Abstract
This prospective study used data from the BRISA Cohort, São Luís, Brazil (n = 1140) and analyzed associations between environmental factors up to the first 1000 days of life and “Childhood Asthma Symptoms”. “Childhood Asthma Symptoms” was a latent variable based on the number of wheezing episodes, emergency care visit due to wheezing, diagnosis of asthma and diagnosis of rhinitis. A theoretical model that included prenatal factors (socioeconomic status, pregestational body mass index-BMI, soft drink and junk food consumption), birth factors (gestational age, smoking and diseases during pregnancy, birth weight and type of delivery), first year of life factors (breastfeeding, environmental aeroallergens and respiratory diseases) and BMI z-score in the second year of life, was analyzed by structural equation modeling. High pregestational BMI, high soft drink consumption, cesarean section without labor, chill in the first three months of life, carpeted floor and child’s exposure to tobacco were associated with higher values of “Childhood Asthma Symptoms”. In contrast, high birth weight, breastfeeding and infant’s age were associated with lower values of “Childhood Asthma Symptoms”. These findings support the hypothesis that environmental factors that are present before conception and up to the first 1000 days of life are associated with asthma.
Similar content being viewed by others
Introduction
Asthma is the most prevalent nontransmissible chronic disease during childhood and is characterized by recurrent episodes of wheezing and shortness of breath that vary in frequency and severity among individuals (www.who.int)1. In children, asthma is a complex syndrome2. To date, the mechanisms involved in the etiology of asthma have not been completely elucidated3,4. However, the first 1000 days of life appear to have a strong influence on foetal programming and epigenetic regulation, thereby increasing the predisposition of children to many chronic diseases, including asthma5.
Accumulating evidence has indicated that epigenetic mechanisms contribute to the aetiology of asthma in children, and asthma can start early in the intrauterine life6. In addition, maternal exposures such as smoking during pregnancy7 and a high maternal pregestational body mass index (BMI)8 have consistently been associated with childhood asthma. At birth, other factors such as caesarian section9,10, especially pre-labour caesarean section10, low birth weight (LBW)11, and preterm birth (PTB)12 are also associated with a higher risk of childhood asthma. In the postnatal period, environmental stimuli including child’s exposure to tobacco and respiratory diseases at first year7,13,14, childhood obesity or rapid weight gain in early life have also been associated with childhood asthma12,13. Conversely, exclusive breastfeeding has been identified as a protective factor against asthma14.
In studies that have investigated environmental factors associated with childhood asthma, a traditional multiple regression approach has frequently been used, analyzing asthma as a dichotomous variable8,9,10,11,12,13,14. However, asthma in preschool children is a condition of difficult diagnosis2. In the present study, “Childhood Asthma Symptoms” was a continuous latent variable, deduced from the observed correlations among four clinical indicators: wheezing episodes, medical emergency visit due to intense wheezing, medical diagnosis of asthma and medical diagnosis of rhinitis. These indicators individually do not measure asthma well, but by using them together measurement error is reduced15.
The association between early life factors and “Childhood Asthma Symptoms” involves complex relationships of multicausality and temporality between variables, which can be better evaluated through structural equation modeling (SEM). Through this method, it is also possible to test, direct and indirect effects (mediation). Even if well-known risk factors for asthma have been studied, the structural equation modeling (SEM) approach allowed us to explore simultaneously direct and indirect effects of different variables at different stages of the life cycle (prenatal, birth and early life factors) in association with “Childhood Asthma Symptoms”. In the present study we tested the hypothesis of the association between prenatal, birth and postnatal factors with “Childhood Asthma Symptoms” in the first 1000 days of life. Thus, the objective of this prospective study was to analyze environmental factors during the prenatal period and the first 1000 days of life associated with “Childhood Asthma Symptoms” by using SEM.
Results
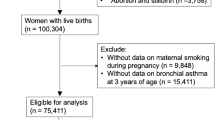
The characteristics of the pregnant women and children of the São Luís BRISA cohort are listed in Tables 1 and 2. Figure 1 presents the flow diagram of the BRISA cohort (2010–2013).
The confirmatory factor analysis (CFA) model that tested the “Childhood Asthma Symptoms” construct validity showed a good fit. A pathway starting from wheezing and progressing towards medical emergency care visit due to intense wheezing (modification index 61.905) was incorporated into the construct and later analyzed by SEM. Validation results for the “Childhood Asthma Symptoms” latent variable are listed in Table 3.
