factors to stimulate endogenous neurogenesis and these factors may also appear in CSF15. Therefore, investigating CSF is a potential way to understand the relationships between neurogenesis and features of stroke. We chose a stroke subtype, SAH, for this pilot study because lumbar drain is a routine management procedure for our SAH patients and therefore we could easily obtain the CSF samples in clinical practice. Here, we showed that the CSF from SAH patients enhanced the proliferation capacity of cultured rat NSCs in a severity-dependent manner, which indicates the existence of proliferation-promoting factors in CSF after SAH. The proliferation capacity positively correlated with clinical outcome, which may imply that more proliferation-promoting factors in CSF is associated with better functional outcome in SAH patients. Therefore, this study for the first time demonstrated the importance of NSC proliferation on the functional outcome of stroke patients, focusing on SAH.
Treatment with post-SAH CSF on cultured NSCs showed that only the CSF samples collected on day 5 post-SAH, and not on day 3 or day 7, can enhance the proliferation capacity of NSCs. Our findings were in accordance with those of previous studies which showed that the proliferation capacity is enhanced at the SVZ regions in a rat model of SAH on days 5–7 post-SAH and CSF collected from SAH rats during days 5–7 post-SAH can enhance NSC proliferation11,12. Since fresh blood containing red blood cells (RBCs) and plasma would appear in CSF of patients with SAH, we also treated the cultured NSCs with human RBCs and plasma to rule out their contributions to NSC proliferation. The PI was not increased in treatment with lytic or non-lytic RBCs (Supplementary Fig. S5) or plasma (Supplementary Fig. S6). Therefore, it is likely that post-SAH brain produces certain factors around 5 days after the onset of SAH which can promote the proliferation capacity of NSCs. Blood-derived neurotrophic factor (BDNF), fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) are important factors contributing to post-insult activation of neurogenesis15,17. In our preview study, BDNF concentration in CSF paralleled the temporal change in NSC neurogenesis after SAH in a rodent model11. BDNF is a potent modulator capable of regulating the functions of neurogenesis18, and might play a key role in post-SAH proliferation of NSCs. In addition, exosomes released from stem cells and some micro RNA, such as miR17–92 cluster and miR-124a are also able to enhance neurogenesis post ischemic stroke19,20,21. The exact proliferation promoting factors in CSF samples collected on day 5 post-SAH are still unclear and require further investigation.
Regarding the mechanisms of SAH-induced production and release of proliferation promoting factors, the hemorrhage-related neuro-inflammatory reactions and high intracranial pressure might play a role since SAH volume is positively associated with the proliferation promoting capacity of post-SAH CSF. Notably, previous studies have shown that neuro-inflammation is able to enhance endogenous neurogenesis22. SAH may be complicated by vasospasm leading to brain ischemia23, which may also induce neurogenesis in post-insult brain5,6,24. However, we did not detect a correlation between the PI and the presence of vasospasm, making the possibility of brain ischemia less likely to be a major contributory mechanism for NSC proliferation.
Using multivariable logistic regression with control of the variables including age, infarct volume, initial GCS, and presence of IVH or vasospasm, the proliferation promoting capacity of post-SAH CSF positively independently correlated with clinical outcome, which indicates that more proliferation promoting factors in CSF is associated with better functional outcome in SAH patients. It is reasonable that with the higher post-SAH production of proliferation promoting factors in CSF, the endogenous NSCs at the SVZ and SGZ of post-SAH brain would receive stronger stimulation to undergo cell proliferation, as we observed in the culture system. Post-stroke neurogenesis can promote tissue remodeling and neural repair, which have been demonstrated in the rodent model of cerebral infarct25. Therefore, in this study, our findings supported the beneficial role of endogenous NSC proliferation on functional recovery in stroke patients, focusing on SAH. Enhancement of endogenous neurogenesis is thus a reasonable and potential therapeutic strategy for SAH treatment. In addition to high initial GCS associated with favorable outcome, a high PI value might be another independent predictor of favorable outcome for SAH patients. We also found that favorable outcome was associated with high proliferation promoting capacity of post-SAH CSF only in the low SAH volume group, not in the high volume group. This means that while more proliferation promoting factors in CSF may enhance post-SAH functional improvement, the benefits are not strong enough to overcome the pernicious effects of massive SAH. In the future, when neurogenesis-related therapy is available for SAH patients, analyzing the NSC proliferation-promoting capacity of CSF might help us to select appropriate cases (according to PI and SAH volume) for further treatment that patients with low PI values and relatively lower SAH volume might be a good candidate to receive certain management to enhance the endogenous NSC proliferation.
