Abstract
Lactic acid bacteria (LAB) are the common probiotics. Here, we investigated the antiviral protective effects of heat-killed LAB strain Lactobacillus casei DK128 (DK128) on influenza viruses. Intranasal treatment of mice with DK128 conferred protection against different subtypes of influenza viruses by lessening weight loss and lowering viral loads. Protection via heat-killed DK128 was correlated with an increase in alveolar macrophage cells in the lungs and airways, early induction of virus specific antibodies, reduced levels of pro-inflammatory cytokines and innate immune cells. Importantly, the mice that were protected against primary viral infection as a result of heat-killed DK128 pretreatment developed subsequent heterosubtypic immunity against secondary virus infection. For protection against influenza virus via heat-killed DK128 pretreatment, B cells and partially CD4 T cells but not CD8 T cells were required as inferred from studies using knockout mouse models. Our study provides insight into how hosts can be equipped with innate and adaptive immunity via heat-killed DK128 treatment to protect against influenza virus, supporting that heat-killed LAB may be developed as anti-virus probiotics.
Similar content being viewed by others
Introduction
Influenza virus can cause serious respiratory disease in humans. Despite the availability of influenza vaccines, it is estimated that influenza virus infections cause 3 to 5 million severe illnesses and 250,000 to 500,000 influenza-related deaths worldwide during epidemics1,2,3. Current influenza vaccines are effective when vaccine strains are well matched with the circulating influenza viruses. A recent outbreak of the new 2009 H1N1 pandemic virus represents an example of the limited efficacy of the current vaccination4,5. Influenza A virus infects various hosts including humans, birds, and pigs. A variety of influenza A viruses are present in many different subtypes based on hemagglutinin (HA) and neuraminidase (NA) proteins on the surface of the virus. At present, 18 different HA (H1-H18) and 11 different NA (N1-N11) subtype molecules are identified, indicating the existence of numerous HA and NA combinations of influenza viruses6. Therefore, it is important to find an alternative measure that would provide protection against influenza virus regardless of strain-specificity. Lactic acid bacteria (LAB) are the most common probiotics which bestow health benefits on the host as micro-organisms. Various fermented vegetables and dairy products contain a variety of LAB, which were shown to provide health benefits1,7,8,9,10.
Some LAB strains as probiotics were reported to partially protect against bacterial infectious diseases, such as Streptococcus pyogenes and Streptococcus pneumoniae 11,12. Also, a variety of LAB strains were shown to protect mice against influenza virus infections by improving survival rates after intranasal or oral pretreatment but not preventing severe disease of weight loss13,14. Yogurt fermented with Lactobacillus was shown to reduce the cases of catching cold in the healthy elderly15 and to prolong the survival periods of mice with influenza virus infection16. In particular, previous studies have demonstrated the protective effects against influenza virus infection by administration of various LAB strains via the oral route3,17,18,19,20 or the intranasal route14,20,21,22,23,24. However, in the previous studies, the probiotic effects of LAB on influenza virus infection include partial protection or prolonged survival periods, accompanying substantial weight loss and resulting in various efficacy depending on the strains and routes of LAB.
It remains unknown whether pretreatments with heat-killed LAB can confer protection by preventing weight loss of animals after influenza virus infection and thus ameliorating morbidity. Furthermore, the antiviral protective mechanisms by LAB are poorly understood. In the present study, we found that heat-killed Lactobacillus casei strain DK128 treatment of mice conferred strain-nonspecific protection against morbidity of weight loss and mortality due to lethal influenza virus infection. Infection permissive protection against primary viral infection via heat-killed DK128 pretreatments was found to equip the mice with cross-protective immunity against secondary lethal infection with a heterosubtypic virus. The possible underlying mechanisms of the antiviral effects of DK128 were investigated.
Results
Intranasal pretreatments with heat-killed DK128 confers protection against influenza H3N2 virus
In our previous study, we have reported that intranasal pretreatments with live Lactobacillus plantarum DK119 could develop resistance to influenza virus H1N1 infection in mice despite a certain degree of morbidity20. LAB DK128, a new isolate from fermented vegetables, was suggested to be a promising probiotic25. To determine whether pretreatments with heat-killed LAB endows mice with resistance to influenza virus, mock and heat-killed DK128-treated mice (BALB/c) were infected with a lethal dose of A/Philippines/82 (H3N2) virus (Fig. 1). BALB/c mice that were treated with heat-killed DK128 at a low dose, 1 × 107 CFU or 1 × 108 CFU showed approximately 12% to 10% weight loss (Fig. 1a,b) but all survived the lethal infection with H3N2 virus. In contrast, mice treated with heat-killed DK128 at a higher dose (1 × 109 CFU) prior to infection did not show weight loss, whereas mock-treated mice displayed severe weight loss reaching to the endpoint by day 8–9 post infection and all died (Fig. 1d,e,f). Thus, the efficacy of protection against influenza virus via heat-killed DK128 appeared to be dependent on the LAB doses of pretreatments. These results are highly significant because mice with heat-killed DK128 pretreatments can be protected against lethal influenza virus infection, resulting in 100% survival and prevention of weight loss.
Pretreatment of mice with heat-killed DK128 confers protection against H3N2 influenza virus (A/Phil/82) infection. BALB/c mice (n = 5/group) were intranasally (IN) pretreated with different doses of heat-killed DK128 at 4-day and 1-day prior to H3N2 influenza virus (2 LD50, A/Philippines/2/1982) infection. (a) Body weight change after H3N2 virus infection of mice with heat-killed DK128 (1 × 107 CFU/50 ul/mouse) pretreatment. (b) Body weight change after H3N2 virus infection of mice with heat-killed DK128 (1 × 108 CFU/50 ul/mouse) pretreatment. (c) Body weight change after H3N2 virus infection of mice with heat-killed DK128 (1 × 109 CFU/50 ul/mouse) pretreatment. (d) Survival rates after H3N2 virus infection of mice with heat-killed DK128 (1 × 107 CFU/50 ul/mouse) pretreatment. (e) Survival rate after H3N2 virus infection of mice with heat-killed DK128 (1 × 108 CFU/50 ul/mouse) pretreatment. (f) Survival rate after H3N2 virus infection of mice with heat-killed DK128 (1 × 109 CFU/50 ul/mouse) pretreatment. PBS.H3N2: mock-treated mice infected with H3N2 influenza virus. DK128.H3N2: mice with heat-killed DK128 pretreatment prior to H3N2 virus infection.