The initial proposed theoretical model did not show a good fit according to the Tucker-Lewis Index - TLI indicator (0.889), which would be desirable to be greater than >0.9515; and also according to the Weighted Root Mean Square Residual - WRMR indicator (1.147), which ideally should be lower than one (<1)15. Then, we followed the modification index suggestion. The higher modification index suggested (value = 108.879) was to include a pathway starting from maternal age towards the junk food variable. This modification originated the final model, which had a good fit according to the TLI indicator (0.951) and the WRMR indicator (0.914) (Table 3). In the final model, each indicator of the Socioeconomic Status (SES) and the “Childhood Asthma Symptoms” constructs had factor loadings greater than 0.5 and all the indicators of these constructs had significant p-values (Table 4).
A higher pregestational BMI (Standardized Coefficient – SC = 0.121; p = 0.032), caesarean section without labour (SC = 0.136; p = 0.046), high soft drink consumption during pregnancy (SC = 0.122; p = 0.044), chill in the first three months of life (SC = 0.311; p < 0.001), carpet covered floor (SC = 0.142; p = 0.023) and child’s exposure to tobacco (SC = 0.198; p = 0.027) were associated with higher values of “Childhood Asthma Symptoms” (Table 5).
Exclusive breastfeeding for six months also had a negative total effect (SC = −0.134; p = 0.038) (Table 5); and also an indirect effect via reducing chill in the first three months of life (SC = −0.035; p = 0.047) resulting in lower “Childhood Asthma Symptoms” values.
Gestational age also had a negative indirect effect on the “Childhood Asthma Symptoms”, with this effect being mediated by birth weight (SC = −0.111; p = 0.025).
Hospitalization due to respiratory tract problems up to the first year of life (otitis, tonsillitis, pneumonia and pharyngitis) was correlated to higher values of “Childhood Asthma Symptoms” (SC = 0.689; p < 0.001).
A correlation was also observed between maternal smoking during pregnancy and child’s exposure to tobacco (SC = 0.290; p < 0.001).
Discussion
The continuous latent variable “Childhood Asthma Symptoms” was based on four indicators, and all had factor loadings greater than 0.5. The CFA model showed good fit indices, indicating that this construct adequately represents what it proposes to measure. Here we used a latent variable, a variable that was derived indirectly based on the observed correlations between the variables number of wheezing episodes, emergency care visit due to intense wheezing, medical diagnosis of asthma and medical diagnosis of rhinitis, to measure “Childhood Asthma Symptoms”. By using a latent variable, it is possible to reduce measurement error15,16. Because asthma is difficult to measure, we used “Childhood Asthma Symptoms” based on symptoms commonly associated with asthma as a latent variable2. By using a latent variable, it was also possible to reduce the probability of type II error (false negative) in the associations between the first 1000 days of life factors and “Childhood Asthma Symptoms”15.
The “Childhood Asthma Symptoms” construct also included indicators of asthma that encompassed allergic rhinitis in children, as suggested by the orientation of the convergent loadings observed in the exploratory factor analysis (EFA) and the CFA. These data support the hypothesis that a “united allergic airway”17 is involved in these two diseases. Correspondingly, epidemiological evidence has shown a strong relationship between allergic rhinitis and asthma17,18 thereby suggesting that these two conditions share common physiopathology aspects18.
A high pregestational BMI, high soft drink consumption during pregnancy, cesarean section without labor, chill in the first three months of life, carpet covered floor and child’s exposure to tobacco were associated with higher “Childhood Asthma Symptoms” values. In contrast, high birth weight and infant’s age were associated with lower “Childhood Asthma Symptoms” values. Exclusive breastfeeding for six months had a negative effect on “Childhood Asthma Symptoms” values, and also an indirect effect via reducing chill in the first three months of life.
A higher maternal pregestational BMI value was one of the factors identified as being associated with increased values of “Childhood Asthma Symptoms”. This result supports the data of a meta-analysis that showed an association between elevated pregestational BMI and a high risk of childhood asthma/wheezing8. Birth by caesarean section without labour was also associated with “Childhood Asthma Symptoms” in the present study. In other studies that used standard multiple regression models, a positive association between caesarean section delivery, especially caesarean section without labour10, and childhood asthma was observed8,9,10,11,12,13,14. Various mechanisms have been proposed to explain this association: a) colonization of a baby’s intestinal tract with bacteria may be affected by a caesarean delivery and this could influence the future risk of childhood asthma9; b) the absence of a hormonal response to delivery involving cortisol and catecholamine among other hormones could lead to a reduction in the immunological response of infants, thereby increasing the future risk of asthma10; and c) caesarean sections could potentially result in a higher risk of respiratory distress syndrome and transitory tachypnea in neonates19, as these are both risk factors for asthma20.