This study had some limitations. First, we could not obtain CSF from healthy volunteers and therefore used CSF from NPH patients as a control instead. However, we cannot guarantee that the results obtained with CSF samples from NPH patients are totally normal. Second, although we clearly demonstrated the existence of proliferation promoting factors in CSF after SAH, we did not identify the specific factors in this study. In addition, we only enrolled patients with SAH but not cerebral infarct or intracerebral hemorrhage, which are more common subtypes of stroke1. Future studies should try to identify the proliferation-promoting factors in CSF and extend the investigation to other types of stroke. Third, in vitro NSC proliferation and actual in vivo neurogenesis may be different. However, in our previous rodent study, we found that the neurogenesis at the SVZ region is enhanced in a rat model of SAH on days 5–7 post-SAH; the CSF collected from SAH rats during days 5–7 post-SAH can promote NSC proliferation11. The result may suggest that in vitro NSC proliferation is likely associated with in vivo post-SAH neurogenesis.
In summary, we provided a novel method using human CSF to study the relationship between NSC proliferation capacity and functional outcome in patients with SAH. The CSF from SAH patients enhanced the proliferation capacity of cultured NSCs, indicating the presence of neurogenesis promoting factors in CSF after SAH. The proliferation capacity positively correlated with clinical outcome, which implies that more proliferation-promoting factors in CSF is associated with better functional outcome in SAH patients. This study thus supports the rationale to enhance the proliferation capacity of NSCs as a reasonable therapeutic strategy for stroke treatment, especially for SAH.
Methods
Patients
We prospectively recruited 36 adult patients with SAH from National Taiwan University Hospital who received lumbar drainage for CSF diversion. Initial head computed tomography (CT) was performed all within 1 day except for one patient (within 2 days) after the onset of SAH. We only included SAH patients with modified Fisher grading score of 3 and 4, which are defined as thick SAH (>1 mm in depth) without and with IVH, respectively26. The patients were all monitored in the neurointensive care unit and treated with the standard protocol which consisted of resuscitation, early surgical or endovascular obliteration of the aneurysm, standard management of intracranial pressure, neurointensive care, and aggressive medical or endovascular therapy for vasospasm if present14. All patients received nimodipine for prevention of vasospasm.
The CSF was collected via lumbar drain on day 5 after SAH. We obtained CSF on days 3, 5, and 7 after SAH in the first six patients for initial analysis of the optimal time to collect the CSF. The CSF samples were immediately centrifuged at 900 g at 4 °C for 20 min before being divided into suitable aliquots and snap-frozen at −80 °C within 30 min. We also collected CSF from six patients with NPH to use as a control. The research was approved by the National Taiwan University Hospital Committee of Human Research and conducted in accordance with human ethics regulations (No. 201605042RINB). Written informed consent was obtained from the patients or from the next of kin of patients who had decreased consciousness levels.
Isolation and culture of neural stem cells
NSCs were obtained from pregnant Wistar rats at the gestational age of 15 days according to a protocol previously described11,27. First, embryos were removed from the rat, and the embryonic cerebral cortices were dissected out, washed, triturated, and cultured in the complete media containing Dulbecco’s Modified Eagle Media (DMEM)/F-12 (Gibco, Pascagoula, MS) supplemented with 1% N2 supplement (Gibco), 20 ng/ml basic fibroblast growth factor (bFGF), and 1% antibiotic solution absence of serum. Cultures were incubated at 37 °C in a humidified atmosphere and 5% CO2 for 6 days by which time primary neurospheres would form. Neurospheres were then cultured on 24-welled tissue culture polystyrene (TCPs, Costar, San Diego, CA) at 200 ± 20 neurospheres/cm2 in the DMEM/F12 medium supplemented with 1% N2 supplemented, 1% antibiotic-antimycotic solution (Gibco), and different concentration (0.25%, 0.5% and 1%) of CSF for 3 days without changing the culture medium. In addition to immunocytochemistry study, the Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich, St. Louis, Missouri) was used to assess the NSC proliferation capacity. Equal amount of NSC was cultured with or without 0.5% CSF. After 3 days, CCK-8 solution was added to culture medium and NSCs were incubated at 37 °C for additional 4 hours. Optical density (OD) was then determined at a wave-length of 450 nm.