Mice with heat-killed DK128 pretreatment control lung viral loads and virus-induced pro-inflammatory cytokines to lower levels
In an independent set of experiments, we determined the efficacy of clearing lung viral loads, which is an important parameter for assessing protective efficacy. At 7 days after infection, mice were sacrificed and lung extract samples were diluted to determine egg infectious titers (Fig. 2a). Heat-killed DK128 (109 CFU) treated mice showed approximately 18-fold lower levels of viral titers than those in mock-treated mice after infection. Infection with a pathogenic influenza virus can cause excessive production of proinflammatory cytokines. IL-6 and TNF-α inflammatory cytokines were determined in BALF and lung samples. At 7 days after infection, IL-6 was detected at significantly lower levels in BALF and lung samples from heat-killed DK128 pretreated mice than those in mock-treated mice after infection (Fig. 2b,c). TNF-α was also significantly lower in BALF from heat-killed DK128 pretreated mice compared to naïve mice (Fig. 2d) despite no statistical difference in the lung TNF-α levels between the two groups (Fig. 2e). Therefore, the induction of proinflammatory cytokines due to viral infection was substantially reduced as a result of heat-killed DK128 treatment.
Pretreatment of BALB/c mice with heat-killed DK128 lowers lung viral loads and inflammatory cytokines upon H3N2 influenza virus infection. BALB/c mice (n = 5/group) were intranasally (IN) pre-treated with heat-killed DK128 (1 × 109 CFU/50 μl/mouse) at 4-day and 1-day prior to infection with H3N2 virus (2 LD50, A/Philippines/2/1982). Mice were sacrificed at 7 days after infection to determine lung virus titers and cytokines in bronchoalveolar lavage fluids (BALF) and lung extracts. (a) Lung virus titers (Log10 EID50 /0.2 ml). (b) IL-6 (pg/ml) in BALF. (c) IL-6 (pg/ml) in lung. (d) TNF-α (pg/ml) in BALF. (e) TNF-α (pg/ml) in lung. PBS: uninfected mouse control. PBS.H3N2: mock-treated mice infected with H3N2 influenza virus. DK128.H3N2: heat-killed DK128 pretreatment prior to H3N2 virus infection. ND indicates not detected. Cytokine levels were described as mean ± SEM. Symbols *, *** denote p < 0.05 and 0.001 respectively by Two-tailed Student’s paired t test. The viral loads in the lungs was described as mean ± SEM. ** denotes p < 0.01 by Two-tailed Student’s paired t test.
Recruitment of virus-induced innate immune cells is differentially modulated by heat-killed DK128 pretreatment
Pathogenic influenza virus can cause severe inflammatory disease in lower respiratory tracts and lungs. Thus, the analysis of immune cell populations recruited to the site of virus infection would provide insight into the possible protective mechanisms mediated by heat-killed DK128 pretreatment. At 7 days after infection with lethal A/Philippines (H3N2) virus, we collected lung and BALF samples to analyze phenotypes of immune cells by multi-color flow cytometry. Alveolar macrophage phenotypic (CD11C+CD11b−F4/80+) cells were observed at higher levels in BALF from mice with heat-killed DK128 pretreatment than those in mock-treated mice with severe weight loss (Fig. 3a).
BALB/c mice with heat-killed DK128 pretreatment differentially modulate infiltrating innate immune cells in bronchoalveolar lavage fluids (BALF) and lungs upon H3N2 influenza virus infection. BALB/c mice (n = 5/group) were intranasally (IN) pretreated with heat-killed DK128 (1 × 109 CFU/50 μl/mouse) at 4-day and 1-day prior to H3N2 virus (2 LD50, A/Philippines/2/1982) infection. Mice were sacrificed day 7 post-infection to determine cell phenotypes and cellularity of infiltrating immune cells in BALF and lungs. (a–d) Infiltrated immune cells in BALF (cellularity/mouse); (a) Alveolar macrophages (CD11c+CD11b−F4/80+), (b) Monocytes (CD11c−CD11b+siglecF-Ly6chiF4/80+), (c) NK cells (F4/80-DX5+), (d) Activated NK cells (F4/80−DX5+CD69+). (e–h) Infiltrating immune cells in the lungs (cellularity/mouse); (e) Alveolar macrophages (CD11c+CD11b−F4/80+), (f) Monocytes (CD11c−CD11b+siglecF-Ly6chiF4/80+), (g) NK cells (F4/80-DX5+), (h) Activated NK cells (F4/80−DX5+CD69+). PBS: uninfected mouse control. PBS.H3N2: mock-treated mice infected with H3N2 influenza virus. DK128.H3N2: heat-killed DK128 pretreatment prior to H3N2 virus infection. The numbers of immune cells (per mouse in BALF or in the lung) were described as mean ± SEM. *, **, and *** denote p < 0.05, 0.1, and 0.001 respectively by One-way ANOVA and Tukey’s multiple comparison test.
In contrast, BALF and lung samples from mice with heat-killed DK128 pretreatment showed lower levels of monocytes (F4/80+CD11c−CD11b+Ly6ChiSiglecF−), natural killer cells (F4/80−DX5+CD69+), and activated natural killer cells (F4/80−DX5+CD69+) (Fig. 3b–d,f–h), while naïve untreated-mice showed significantly enhanced recruitment of monocytes, natural killer cells, and activated natural killer cells in response to the lethal infection with A/Philippines (H3N2) virus (Fig. 3b–d,f–h). Cellular analysis of BALF and lungs suggest that LAB DK128-mediated modulation of innate immune cells may prevent pulmonary inflammation, contributing to protection against influenza virus infection.
To further detail the possible role of alveolar macrophages, we applied clodronate-liposome to deplete airway macrophages in mice with heat-killed LAB treatment prior to influenza virus lethal infection (Supplementary Fig. 1). We confirmed that over 90% alveolar macrophages were depleted in the lungs after clodronate-liposome treatment prior to influenza virus infection (Supplementary Fig. 1a). Upon lethal infection with influenza virus, the clodronate-treated LAB DK128 mice displayed severe weight loss of over 20% and a survival rate of 25% whereas control LAB DK128 mice (100%) survived lethal infection and prevented weight loss (DK.H3N2, Supplementary Fig. 1b,c). All mice without heat-killed LAB treatment died of infection (data not shown). These data indicate the protective roles of alveolar macrophages that were elevated by prior treatment with heat-killed LAB.