Meta-analysis results have also shown that preterm birth significantly increases the risk of childhood asthma12. While traditional multiple regression models were previously employed, our data was analyzed by SEM. In our study, a higher GA was indirectly associated with lower “Childhood Asthma Symptoms” values, which was almost completely mediated by birth weight. In addition, a higher birth weight was associated with lower “Childhood Asthma Symptoms” values. Therefore, the results of the present study support the findings of a previous meta-analysis in which low birth weight was found to significantly increase the risk of childhood asthma11.
The present data did not show an association between junk food consumption during pregnancy and “Childhood Asthma Symptoms”. However, a higher tertile of soft drink consumption during the prenatal period was associated with higher “Childhood Asthma Symptoms” values. The sugar added to soft drinks may explain this association. In a study of 53 countries, an association between per capita sugar consumption during the perinatal period and subsequent risk of severe asthma symptoms in children was observed21. It is also possible that non-sugar ingredients contributed to the observed association between soft drinks and “Childhood Asthma Symptoms” in the present study. In another cohort study, consumption of artificially sweetened soft drinks during pregnancy was associated with childhood asthma22.
It is possible that nutritional variables during the first 1000 days of life influence epigenetic regulatory mechanisms and this can lead to a predisposition towards many nontransmissible chronic diseases, including asthma5. For example, foetal exposure to certain feeding patterns has been shown to induce a proinflammatory milieu that has the potential to affect foetal immunity and pulmonary development, thereby affecting the onset of childhood asthma8.
Maternal infection variables were not associated with “Childhood Asthma Symptoms” in our study, possibly because serious infections in pregnancy were not frequently reported (only 3.4% of pregnant women - n = 39 - reported pneumonia) and other non-serious airways infections may have been underreported by pregnant women. However, regarding child’s respiratory infections, chill in the first three months of life was associated with higher “Childhood Asthma Symptoms” values and hospitalization due to respiratory tract problems up to the first year of life (pneumonia, otitis, tonsillitis, pharyngitis, influenza and sinusitis) was correlated to higher values of “Childhood Asthma Symptoms”. It is possible that some of the reports of wheezing are consequences of infectious conditions, for example, respiratory syncytial virus (RSV). It is well documented that RSV is the most common trigger of bronchiolitis and that rhinovirus is the biological agent which is mainly associated with childhood asthma14. Thus, children’s infections may even result in lifelong asthma.
In our study, post-birth environmental stimuli (carpet covered floor, pets and mold/mildew) were associated to higher “Childhood Asthma Symptoms” values. In agreement with our results, exposure to allergens in early childhood has been implicated in asthma pathogenesis in children14 and birth cohort results have also shown that carpet covered floor in the child’s bedroom is associated with greater prevalence of wheezing in childhood23.
Also in relation to post-birth environmental aeroallergens, our data showed that child’s exposure to tobacco was associated to higher “Childhood Asthma Symptoms” values. Passive exposure to smoking in the postnatal period has been showed to increase the risk of asthma in early chidhood7. However, different from what was expected7, maternal smoking during pregnancy was not associated with higher values of “Childhood Asthma Symptoms”. This could be explained by the lower maternal smoking rate in the present study (2.6%), compared to Brazilian national24 data (9.6%) and US population-data25 (12%); furthermore, a lower smoking rate has been showed in the city of São Luís compared to other Brazilian cities26. Additionally, our data also showed a correlation between maternal smoking during pregnancy and child’s exposure to tobacco.
Children who were exclusively breastfed for six months had lower “Childhood Asthma Symptoms” values, mediated by chill in the first three months of life. It is well established that infant breastfeeding is strongly associated with lower risks of respiratory illnesses in later childhood and adolescence. A systematic review showed that breastfeeding is a protective factor against childhood asthma14. Nursing provides exposure to maternal antibodies and favors the production of anti-inflammatory cytokines that appear to protect children against asthma14.