The animal experimental procedures were approved by the National Taiwan University Institutional Laboratory Animal Care committee and the Utilization Committee (No. 20160109). All procedures met the requirements of the Animal Welfare Protection Act of the Department of Agriculture, Executive Yuan, Taiwan.
Immunocytochemistry
In immunocytochemical characterization, cells were fixed with ice-cold methanol for 20 min, blocked with 1% bovine serum albumin for 30 minutes at room temperature, and then incubated with primary antibodies overnight at 4 °C. For BrdU labeling, NSCs were pretreated with BrdU (Roche, Basel, Switzerland) at 10 μM for 3 days, fixed with methanol, and then treated with 4% Formaldehyde/1% Triton X100 for 10 min and 1 M HCl for 10 min before blocked. The primary antibodies used in this study were rabbit anti-Ki67 polyclonal antibody (a proliferation marker; 1:300, Abcam, Cambridge, MA), mouse anti-BrdU monoclonal antibody (a proliferation marker; 1:500, Cell Signaling technology, Danvers, MA), and mouse anti-nestin monoclonal antibody (a NSC marker; 1: 300, Merck Millipore, Billerica, MA). After wash, cells were incubated with secondary antibodies and Hoechst 33258 (diluted in PBS) for 2 hours at room temperature. The secondary antibodies used in the experiment were Alexa Fluor 488-conjugated goat anti-ribbit IgG and CyTM3-conjugated donkey anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA). The immunostained cells were visualized by confocal LSM880 microscope (Carl Zeiss AG, Oberkochen, Germany) with the optical thickness of 2 μm. For each condition, we analyzed at least three neurospheres with at least three planes for each neurosphere. The sub-PI of each plane was calculated as the areas with Ki-67 (or BudU) immunoreactive cells divided by the areas with Hoechst 33258-stained cells; the areas were determined automatically using MetaMorph software (Leica Microsystems GmbH, Wetzlar, Germany). All the sub-PI values were then averaged to get a final PI for each condition.
SAH severity and outcome analysis
The severity of SAH was determined by the volume of blood that appeared in the subarachnoid cisterns (basal, ambient, sylvian, suprasellar, and frontal interhemispheric cisterns) on initial axial head CT (thickness, 0.5 cm). The area of hemorrhage on each film was measured manually using software of ImageJ. The median SAH volume in the subarachnoid cistern of 36 patients was 15.2 ml; therefore, we set the cutoff value for high and low SAH volume at 15 ml. The location of aneurysm is stratified into anterior circulation (internal carotid artery, anterior or middle cerebral arteries, anterior communicating artery, and anterior choroidal artery) and posterior circulation (vertebral or basilar arteries, posterior cerebral artery, posterior communicating artery, and posterior inferior cerebellar artery). A follow-up head CT was performed around 1 week post-SAH in all patients to detect potential new intracerebral hypodense lesions, which indicate post-SAH cerebral infarction. An additional head CT with CT angiography was performed if neurological deterioration developed or when vasospasm was suspected during post-SAH care within 2 weeks. The existence of vasospasm was defined as the presence of post-SAH cerebral infarct detected with head CT or vasospasm detected with CT angiography. The functional outcome was evaluated using the mRS at 3 months after onset of SAH. An mRS score less than 3 (able to look after own affairs without assistance) was defined as a favorable functional outcome28. The researcher who measured the PI was blinded to the clinical condition of SAH patients and the researchers who analyzed the SAH volume or accessed the mRS were blinded to the PI values.
Statistical analysis
The results are reported as mean ± standard deviation (SD). To compare the PI between groups, the Kruskal-Wallis test with the Mann-Whitney test as post-hoc analysis was used. We correlated the PI value with clinical data, including age, gender, initial GCS, SAH volume, IVH, vasospasm, location of aneurysm, and mRS using linear regression analysis. To analyze the outcome, the Mann-Whitney test and Fisher’s exact test were used to determine the differences between groups for continuous data and categorical data, respectively. A logistic regression model was used to analyze the determinants of favorable outcome. The model included SAH volume, initial GCS, PI value, age, presence of IVH, and presence of vasospasm. A p-value of less than 0.05 was considered statistically significant. The SPSS statistical software (version 10.0, SPSS) was used for the statistical analyses.