Heat-killed DK128 pretreatment of mice mediates earlier induction of virus specific antibodies upon infection
B cells are responsible for producing antibodies that play a critical role in establishing long-lived adaptive immunity. To better understand the possible roles of antibodies in conferring protection in the heat-killed DK128 pretreated mice, we compared virus specific antibody responses in heat-killed DK128 (108 CFU) treated and untreated BALB/c mice at 6 days after infection of with a low dose (1.5 x LD50) of H3N2 virus (Fig. 4). Heat-killed DK128 pretreated mice displayed a moderate level of weight loss (6–9%) whereas mock control mice exhibited severe weight loss (20–25%) resulting in partial survival rates upon H3N2 virus infection (Fig. 4a,e). Interestingly, the mice with heat-killed DK128 pretreatment raised significantly higher levels of IgG, IgG1, and IgG2a antibodies at an earlier time point of day 6 post infection compared to those in mock untreated mice with infection (Fig. 4b,c,d). At a later time point of 14 days after infection, H3N2 virus infected naïve untreated mice that exhibited severe weight loss reached the high levels of IgG, IgG1, and IgG2a antibodies comparable to those from heat-killed DK128 treated mice (Fig. 4f,g,h). These results suggest that mice with heat-killed DK128 pretreatment are effective in inducing early IgG antibodies, possibly contributing to controlling viral replication as well as reducing weight loss whereas the untreated mice are ineffective in producing IgG antibodies at early times post infection, resulting in high viral replication and severe weight loss, and likely to die.
Heat-killed DK128 pretreatment mediates early induction of IgG antibodies after H3N2 virus infection. BALB/c mice (n = 5/group) were intranasally (IN) pretreated with heat-killed DK128 (1 × 108 CFU/50 μl/mouse) at 4-day and 1-day prior to infection with H3N2 virus (1.5 LD50, A/Philippines/2/1982). At 6 and 14 days after H3N2 virus infection, serum was collected and the levels of IgG and IgG isotypes specific for H3N2 virus were measured by enzyme-linked immunosorbent assay (ELISA). (a) Body weight changes in mice after H3N2 virus infection. (b–d) Antibody responses at 6 days after H3N2 virus infection; (b) IgG, (c) IgG1, (d) IgG2a. (e) Survival rates of mice after H3N2 virus infection. (f–h) Antibody responses at 14 days after H3N2 virus infection; (e) IgG, (f) IgG1, (g) IgG2a. PBS.H3N2: untreated mice with H3N2 virus infection, DK128.H3N2: mice with heat-killed DK128 pretreatment prior to H3N2 virus infection.
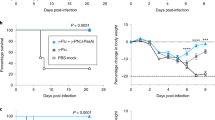
Protected BALB/c mice against H3N2 virus primary infection by heat-killed DK128 pretreatment acquire heterosubtypic immunity against secondary infection later
It is highly desirable for the hosts to acquire immunity against primary and secondary heterosubtypic viral infections without morbidity (weight loss). To determine cross-protective immunity against subsequent secondary infection, BALB/c mice that were protected against H3N2 virus primary infection as a result of the heat-killed DK128 pretreatment (Fig. 5a,c) were lethally challenged with heterosubtypic H1N1 (A/California/2009) pandemic virus at 4 weeks later (Fig. 5b). Naïve mice showed over 20% weight loss and 0% survival rates after infection with H1N1 pandemic virus (Fig. 5d). Mice that were protected against H3N2 A/Philippines/2/1982 virus primary infection as a result of heat-killed DK128 pretreatment displayed only transient 2–5% weight loss and 100% protection after secondary infection with heterosubtypic H1N1 virus (Fig. 5b,d).
Mice with heat-killed DK128 mediated protection against H3N2 virus primary infection show immunity to heterosubtypic H1N1 virus secondary infection. BALB/c mice (n = 5/group) were intranasally (IN) pretreated with heat-killed DK128 (109 CFU/50 μl/mouse) at 4-day and 1-day prior to infection with H3N2 virus (2 LD50, A/Philippines/2/1982). Body weight change and survival rate were monitored for 14 days. After primary virus (H3N2) infection, the protected mice were secondary infected with heterosubtypic virus (H1N1, 5 LD50, A/California/04/2009) and then weight loss and survival rate were monitored for 14 days. (a) Body weight changes after primary infection of BALB/c mice with H3N2 virus. (b) Body weight changes after secondary infection of BALB/c mice with H1N1 virus. (c) Survival rates after primary infection of BALB/c mice with H3N2 virus. (d) Survival rates after secondary infection of BALB/c mice with H1N1 virus. PBS.H3N2: H3N2 virus infection, DK128.H3N2: mice with heat-killed DK128 pretreatment prior to H3N2 virus infection, PBS.H1N1: H1N1 virus infection, DK128.H3N2.H1N1: The protected-mice against primary H3N2 virus via heat-killed DK128 pretreatment were then infected with secondary H1N1 virus.
To better understand the potential immune correlates conferring protection against secondary infection, an assay of hemagglutination inhibition (HI) was performed to determine cross protective HA antibodies. Consistent with IgG antibody levels (Fig. 4), sera from mice survived H3N2 primary infection regardless of LAB treatment showed high titers of HI activity against the homologous H3N2 virus (Supplementary Fig. 2a). Interestingly, sera from mice that were protected against H3N2 infection via prior LAB treatment showed substantial levels of HI activity against heterosubtypic H1N1 virus at a 2-fold higher level when compared to sera from mice survived H3N2 infection without prior LAB treatment (Supplementary Fig. 2b). Therefore, mice that were protected from primary infection (H3N2) via heat-killed DK128 pretreatment have acquired heterosubtypic immunity against secondary heterosubtypic infection (H1N1), most likely due to the elevated host immune responses during primary infection.