During the second year of life, no effect of BMI z-scores on “Childhood Asthma Symptoms” was observed. Previously, systematic reviews demonstrated that overweight or obesity appeared not to be associated with an increased risk of childhood asthma13. However, the causal pathway and temporal aspects of this relationship remain to be elucidated, and also warrant more in-depth epidemiological investigations13. Adjustment in our model for variables that precede childhood BMI (e.g., pregestational BMI, consumption of soft drinks during pregnancy, caesarian section without labour, and birth weight) may account for the absence of an observed association between BMI z-scores and “Childhood Asthma Symptoms” in contrast with the results of previous studies12,13.
There were three significant strengths of the current prospective study. One, data were collected at three distinct time points: during the intrauterine period, following birth, and at the second year of life. Thus, a temporal analysis of independent variables in regard to asthma was performed. Second, the latent variable “Childhood Asthma Symptoms” encompassed four indicators, and measurement errors in the determination of asthma were minimized. Third, SEM was used to evaluate the associations.
Among the limitations of the present study we point out the use of a convenience sample in the BRISA Prenatal Cohort, due to the difficulty of obtaining a random sample representative of the population of pregnant women in São Luís, Brazil. To analyze the representativeness of our data with relation to the general population, we compared the frequencies of some variables in our sample (the BRISA Prenatal Cohort) with the random sample of “the BRISA Birth Cohort”27, a population-based study that was conducted during the same period in the city of São Luís, by the chi-square test. We observed that only the intermediate categories of maternal schooling were different in the two cohorts (5 to 8 years of study and 9 to 11 years of study), showing that in our sample the pregnant women were slightly better educated compared to the population-based sample. However, the frequencies of the other variables, ie. smoking during pregnancy, gestational age and birth weight did not differ in the two cohorts, reinforcing the external validity of our data.
In the present study we did not perform allergy tests. Although they would be desirable and would allow better characterization of the sample with relation to the allergic status of the children, considerations about their costs due to the high sample size, and their accuracy in the first years of life28 weighed more on the choice.
Conclusions
High pregestational BMI, high soft drink consumption during pregnancy, and cesarean section without labor, chill in the first three months of life, carpet covered floor, and child’s exposure to tobacco were factors associated with higher “Childhood Asthma Symptoms” values. In contrast, high birth weight, exclusive breastfeeding for six months and infant’s age were associated with lower “Childhood Asthma Symptoms” values. These findings highlight that asthma prevention for children might start before conception. Moreover, guidelines for healthy eating habits might be included in obstetrical practice along with an emphasis on the need to reduce caesarian section rates without medical indication and the importance of exclusive breastfeeding for six months for the prevention of asthma and respiratory diseases in the first year of life. The repercussions of these guidelines might not only improve the health of mother and child, but might also have a lifelong impact as a result of a reduced incidence of asthma.
Methods
Study design
This was a prospective cohort study entitled: “Etiological factors of preterm birth and consequences of perinatal factors on the child’s health: birth cohorts in two Brazilian cities, Ribeirão Preto and São Luís - BRISA”27. In this analysis we used data available from São Luís, a city located in the northeast region of Brazil29.
Participants and sample
This prenatal cohort consisted of a convenience sample due to the difficulty in obtaining a random sample representative of the population of pregnant women in São Luís, Brazil. Thus, pregnant women attending prenatal outpatient clinics, public, or private hospitals of São Luís were invited to participate in this study. Inclusion criteria were: completion of a first ultrasound exam at less than 20 weeks of gestational age (GA) and an intent to give birth at one of the maternity hospitals in the municipality where the prenatal interview was held. Only women with singleton pregnancies were included.
Data collection
A prenatal questionnaire was used to collect the following baseline information: maternal age (years), monthly family income (multiples of the minimum wage), maternal schooling (years of study), occupation of the family head, economic class according to the Criterion of Economic Classification Brazil (A-B being the wealthiest, C, D-E being the poorest)30, soft drink consumption, junk food intake, reported weight before pregnancy, and measured height31.
Soft drink consumption during pregnancy was determined based on two questions: 1) “On how many days of the week do you consume soft drinks?” and 2) “How many times a day do you ingest soft drinks?”. Junk food intake was determined based on the Block scoring system32 that considered the consumption of hamburgers, cheeseburgers, ham and cheese hot sandwiches, sausages, hot dogs, salame, ham, baloney, cold cuts, french fries, packaged snacks, and popcorn.