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017).
George, P. M. & Steinberg, G. K. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87, 297–309 (2015).
Janowski, M., Wagner, D. C. & Boltze, J. Stem cell-based tissue replacement after stroke: factual necessity or notorious fiction? Stroke 46, 2354–2363 (2015).
Eriksson, P. S. et al. Neurogenesis in the adult human hippocampus. Nature medicine 4, 1313–1317 (1998).
Jin, K. et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proceedings of the National Academy of Sciences of the United States of America 98, 4710–4715 (2001).
Jin, K. et al. Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences of the United States of America 103, 13198–13202 (2006).
Thored, P. et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24, 739–747 (2006).
Jin, K., Wang, X., Xie, L., Mao, X. O. & Greenberg, D. A. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proceedings of the National Academy of Sciences of the United States of America 107, 7993–7998 (2010).
Sun, F., Wang, X., Mao, X., Xie, L. & Jin, K. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS One 7, e46326 (2012).
Rinkel, G. J. E. & Algra, A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. The Lancet Neurology 10, 349–356 (2011).
Lee, W. D. et al. Subarachnoid hemorrhage promotes proliferation, differentiation, and migration of neural stem cells via BDNF upregulation. PLoS One 11, e0165460 (2016).
Mino, M. et al. Temporal changes of neurogenesis in the mouse hippocampus after experimental subarachnoid hemorrhage. Neurological research 25, 839–845 (2003).
Sgubin, D., Aztiria, E., Perin, A., Longatti, P. & Leanza, G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J Neurosci Res 85, 1647–1655 (2007).
Connolly, E. S. Jr. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43, 1711–1737 (2012).
Zappaterra, M. W. & Lehtinen, M. K. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cellular and molecular life sciences: CMLS 69, 2863–2878 (2012).
Scholzen, T. & Gerdes, J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology 182, 311–322 (2000).
Ruan, L. et al. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Progress in neurobiology 115, 116–137 (2014).
Chen, A., Xiong, L. J., Tong, Y. & Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 1, 167–176 (2013).
Xin, H. et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 33, 1711–1715 (2013).
Uziel, T. et al. The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proceedings of the National Academy of Sciences of the United States of America 106, 2812–2817 (2009).
Liu, X. S. et al. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6, e23461 (2011).
Chapman, K. Z. et al. Inflammation without neuronal death triggers striatal neurogenesis comparable to stroke. Neurobiol Dis 83, 1–15 (2015).
Macdonald, R. L. Delayed neurological deterioration after subarachnoid haemorrhage. Nature reviews. Neurology 10, 44–58 (2014).
Jin, K. et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Molecular and Cellular Neuroscience 24, 171–189 (2003).
Koh, S. H. & Park, H. H. Neurogenesis in stroke recovery. Translational stroke research 8, 3–13 (2017).
Claassen, J. et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 32, 2012–2020 (2001).
Li, Y. C., Tsai, L. K., Wang, J. H. & Young, T. H. A neural stem/precursor cell monolayer for neural tissue engineering. Biomaterials 35, 1192–1204 (2014).
Van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J. & van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19, 604–607 (1988).
Acknowledgements
We thank Hsu-Hua Man in the imaging core at the First Core Labs, National Taiwan University College of Medicine, for technical assistance. This research was supported by a grant (MOST 104-2221-E-002-129-MY3) from the National Science Council, Council of Agriculture, Taipei, Taiwan and a grant (106-003362) from the National Taiwan University Hospital, Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
T.H.Y. and L.K.T. designed the experiments. Y.A.C. and K.C.W. performed the experiments. Y.A.C. and L.K.T. prepared the figures. Y.A.C., D.Z.L., T.H.Y. and L.K.T. analyzed the data. Y.A.C. and L.K.T. wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YA., Wang, KC., Liu, DZ. et al. The Proliferation Capacity of Cultured Neural Stem Cells Promoted by CSF Collected from SAH Patients Correlates to Clinical Outcome. Sci Rep 8, 1109 (2018). https://doi.org/10.1038/s41598-018-19371-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19371-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.