C57BL/6 mice with heat-killed DK128 pretreatment acquire immunity against primary H1N1 and secondary rgH5N1 virus
It is highly desirable if the protective effects of heat-killed LAB treatment would be expected in a strain non-specific pattern. We tested whether heat-killed DK128 pretreatment would mediate protection against a different subtype of influenza viruses in a different mouse strain. C57BL/6 mice (n = 5) were treated with heat-killed DK128 (1 × 109 CFU) at days -4 and -1 prior to infection with H1N1 virus (A/California/2009). As we observed with BALB/c mice, heat-killed DK128 treated C57BL/6 mice showed protection against lethal H1N1 virus infection with minimal weight loss of approximately 5% (Fig. 6a,d). In contrast, the mock control group did not survive viral infection and all died by day 9 post infection (Fig. 6a,d). Additionally, we determined whether heat-killed DK128-treated C57BL/6 mice that were protected against H1N1 virus primary infection without weight loss would be also protected against secondary infection with rgH5N1 virus (Fig. 6b,e). C57BL/6 mice that were protected against primary H1N1 virus via heat-killed DK128 pretreatment were completely protected against lethal infection with the secondary rgH5N1 virus which was lethal for all naïve mice (Fig. 6b,e). These results suggest that heat-killed DK128 pretreatment can equip the mice with the capacity to confer protective immunity against a broader range of influenza A virus primary and secondary infections. Sera from mice survived H1N1 primary infection showed high titers of HI activity against the homologous H1N1 virus (Supplementary Fig. 2c) as well as significant levels of HI activity against heterosubtypic rgH5N1 virus, which are higher than those of naïve serum control (Supplementary Fig. 2b). Also, sera of H1N1 infection showed cross reactive binding antibodies against rgH5N1 virus (data not shown). Thus, cross-reactive immune responses developed during primary infection appear to be partially responsible for conferring protection against secondary infection.
B cells are required to establish heat-killed DK128 mediated long-lasting immunity. Wilde type (C57BL/6) and B cell deficient (µMT) mice (n = 5/group) received intranasal pretreatment of heat-killed DK128 (109 CFU/50 μl/mouse) at 4-day and 1-day prior to H1N1 (2 LD50, A/California/04/2009) virus for primary infection. Body weight change and survival rate were monitored for 14 days. The protected-mice against primary H1N1 virus via heat-killed DK128 pretreatment were then infected with secondary heterosubtypic virus rgH5N1 (5 LD50, reassortant with HA from A/Indonesia/05/2005), and then weight changes and survival rates were monitored for 14 days. (a) Body weight changes after primary infection of C57BL/6 mice with H1N1 virus. (b) Body weight changes after secondary infection of C57BL/6 mice with rgH5N1 virus. (c) Body weight changes in µMT mice with H1N1 virus infection. (d) Survival rates of C57BL/6 mice with H1N1 virus infection. (e) Survival rates of C57BL/6 mice with rgH5N1 secondary virus infection. (f) Survival rate of µMT mice with H1N1 primary virus infection. PBS.H1N1: H1N1 virus infection, DK128.H1N1: mice with heat-killed DK128 pretreatment prior to H1N1 virus infection. PBS.H5N1: rgH5N1 virus infection, DK128.H1N1.H5N1: The protected-mice against primary H1N1 virus via heat-killed DK128 pretreatment were then infected with secondary rgH5N1 heterosubtypic virus.
B cells are required for establishing protective immunity in mice with heat-killed DK128 treatment
We observed that heat-killed DK128 pretreated mice elicited earlier induction of IgG isotype-switched antibodies specific for the infecting virus (Fig. 4), indicating the important roles of B cells. Using a B-cell deficient (µMT) mouse model, we further determined whether B-cells would play a critical role in establishing protective immunity against influenza virus infection via heat-killed DK128 pretreatments. B-cell deficient (µMT) mice (n = 5) were treated with heat-killed DK128 (109 CFU per mouse) at day 4 and day 1 prior to infection with H1N1 pandemic virus. Compared to the mock-treated µMT mice after infection, heat-killed DK128 pretreated µMT mice showed a delay of 3 to 5 days in displaying severe weight loss (Fig. 6c,f). Despite a prolonged delay in weight loss, the heat-killed DK128 treated µMT mice were deemed to progressive morbidity and did not survive H1N1 virus infection. These results suggest that B cells are essential for establishing sustained long-lasting protection although a significant delay in weight loss might be due to innate immunity mediated by DK128 pretreatment.
T cells are not required for protection against primary infection by heat-killed DK128 pretreatment but CD4 T cells partially contribute to preventing severe weight loss
In contrast to B cell-deficient mice, CD8 T cell-deficient (CD8KO) mice that received heat-killed DK128 pretreatment showed complete protection against H1N1 virus primary infection without weight loss (Fig. 7a,e). The CD4 T cell-deficient (CD4KO) mice that were pretreated with heat-killed DK128 were protected against H1N1 virus primary infection, displaying approximately 8% body weight loss and all CD4KO mice (100%) survived lethal infection (Fig. 7c,g). However, all mice died by day 9 post infection in the untreated mock control CD4KO and CD8KO groups (Fig. 7e,g).
CD4 T cells contribute to preventing weight loss in heat-killed DK128 pretreated mice after primary or secondary infection. CD8-deficient (CD8KO) and CD4-deficient (CD4KO) mice (n = 5/group) received intranasal pretreatment of heat-killed DK128 (109 CFU/50 μl/mouse) prior to H1N1 (2 LD50, A/California/04/2009) virus for primary infection, and then weight changes and survival rates were monitored for 14 days. The protected-mice against primary H1N1 virus via heat-killed DK128 pretreatment were then infected with secondary heterosubtypic virus rgH5N1 (5 LD50, A/Indonesia/05/2005), and then weight changes and survival rates were monitored for 14 days. (a) Body weight changes of CD8KO mice after primary infection with H1N1 virus. (b) Body weight changes of protected CD8KO mice against H1N1 virus after secondary infection with rgH5N1 virus. (c) Body weight changes of CD4KO mice after primary infection with H1N1 virus. (d) Body weight changes of CD4KO mice after secondary infection with rgH5N1 virus. (e) Survival rates of CD8KO mice after primary infection with H1N1 virus. (f) Survival rates of CD8KO mice after secondary infection with rgH5N1. (g) Survival rates of CD4KO mice after primary infection with H1N1 virus. (h) Survival rates of CD4KO mice after secondary infection with rgH5N1. PBS.H1N1: H1N1 virus infection, DK128.H1N1: mice with heat-killed DK128 pretreatment prior to H1N1 virus infection, PBS.H5N1: H5N1 virus infection, DK128.H1N1.H5N1: Protected mice against primary H1N1 virus via heat-killed DK128 pretreatment were exposed to secondary infection with rgH5N1.