The initial birth questionnaire was reviewed at the first follow-up at birth to obtain the most accurate information regarding: maternal age (years), smoking during pregnancy, self-reported diabetes, hypertension based on medical diagnosis and respiratory diseases during pregnancy: bronchitis, asthma, bronchiolitis, wheezing, otitis, pneumonia, tonsillitis and pharyngitis, type of delivery (vaginal or caesarian), whether the mother had started labour with pain, GA-based obstetrical ultrasound (OU), date of last menstruation (DLM), and birth weight obtained from the neonate’s medical records.
At the second year of life follow-up, the following information was obtained: infant’s age (months), sex, skin color, respiratory symptoms related to “Childhood Asthma Symptoms”, status of exclusive breastfeeding, child’s respiratory infections during the first year of life, infant’s weight and height, environmental stimuli that included allergens (exposure to carpet covered floor, pets and mold/mildew) and child’s exposure to tobacco. Exclusive breastfeeding was defined according to the response obtained to the following question: “Up to what age was your baby exclusively breastfed, with the exclusion of tea, water, other types of milk, other drinks, and foods?”. Data regarding child’s respiratory infections during the first year of life were obtained by the following questions: chill in the first three months of life (cold, runny nose, sneezing, nasal obstruction, cough, with or without fever) and hospitalization due to respiratory tract problems up to the first year of life (pneumonia, otitis, tonsillitis, pharyngitis, influenza and sinusitis). The baby’s weight was measured with a digital scale with 0.1 kg precision (Tanita®, Arlington Heights, IL, USA). Height for mother and child was measured with a portable stadiometer with 0.1 cm precision (Alturexata®, Belo Horizonte, Minas Gerais, Brazil)31,33.
Endogenous/dependent variables
Maternal age (years) was used as a discrete numerical variable in our model. In contrast, pregestational BMI32 was used as a continuous numerical variable. Soft drink consumption was obtained by multiplying the frequency of weekly intake (0–7×/week) by daily intake (1–6×/day) and categorized into tertiles. Junk food intake was evaluated as the sum of the food items previously described and then was categorized as <3×/month, 1–2×/week, or ≥5×/week. Smoking, hypertension, diabetes and respiratory diseases during the current pregnancy were self-reported and treated as dichotomous variables (yes or no).
Type of delivery was treated as a categorical variable (vaginal, caesarian with labour, and caesarian without labour), with the latter category referring to pregnant women who did not start labour with pain and underwent a caesarian section. GA was calculated based on two criteria: DLM or OU obtained at a GA <20 weeks. When GA was measured according to DLM and differed by ≤10 days compared with the value estimated by OU, GA was calculated according to the DLM; when the difference in GA was >10 days between the use of DLM versus OU, GA was estimated according to the OU34. GA was categorized as: preterm (<37 gestational weeks), early term (37–38 gestational weeks), full term and/or late term (39–41 gestational weeks), and post-term (≥42 gestational weeks)35.
Child’s sex was a dichotomous variable (male = 1 or female = 2). Skin color was reported by the child’s mother as white, oriental, miscegenation of black and white Brazilians (mullato/brown), or black. Infant’s age was used as a discrete numerical variable.
Birth weight and BMI z-score36 were treated as continuous numerical variables, while exclusive breastfeeding was treated as a dichotomous categorical variable: <6 months or ≥6 months.
Chill in the first three months of life, hospitalization due to respiratory tract problems up to the first year of life, environmental stimuli that included allergens (carpet covered floor, pets and mold/mildew), child’s exposure to tobacco were self-reported and treated as dichotomous variables (yes or no).
Statistical analysis
Because of the occurrence of losses to follow-up, all variables were compared between the children who were seen for a second follow-up and those who were not, using the Chi-square test. Fewer children that were born at an earlier GA attended the second follow-up visit. On this basis, the sample was weighted by calculating the probability of a children being seen at the second follow-up visit as a function of GA using a logistic regression model. The inverse of this probability of selection was then calculated and this variable was used to weight the SEM estimates.
“Childhood Asthma Symptoms” latent variable
The “Childhood Asthma Symptoms” latent variable consisted of four indicators: number of wheezing episodes, emergency care visit due to intense wheezing, medical diagnosis of asthma and medical diagnosis of rhinitis. The response options for each of these questions were dichotomous (no or yes), except for the number of wheezing episodes which were categorized as: 0, <3, 3–6, and >6 episodes. These indicators were selected based on the convergent loadings (>0.50) in an EFA. This latent variable had its construct validity evaluated later by CFA using the Mplus software (version 7.0). Model fit was assessed based on the following fit indices: a) a p-value < 0.05 in the Chi-square test (χ 2)15; b) p > 0.05 and an upper 90% confidence interval limit <0.08 for the Root Mean Square Error of Approximation (RMSEA); c) Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) values >0.95, and d) a WRMR value <115. The modindices command was used to calculate the modification indices and thus to identify new paths that could improve the model fit16.
SEM
The Fig. 2 presents the proposed theoretical model to analyze the environmental factors associated with “Childhood Asthma Symptoms” during the prenatal period and the first 1000 days of life using SEM.
Proposed theoretical model for the environmental factors associated with “Childhood Asthma Symptoms” during the prenatal period and the first 1000 days of life: BRISA cohort, São Luís, Brazil, 2010–2013. The Socioeconomic Status (SES) would be a more distal determinant (exogenous variable) exerting its effects on the development of “Childhood Asthma Symptoms” in early childhood38 and on the remaining dependent variables of the model. Maternal age potentially affects pregestational body mass index (BMI)39 and “Childhood Asthma Symptoms”8. Maternal respiratory infections may be associated with “Childhood Asthma Symptoms”14. Smoking during pregnancy may result in “Childhood Asthma Symptoms”7 and may also result in low birth weight (LBW)40; soft drink and junk food consumption potentially affect blood pressure41,42 and diabetes43, which may lead to caesarean delivery, which has been associated with “Childhood Asthma Symptoms”9,10, LBW11 and preterm birth (PTB)12,13. Girls have a lower risk of “Childhood Asthma Symptoms” and black race is associated with an increased risk of “Childhood Asthma Symptoms”3,44. Exclusive breastfeeding may have a protective effect on “Childhood Asthma Symptoms”14. Child’s respiratory infections during the first year of life may be associated with “Childhood Asthma Symptoms”14, while higher child’s BMI z-score has been associated with “Childhood Asthma Symptoms”12,13. Environmental stimuli in early childhood (exposure to carpet covered floor, pets and mold/mildew), including exposure to tobacco have been associated with “Childhood Asthma Symptoms”7,14,23.
In the SEM the Weighted Least Squares Mean and Variance Adjusted (WLSMV) estimator and theta parameterization were used37. The same previously described fit indices15,16 were considered to determine whether the model showed good fit. The Chi-square, degrees of freedom, and p values were evaluated, but were not adopted as parameters to assess model fitting due to their sensitivity to sample size.
The modindices command was also used to test if a new path could improve model fit. When the modification index was >10 and the suggested modification were considered plausible from a theoretical standpoint a new model including that path was generated16. The total, direct and indirect effects were estimated.
Ethical aspects
The Research Ethics Committee of the University Hospital of the Federal University of Maranhão, Brazil (protocol no. 4771/2008-30), approved this study. All methods were performed in accordance with relevant guidelines and regulations of that Research Ethics Committee. The written informed consent was obtained from all women and for those younger than 18 years an accompanying adult also signed the consent form. The confidentiality, image protection and non-stigmatization were guaranteed to all participants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
World Health Organization (WHO). Available: http://www.who.int/features/factfiles/asthma/asthma_facts/en/ Accessed 2016 Mar 25.
Papadopoulos, N. G. et al. International consensus on (ICON) pediatric asthma. Allergy 67, 976–97, https://doi.org/10.1111/j.1398-9995.2012.02865.x (2012).
Milligan, K. L., Matsui, E. & Sharma, H. Asthma in Urban Children: Epidemiology, Environmental Risk Factors, and the Public Health Domain. Curr Allergy Asthma Rep. 16, 33, https://doi.org/10.1007/s11882-016-0609-6 (2016).
Cheng, R. Y. et al. Alterations of the lung methylome in allergic airway hyper-responsiveness. Environ Mol Mutagen 55, 244–55, https://doi.org/10.1002/em.21851 (2014).
Adair, L. S. Long-term consequences of nutrition and growth in early childhood and possible preventive interventions. Nestle Nutr Inst Workshop Ser. 78, 111–20, https://doi.org/10.1159/000354949 (2014).
Weinmann, T. et al. Establishing a birth cohort to investigate the course and etiology of asthma and allergies across three generations - rationale, design, and methods of the ACROSSOLAR study. BMC Public Health 15, 1210, https://doi.org/10.1186/s12889-015-2555-y (2015).