Next, we determined the efficacy of subsequent protection against rgH5N1(A/Indonesia/05/2005) virus secondary infection in T cell-deficient mice that were well protected against H1N1 virus primary infection via heat-killed DK128 pretreatment (Fig. 7b,d). The CD8KO mice that were protected against H1N1 virus primary infection via heat-killed DK128 pretreatment without weight loss were well protected against rgH5N1 secondary virus, accompanying only a slight weight loss of 2–3% (Fig. 7b) under a condition with lethal infection (Fig. 7f). Whereas the CD4KO mice that survived H1N1 virus primary infection via killed-DK128 exhibited substantial weight loss of approximately 15–18% after secondary lethal infection with rgH5N1 virus under lethal infection (Fig. 7d,h). These results suggest that T cells are not needed for survival protection via heat-killed DK128 pretreatment against primary infection. However, CD4 T cells are required for establishing sufficient immunity to prevent severe weight loss during primary and secondary viral infections.
Discussion
LAB as probiotics have been mostly studied using live bacteria that improve survival rates or partial protection against influenza virus. In the present study, we investigated the effects of heat-killed LAB DK128 on protective efficacy against heterologous influenza virus primary and secondary infections, and modulation of innate immune cells post infection. We found that mice that were pretreated with heat-killed DK128 were well protected against lethal influenza virus infection independent of strain specificity by lessening weight loss. The mice that were heat-killed DK128 treated and protected against influenza virus primary infection developed immunity contributing to heterosubtypic cross protection against subsequent secondary infection with heterosubtypic virus. In addition, B cells but not T cells were required for establishing prolonged immunity conferring protection via pretreatment with heat-killed DK128. These findings would significantly improve our understanding of mechanisms by which hosts can develop innate and adaptive immunity against lethal viral infections. It suggests a potential usage of LAB as antiviral probiotics.
In general, the hosts that experienced and survived severe disease due to natural viral infection are expected to develop strong immunity after recovery. In this study, heat-killed DK128 treated mice without experiencing severe illness during primary viral infection can develop strong immunity. It is significant that heat-killed DK128 pretreated and well protected mice against primary infection also developed cross protective immunity against secondary heterosubtypic virus. Rapid induction of IgG isotype switched antibodies might be a key mechanism for equipping the hosts with early immunity against pathogens. This study suggests that heat-killed DK128 treated mice can induce IgG1 and IgG2a antibodies at an earlier time and at higher levels while not exhibiting a sign of severe disease, such as body weight loss, compared with the mock-treated mice after lethal infection. Vaccination against seasonal influenza viruses usually induces strain-specific immune responses to vaccine strains26, while influenza virus infection induces broader cross-reactive and long-lasting immune responses when the host is re-infected by the same virus subtypes27,28. In this study, mice with pretreatment of heat-killed DK128 were protected against primary and heterosubtypic secondary influenza virus infections. Sera from mice protected against primary H1N1 virus or H3N2 virus via heat-killed LAB treatment exhibited high levels of homologous and heterosubtypic hemagglutination inhibiting activity during infection. Cross reactive antibodies elevated during primary infection are most likely contributing to conferring heterosubtypic protection during secondary lethal infections. In previous studies, mice that survived influenza virus infection and severe disease of weight loss are known to induce cross protective T cell responses, partially contributing to heterosubtypic protection during subsequent secondary lethal infections29,30. As expected, the survived control mice in the PBS.H3N2 group recovered from severe weight loss were protected during secondary virus infection (data not shown).
The importance of B cells was further evidenced by the finding that B cell-deficient mice were not able to establish long-lasting protection. A delay in weight loss was observed probably due to the induction of innate immunity as a result of pretreatment with heat-killed DK128. The CD8KO mice with heat-killed DK128 pretreatment did not show any weight loss, indicating that CD8 T cells might not play a critical role in conferring protection mediated by DK128 treatment. Meanwhile, CD4KO mice with heat-killed DK128 treatment showed a moderate weight loss (~5–8%) during primary infection and substantial weight loss of ~15–18% during secondary infection in contrast to wild type or CD8KO mice. Therefore, our study suggests that B cells are playing a critical role in establishing heat-killed DK128-mediated immunity; and CD4 T cells are partially involved in this protection probably helping the B cells in generating long lasting immunity.
Interestingly, we observed that alveolar macrophages were increased in the BALF and maintained in the lungs from the heat-killed DK128-treated mice whereas naïve mice showed a significantly lower level of macrophages in the lungs after viral infection. Better understanding of the innate immune cell mobilization during early time of infection provides a clue in the mechanism of protection. Alveolar macrophages are located at the interphase between air and lung tissue providing the first line of innate immune defense against influenza viruses31. Alveolar macrophages appear to play a critical role in conferring protection mediated by prior treatment with heat-killed LAB. Upon influenza virus infection, alveolar macrophages were depleted to lower levels correlating with disease and mortality31. Alveolar macrophages were reported to release inflammatory cytokines to control viral replication but did not support productive viral replications within the respiratory tract32,33. The increased populations of alveolar macrophages in BAL from heat-killed DK128 pretreated mice after H3N2 virus infection are likely to be partially responsible for conferring protection, which is further supported by macrophage depletion experiment results (Supplementary Fig. 1). Upon lethal infection with influenza virus, the clodronate-treated LAB mice displayed severe weight loss of over 20%. The importance of alveolar macrophages in LAB-mediated protection against influenza virus is consistent with a report that clodronate-mediated depletion of alveolar macrophages abolished the live LAB-mediated protection20. Other innate immune cells, such as monocytes and activated natural killer cells, were regulated to a lower level in the airways and lungs of the heat-killed DK128 treated mice, whereas the naïve mice recruited high levels of these inflammatory cells upon lethal infection. In addition, previous studies demonstrated that alveolar macrophages play a critical role in controlling lung viral loads and protection against influenza virus infections34,35. Heat-killed DK128 treatment likely modulates innate immune cells differentially depending on the cell types in lung microenvironment, which may contribute to controlling lung viral loads and protection during primary influenza virus infection.