Vardavas, C. I. et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J. pii: ERJ-01016-2015, https://doi.org/10.1183/13993003.01016-2015 (2016).
Forno, E., Young, O. M., Kumar, R., Simhan, H. & Celedón, J. C. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 134, e535–46, https://doi.org/10.1542/peds.2014-0439 (2014).
Thavagnanam, S., Fleming, J., Bromley, A., Shields, M. D. & Cardwell, C. R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy 38, 629–33, https://doi.org/10.1111/j.1365-2222.2007.02780.x (2008).
Hyde, M. J., Mostyn, A., Modi, N. & Kemp, P. R. The health implications of birth by Caesarean section. Biol Rev Camb Philos Soc. 87, 229–43, https://doi.org/10.1111/j.1469-185X.2011.00195.x (2012).
Xu, X. F. et al. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr. 14, 275, https://doi.org/10.1186/1471-2431-14-275 (2014).
Sonnenschein-van der Voort, A. M. et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 133, 1317–29, https://doi.org/10.1016/j.jaci.2013.12.1082 (2014).
Mebrahtu, T. F., Feltbower, R. G., Greenwood, D. C. & Parslow, R. C. Childhood body mass index and wheezing disorders: a systematic review and meta-analysis. Pediatr Allergy Immunol. 26, 62–72, https://doi.org/10.1111/pai.12321 (2015).
Noutsios, G. T. & Floros, J. Childhood asthma: causes, risks, and protective factors; a role of innate immunity. Swiss Med Wkly 144, w14036, https://doi.org/10.4414/smw.2014.14036 (2014).
Kline, R. B. Principles and practice of structural equation modeling. New York: The ebook (THE GUILFORD PRESS, 2016).
Wang, J. & Wang, X. Structural equation modeling: applications using Mplus (Noida: Thomson Digital, 2012).
Feng, C. H., Miller, M. D. & Simon, R. A. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol. Allergy 26, 187–90, https://doi.org/10.2500/ajra.2012.26.3762 (2012).
Khan, D. A. Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc. 35, 357–61, https://doi.org/10.2500/aap.2014.35.3794 (2014).
Bergenhenegouwen, L. A. et al. Vaginal delivery versus caesarean section in preterm breech delivery: a systematic review. Eur J Obstet Gynecol Reprod Biol. 172, 1–6, https://doi.org/10.1016/j.ejogrb.2013.10.017 (2014).
Wegienka, G., Zoratti, E. & Johnson, C. C. The role of the early-life environment in the development of allergic disease. Immunol Allergy Clin North Am. 35, 1–17, https://doi.org/10.1016/j.iac.2014.09.002 (2015).
Thornley, S., Stewart, A., Marshall, R. & Jackson, R. Per capita sugar consumption is associated with severe childhood asthma: an ecological study of 53 countries. Prim Care Respir J. 20, 75–8, https://doi.org/10.4104/pcrj.2010.00087 (2011).
Maslova, E., Strøm, M., Olsen, S. F. & Halldorsson, T. I. Consumption of artificially sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoSOne. 8, e57261, https://doi.org/10.1371/journal.pone.0057261 (2013).
Herr, M. et al. Influence of host and environmental factors on wheezing severity in infants: findings from the PARIS birth cohort. Clin Exp Allergy. 42, 275–83, https://doi.org/10.1111/j.1365-2222.2011.03933.x (2012).
Theme-Filha, M. M. et al. Factors associated with unintended pregnancy in Brazil: cross-sectional results from the Birth in Brazil National Survey, 2011/2012. Reprod Health. 13(Suppl 3), 118, https://doi.org/10.1186/s12978-016-0227-8 (2016).
Joubert, B. R. et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet. 98, 680–96, https://doi.org/10.1016/j.ajhg.2016.02.019 (2016).
Veloso, H. J. F. & da Silva, A. A. M. Prevalence and factors associated with abdominal obesity and excess weight among adults from Maranhão, Brazil. Rev Bras Epidemiol. 13, 400–12 (2010).
da Silva, A. A. et al. A protocol to identify non-classical risk factors for preterm births: the Brazilian Ribeirão Preto and São Luís prenatal cohort (BRISA). Reprod Health 11, 79, https://doi.org/10.1186/1742-4755-11-79 (2014).
Moeller, A. et al. Monitoring Asthma in Children. Monitoring asthma in childhood: lung function, bronchial responsiveness and inflammation. Eur Respir Rev. 24, 204–15, https://doi.org/10.1183/16000617.00003914 (2015).
Atlas of Human Development, Brazil http://www.atlasbrasil.org.br/2013/perfil/sao-luis_ma (2013).
Associação Brasileira de Empresas de Pesquisas. Critério de Classificação Econômica Brasil. 2010. São Paulo: ABEP; 2010. Available: file:///C:/Users/Dell/Downloads/03_cceb_2012_base_lse_2010.pdf. http://www.abep.org/criterio-brasil (2015).
Lohman, T. G., Roche, A. & Martorell, R. Anthropometric standardization reference manual. (Champaign: Human Kinetics Books, 1988).
Block, G. et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124, 453–69 (1986).
World Health Organization. Physical status: the use and interpretation of anthropometry. Genebra: WHO (Technical Report Series n. 854) (1995).
Verburg, B. O. et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol 31, 388–96, https://doi.org/10.1002/uog.5225 (2008).
Spong, C. Y. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 309, 2445–6, https://doi.org/10.1001/jama.2013.6235 (2013).
WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age http://www.who.int/childgrowth/standards/en (2017).
Muthén, L. K. & Muthén, B. O. Mplus: statistical analysis with latent variables. User’s guide. (1998–2010). 6.ed. (Los Angeles: Muthén&Muthén, 2010).
Lodge, C. J. et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 104, 38–53, https://doi.org/10.1111/apa.13132 (2015).
Brisbois, T. D., Farmer, A. P. & McCargar, L. J. Early markers of adult obesity: a review. Obes Rev. 13, 347–67, https://doi.org/10.1111/j.1467-789X.2011.00965.x (2012).
Smith, L. K. et al. Associations between late and moderately preterm birth and smoking, alcohol, drug use and diet: a population-based case-cohort study. Arch Dis Child Fetal Neonatal Ed. 100, F486–91, https://doi.org/10.1136/archdischild-2014-307265 (2015).
Schoenaker, D. A., Soedamah-Muthu, S. S. & Mishra, G. D. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med. 12, 157, https://doi.org/10.1186/s12916-014-0157-7 (2014).
Cheungpasitporn, W. et al. Sugar and artificially sweetened soda consumption linked to hypertension: a systematic review and meta-analysis. Clin Exp Hypertens. 37, 587–93, https://doi.org/10.3109/10641963.2015.1026044 (2015).
Langsetmo, L. et al. Dietary patterns in men and women are simultaneously determinants of altered glucose metabolism and bone metabolism. Nutr Res. 36, 328–36, https://doi.org/10.1016/j.nutres.2015.12.010 (2016).
Vo, P., Bair-Merritt, M., Camargo, C. A. Jr., Eisenberg, S. & Long, W. Individual Factors, Neighborhood Social Context and Asthma at Age 5 Years. J Asthma. 54, 265–272, https://doi.org/10.1080/02770903.2016.1216563 (2016).
Ribeiro, M. R. C. et al. Effects of Socioeconomic Status and Social Support on Violence against Pregnant Women: A Structural Equation Modeling Analysis. PLoS One. 12(1), e0170469, https://doi.org/10.1371/journal.pone.0170469 (2017).
Acknowledgements
Maranhão State Research and Scientific and Technological Development Foundation (FAPEMA), São Paulo State Research Foundation (FAPESP), and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: J.X.P.T.N., C.C.C.R., R.F.L.B., M.T.S.S.B.A., V.M.F.S., L.L.P., V.C.C., E.O.V., H.B., M.A.B., A.A.M.S. Analyzed and interpreted the data: J.X.P.T.N., C.C.C.R., L.L.P., E.O.V., H.B., M.A.B., A.A.M.S. Drafted the manuscript: J.X.P.T.N., C.C.C.R., L.L.P., E.O.V., H.B., M.A.B., A.A.M.S. Critical reading of the manuscript and final approval of the version to be published: J.X.P.T.N., C.C.C.R., R.F.L.B., M.T.S.S.B.A., V.M.F.S., L.L.P., V.C.C., E.O.V., H.B., M.A.B., A.A.M.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nascimento, J.X.P.T., Ribeiro, C.C.C., Batista, R.F.L. et al. The First 1000 Days of Life Factors Associated with “Childhood Asthma Symptoms”: Brisa Cohort, Brazil. Sci Rep 7, 16028 (2017). https://doi.org/10.1038/s41598-017-16295-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16295-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.