Live LAB is more often studied probably because live Lactobacillus are expected to be more effective in improving survival rates of mice than dead bacteria as demonstrated by both oral and intranasal administration24,36. The efficacy of protection by LAB is variable in a wide range of improving survival protection to just delaying the death depending on the strains of choice3,17,19,20,21,22,24,37. In terms of safety concerns, heat-killed LAB would be a desirable choice for developing safer probiotics. Dendritic cells are professional antigen presenting innate immune cells playing a critical role in generating cell-mediated immunity. Induction of proinflammatory cytokines is considered a possible mechanism of LAB-mediated antiviral effects38,39 although high levels of inflammatory cytokines caused inflammatory pulmonary disease in mice that were lethally infected with pathogenic influenza viruses40,41. The LAB experimental protocols are various and pretreatment periods are from few days to several weeks via either intranasal or oral routes14,16,20,37,42,43. In this study, we found that mice that were pretreated with heat-killed DK128 were protected against lethal infection and protected against severe weight loss. Even a single treatment resulted in effective protection against lethal infection as well as preventing weight loss (data not shown). These results are notable that DK128 strain as a novel strain of Lactobacillus tested in this study might have potent protective effects on equipping the hosts with immunity against influenza virus in a form of dead LAB.
A possible mechanism of heat-killed LAB DK128 antiviral effects is that innate immune cells and cytokines induced by heat-killed DK128 pretreatment control or delay viral replication to a lower level which does not cause disease. Even the B cell deficient mice with heat-killed DK128 pretreatment showed a delay of 3–5 days before displaying a sign of severe weight loss and mortality. Thus, the immediate control of viral replication by protective innate immunity might provide sufficient time for hosts to establish heterosubtypic cross protective immunity via B cells. This idea is further supported by the important roles of CD4 T cells in establishing heterosubtypic immunity during primary and secondary infection probably by providing the help to B cells. Also, moderate innate inflammation induced by heat-killed DK128 pretreatment may provide favorable microenvironment to expedite the earlier induction of adaptive immunity. Further studies will be required in testing this hypothesis.
Oral administration of live LAB was also reported to provide beneficial effects on mitigating severe disease due to influenza virus infection in mice14. It remains to be determined whether heat-killed LAB would confer similar protection against influenza virus via daily diet. Air transmission of influenza virus spreads rapidly among susceptible humans as exampled by the 2009 H1N1 pandemic44. FluMist® is licensed influenza nasal spray vaccine formulations. Our current study provides a piece of evidence that heat-killed LAB could potentially be administered via a nasal spray form as a prophylactic drug against non-specific influenza virus infections.
Methods
Preparation of Lactobacillus casei DK128 and virus
Lactobacillus casei (DK128) obtained from Dankook University in South Korea were isolated from fermented Korean vegetable food “Kimchi” as described25. MRS (de Man, Rogosa and Sharpe) broth (Becton Dickinson, Sparks, MD) was used to culture DK128 for 17–24 hr at 37 °C. LAB DK128 was harvested by spinning down at 8,000 rpm at 4 °C for 5 minutes, washed twice with phosphate-buffered saline (PBS), and then resuspended in PBS. Heat-killed DK128 was prepared by heating DK128 at 95 °C for 30 minutes and inactivation was confirmed by the absence of colony formation on the MRS agar plate. The reverse genetic reassortant rgH5N1 virus contains HA from A/Indonesia/5/2005 (H5N1), and the remaining 7 backbone genes from A/Puerto Rico/8/3445,46. Influenza viruses A/Philippines/2/1982 (H3N2), A/California/04/2009 (H1N1), and rgH5N1 were grown in embryonated chicken eggs and stored in −80 °C as described20,45,47. Heat-killed LAB would not cause disease in humans and belong to biosafety level 1.
Treatment of mice with LAB DK128 and infection with influenza viruses
Female BALB/c mice (6–8 weeks old, Harlan Laboratories) and C57BL/6 mice (Jackson Laboratory) were used (n = 5 each group) in this study. For intranasal administration of heat-killed LAB DK128, mice were anesthetized with isoflurane using an oxygen controlled machine (Baxter, Deerfield, IL) and then administered heat-killed LAB DK128 (107 to 109 CFU, colony forming units, per mouse). For primary virus infection, naïve or LAB treated mice were infected with A/Philippines/82 H3N2 virus, H1N1 pandemic virus (A/California/2009), or rgH5N1(A/Indonesia/05/2005) virus at a lethal dose (2 x LD50, 50% lethal dose). A control group was infected with the same dose of H3N2, H1N1, or rgH5N1 virus without LAB DK128 probiotic treatment. For mutant mice, B cell-deficient mice (B6.129S2-Ighm tm1Cgn/J), CD4-deficient mice (B6.129S2-Cd4tm1Mak/J), and CD8-deficient mice (B6.129S2-Cd8atm1Mak/J) were purchased from Jackson Laboratory (Bar Harbor, Maine). These mutant mice were intranasally pre-treated with heat-killed LAB DK128 (109 CFU per mouse) prior to lethal infection with influenza virus. All infected animals were daily monitored, and their weight and survival rates were recorded by following humanely handling animal procedures. Animal experiments presented in this study were approved by the Georgia State University (GSU) Institutional Animal Care and Use protocol (IACUC) review boards. All animal experiments and husbandry have been carried out under the guidelines of the GSU IACUC. All influenza virus infection experiments were carried out in the biosafety level 2 animal facility.
Lung and bronchoalveolar lavage fluid (BALF) cells and sample preparations
Mice of each group were euthanized at the indicated date post infection. The lungs were removed aseptically and homogenized in Roswell Park Memorial Institute (RPMI) medium 1640 (Fisher Scientific, Corning cellgro, USA) using 0.4 um cell strainer. The homogenates were then centrifuged at 1,700 rpm for 10 minutes. The supernatants were stored at −80 °C for other assays. The cell pellet was resuspended in 44% then carefully layered on to 66% Percoll and centrifuged at 2,700 rpm for 15 minutes. After the centrifugation, two layers were formed and the interspace between the two Percoll concentrations were collected using the sterilized transfer pipet. The layers were then washed in cold PBS and ready for counting cells to check their viability assessed by Trypan blue (Sigma). The bronchoalveolar lavage (BAL) fluid was collected from the trachea using 18-gauge Excel Safelet Catheter. A small incision in the trachea was made to allow passage of 18-gauge lavage tube into trachea. 800 ul of cold PBS was injected into lung through the incision for two times and the BALF was collected in 5 ml tube. The BALF was then centrifuged at 1,700 rpm for 5 minutes. The supernatant was stored at −80 °C for cytokine ELISA and the cell pellet was lysed using red blood cell lysis buffer and resuspended in PBS to stain for FACS analysis.
Flow cytometry analysis of innate immune cells
The prepared lung and BAL cells were stained with different surface markers. The cell surface markers for flow cytometry analysis included CD11c+CD11b−F4/80+MHCIIhi (alveolar macrophage), F4/80−DX5+CD69+ (natural killer Cells), F4/80−DX5+CD69+(activated natural killer cells), and CD11c−CD11b+siglecF−Ly6chiF4/80+ (monocytes) as described48. Stained cell samples were performed by Becton-Dickenson LSR flow cytometer and data analysis by Flowjo Software Program (Tree Star Inc.).
Antibody and cytokine ELISA
To measure antibody responses in serum samples, the antigen of inactivated virus (H3N2 A/Philippines/82, H1N1 A/California/09, rgH5N1) was coated onto the 96-well ELISA plates for overnight at 4 °C and washed with phosphate buffered saline Tween 20 (0.05%). Blocking was done with 3% bovine serum albumin containing PBS (3% BSA) at room temperature (RT) for 1.5 hr. The serially diluted serum samples were added to each well and incubated at RT for 1.5 hr. After washing, secondary antibodies were added and incubated at RT for 1hr. Then tetramethylbenzidine (TMB) substrate was added to detect horseradish peroxidase (HRP) enzyme conjugate activity, which was measured by optical density at 450 nm. Levels of cytokines in BALF and lung were determined according to Read-Set-Go IL-6 and TNF-α cytokine kits by ELISA as described (eBioscience, San Diego, CA) as previously described20,48.
Lung viral titers
Virus titers in the lung homogenates were determined by infectivity doses of serially diluted samples in embryonated chicken eggs as previously described45,46.
Statistical analysis
All data were statistically analyzed using a GraphPad Prism version 5.01 software (GraphPad Software Inc, La Jolla, CA, USA). The statistical significances of two different groups were stated using two-tailed student’s paired t test and one-way ANOVA. A P value < 0.05 was considered to be significant.
References
Drouault, S. & Corthier, G. Health effects of lactic acid bacteria ingested in fermented milk. Veterinary research 32, 101–117, https://doi.org/10.1051/vetres:2001115 (2001).
Hosoi, T. et al. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). International journal of food microbiology 82, 255–264 (2003).
Maeda, N. et al. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. International immunopharmacology 9, 1122–1125, https://doi.org/10.1016/j.intimp.2009.04.015 (2009).
Hancock, K. et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 361, 1945–1952, doi:NEJMoa090645310.1056/NEJMoa0906453 (2009).
Smith, L. R. et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 28, 2565–2572, doi:S0264-410X(10)00061-710.1016/j.vaccine.2010.01.029 (2010).
Tong, S. et al. New world bats harbor diverse influenza A viruses. PLoS Pathog 9, e1003657, https://doi.org/10.1371/journal.ppat.1003657 (2013).
Gorbach, S. L. Lactic acid bacteria and human health. Annals of medicine 22, 37–41 (1990).
Tan, G. S. et al. Broadly-Reactive Neutralizing and Non-neutralizing Antibodies Directed against the H7 Influenza Virus Hemagglutinin Reveal Divergent Mechanisms of Protection. PLoS Pathog 12, e1005578, https://doi.org/10.1371/journal.ppat.1005578 (2016).
Hanniffy, S. et al. Potential and opportunities for use of recombinant lactic acid bacteria in human health. Advances in applied microbiology 56, 1–64, https://doi.org/10.1016/S0065-2164(04)56001-X (2004).
Parvez, S., Malik, K. A., Ah Kang, S. & Kim, H. Y. Probiotics and their fermented food products are beneficial for health. Journal of applied microbiology 100, 1171–1185, https://doi.org/10.1111/j.1365-2672.2006.02963.x (2006).
Alexandre, Y., Le Berre, R., Barbier, G. & Le Blay, G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol 14, 107, https://doi.org/10.1186/1471-2180-14-107 (2014).
Racedo, S. et al. Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection in mice. Microbes Infect 8, 2359–2366, https://doi.org/10.1016/j.micinf.2006.04.022 (2006).
Corr, S. C., Gahan, C. G. & Hill, C. Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS immunology and medical microbiology 50, 380–388, https://doi.org/10.1111/j.1574-695X.2007.00264.x (2007).
Youn, H. N. et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral research 93, 138–143, https://doi.org/10.1016/j.antiviral.2011.11.004 (2012).
Makino, S. et al. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br J Nutr 104, 998–1006, https://doi.org/10.1017/S000711451000173X (2010).
Nagai, T., Makino, S., Ikegami, S., Itoh, H. & Yamada, H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. International immunopharmacology 11, 2246–2250, https://doi.org/10.1016/j.intimp.2011.09.012 (2011).
Kobayashi, N. et al. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. International immunopharmacology 11, 199–203, https://doi.org/10.1016/j.intimp.2010.11.019 (2011).
Hori, T., Kiyoshima, J., Shida, K. & Yasui, H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clinical and diagnostic laboratory immunology 9, 105–108 (2002).
Yasui, H., Kiyoshima, J. & Hori, T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clinical and diagnostic laboratory immunology 11, 675–679, https://doi.org/10.1128/CDLI.11.4.675-679.2004 (2004).
Park, M. K. et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PloS one 8, e75368, https://doi.org/10.1371/journal.pone.0075368 (2013).
Harata, G. et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Letters in applied microbiology 50, 597–602, https://doi.org/10.1111/j.1472-765X.2010.02844.x (2010).
Hori, T., Kiyoshima, J., Shida, K. & Yasui, H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clinical and diagnostic laboratory immunology 8, 593–597, https://doi.org/10.1128/CDLI.8.3.593-597.2001 (2001).
Izumo, T. et al. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. International immunopharmacology 10, 1101–1106, https://doi.org/10.1016/j.intimp.2010.06.012 (2010).
Youn, H. N. et al. Effect of intranasal administration of Lactobacillus fermentum CJL-112 on horizontal transmission of influenza virus in chickens. Poultry science 91, 2517–2522, https://doi.org/10.3382/ps.2012-02334 (2012).
Cho, Y. H., Hong, S. M. & Kim, C. H. Isolation and Characterization of Lactic Acid Bacteria from Kimchi, Korean Traditional Fermented Food to Apply into Fermented Dairy Products. Korean J Food Sci 33, 75–82 (2013).
Lee, Y. N., Lee, Y. T., Kim, M. C., Gewirtz, A. T. & Kang, S. M. A Novel Vaccination Strategy Mediating the Induction of Lung-Resident Memory CD8 T Cells Confers Heterosubtypic Immunity against Future Pandemic Influenza Virus. J Immunol 196, 2637–2645, https://doi.org/10.4049/jimmunol.1501637 (2016).
Liu, L. et al. Induction of Broadly Cross-Reactive Stalk-Specific Antibody Responses to Influenza Group 1 and Group 2 Hemagglutinins by Natural H7N9 Virus Infection in Humans. J Infect Dis 215, 518–528, https://doi.org/10.1093/infdis/jiw608 (2017).
Rajendran, M. et al. Analysis of Anti-Influenza Virus Neuraminidase Antibodies in Children, Adults, and the Elderly by ELISA and Enzyme Inhibition: Evidence for Original Antigenic Sin. MBio 8, https://doi.org/10.1128/mBio.02281-16 (2017).
Kees, U. & Krammer, P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med 159, 365–377 (1984).
Kreijtz, J. H. et al. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine 27, 4983–4989, https://doi.org/10.1016/j.vaccine.2009.05.079 (2009).
Smith, A. M. & Smith, A. P. A Critical, Nonlinear Threshold Dictates Bacterial Invasion and Initial Kinetics During Influenza. Sci Rep 6, 38703, https://doi.org/10.1038/srep38703 (2016).
Tate, M. D., Pickett, D. L., van Rooijen, N., Brooks, A. G. & Reading, P. C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol 84, 7569–7580, https://doi.org/10.1128/JVI.00291-10 (2010).
Tate, M. D., Schilter, H. C., Brooks, A. G. & Reading, P. C. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral immunology 24, 77–88, https://doi.org/10.1089/vim.2010.0118 (2011).
Fleming-Dutra, K. E. et al. Effect of the 2009 influenza A(H1N1) pandemic on invasive pneumococcal pneumonia. The Journal of infectious diseases 207, 1135–1143, https://doi.org/10.1093/infdis/jit008 (2013).
Tumpey, T. M. et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 79, 14933–14944 (2005).
Yeo, J. M. et al. Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. International immunopharmacology 18, 50–54, https://doi.org/10.1016/j.intimp.2013.10.020 (2014).
Kikuchi, Y. et al. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PloS one 9, e86416, https://doi.org/10.1371/journal.pone.0086416 (2014).
Al Kassaa, I., Hamze, M., Hober, D., Chihib, N. E. & Drider, D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb Ecol 67, 722–734, https://doi.org/10.1007/s00248-014-0384-7 (2014).
Kishino, S., Ogawa, J., Yokozeki, K. & Shimizu, S. Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Appl Microbiol Biotechnol 84, 87–97, https://doi.org/10.1007/s00253-009-1949-0 (2009).
Chan, M. C. et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res 6, 135, https://doi.org/10.1186/1465-9921-6-135 (2005).
Chan, R. W., Leung, C. Y., Nicholls, J. M., Peiris, J. S. & Chan, M. C. Proinflammatory cytokine response and viral replication in mouse bone marrow derived macrophages infected with influenza H1N1 and H5N1 viruses. PloS one 7, e51057, https://doi.org/10.1371/journal.pone.0051057 (2012).
Kawase, M. et al. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS immunology and medical microbiology 64, 280–288, https://doi.org/10.1111/j.1574-695X.2011.00903.x (2012).
Nakayama, Y. et al. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci Rep 4, 4638, https://doi.org/10.1038/srep04638 (2014).
Fraser, C. et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324, 1557–1561, doi:1176062 10.1126/science.1176062 (2009).
Song, J. M., Van Rooijen, N., Bozja, J., Compans, R. W. & Kang, S. M. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci USA 108, 757–761, https://doi.org/10.1073/pnas.1012199108 (2011).
Song, J. M. et al. Proteomic characterization of influenza H5N1 virus-like particles and their protective immunogenicity. J Proteome Res 10, 3450–3459, https://doi.org/10.1021/pr200086v (2011).
Quan, F. S., Huang, C., Compans, R. W. & Kang, S. M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol 81, 3514–3524, https://doi.org/10.1128/JVI.02052-06 (2007).
Lee, Y. T. et al. Innate and adaptive cellular phenotypes contributing to pulmonary disease in mice after respiratory syncytial virus immunization and infection. Virology 485, 36–46, https://doi.org/10.1016/j.virol.2015.07.001 (2015).
Acknowledgements
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.), AI119366 (S.M.K.), NIDDKR01 1R01DK105118 (J.S.), DOD CDMRP BC160450P1 (J.S.), and partially by a research grant to Georgia State University from TOBICO.
Author information
Authors and Affiliations
Contributions
Y.J.J. and S.M.K. designed and led this project. Y.H.C., S.M.H., J.H.J., J.S.O., C.H.K. contributed to providing reagents. Y.T.L., V.N., E.J.K., K.H.K. and M.K.P. were involved in developing the assay methods and carrying out experiments. Y.J.J. and S.M.K. analyzed the data. Y.J.J., J.S., and S.M.K. contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, YJ., Lee, YT., Ngo, V.L. et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci Rep 7, 17360 (2017). https://doi.org/10.1038/s41598-017-17487-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17487-8
This article is cited by
-
Construction of recombinant Lactococcus expressing thymosin and interferon fusion protein and its application as an immune adjuvant
Microbial Cell Factories (2024)
-
Identification and characterization of a marine bacterium extract from Mameliella sp. M20D2D8 with antiviral effects against influenza A and B viruses
Archives of Virology (2024)
-
Bioactive metabolites in functional and fermented foods and their role as immunity booster and anti-viral innate mechanisms
Journal of Food Science and Technology (2023)
-
In Vitro Inactivation of Respiratory Viruses and Rotavirus by the Oral Probiotic Strain Weissella cibaria CMS1
Probiotics and Antimicrobial Proteins (2022)
-
Antigen-capture ELISA and immunochromatographic test strip to detect the H9N2 subtype avian influenza virus rapidly based on monoclonal antibodies
Virology Journal (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.