Abstract
Biodegradable polymer biolimus-eluting stents (BP-BES) are third-generation drug-eluting stents (DES) composed of biodegradable polymers that may improve prognosis after percutaneous coronary intervention (PCI). After five years of follow-up, BP-BES showed conflicting results compared to durable polymer drug-eluting stents (DP-DES). We performed a meta-analysis of the outcomes of studies on BP-BES and DP-DES after percutaneous coronary intervention (PCI) at five years of follow-up. Eligible studies were retrieved from PubMed, Embase and the Cochrane Library and reported the results of all-cause mortality, myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR) and stent thrombosis (ST) at five years of follow-up. Five studies of a total of 4687 patients were included in the meta-analysis. At five years of follow-up, BP-BES was associated with lower rates of major adverse cardiac events (MACE) (OR = 0.83, 95%CI = [0.71, 0.97]), TLR (OR = 0.77, 95%CI = [0.62, 0.96]) and ST (OR = 0.60, 95%CI = [0.43 to 0.84]), whereas no significant differences in mortality, MI, or TVR rates were detected. Our results demonstrated that at five years of follow-up, BP-BES can significantly reduce the risk of MACE, TLR and ST, which indicate that safety and efficacy were increased after PCI.
Similar content being viewed by others
Introduction
Application of drug-eluting stents (DES) has had a great impact on percutaneous coronary intervention (PCI). Compared with bare metal stents (BMS), a reduced risk of restenosis and target lesion revascularization were observed with DES in previous clinical trials1,2,3,4. Because of their efficacy in limiting neointimal hyperplasia, DES were treated as a standard therapy in PCI. Self-perpetuating inflammation and late stent thrombosis (ST) were associated with the durable polymer used in the first- and second-generation DES5,6,7,8,9,10,11. To overcome these adverse events, biodegradable polymer drug eluting stents (BP-DES) were developed.
Biolimus is a highly lipophilic sirolimus analogue that inhibits the proliferation of smooth muscle cells by binding to the FK-binding protein and subsequently inhibiting mammalian target of rapamycin (mTOR)12. Biolimus-eluting stents (BES) are third-generation DES and elute biolimus from a polylactic acid (PLA) biodegradable polymer applied to the stent’s abluminal surface13. BES include Nobori stents (Terumo, Tokyo, Japan) and Biomatrix stents (Biosensors Europe SA, Morges, Switzerland). After implantation, the biodegradable polymer gradually dissolves into water and carbon dioxide within nine months14,15, and alleviates self-perpetuating inflammation and late stent thrombosis. Therefore, biodegradable polymer biolimus-eluting stents (BP-BES) may play an important role in reducing the risk of persistent inflammation and ST.
Some studies have compared BP-BES and durable polymer drug-eluting stents (DP-DES) in terms of prognosis at five years of follow-up after PCI. However, the results were conflicting. BP-BES had an advantage in improved MACE as reported by Zhang et al.16. However, Chevalier et al.17 reported opposite results. The aim of this study was to perform a meta-analysis of the outcomes associated with BP-BES and DP-DES for the treatment of PCI at five years of follow-up.
Results
Characteristics of the Included Studies
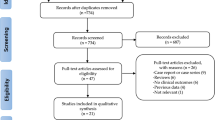
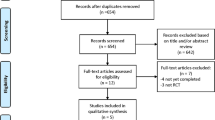
One-thousand-six hundred-fifty-two articles were obtained by online and manual searches. After removing duplicates and screening titles and abstracts, six independent trials that contained data for BP-BES versus DP-DES were included. The LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) trial had four and five years of follow-up, and we obtained the latest data13,18. Finally, five studies13,16,17,19,20 were selected that included 4687 patients (2002 randomized to BP-BES and 2685 to DP-DES). (Fig. 1) (as seen in the flow chart).
The characteristics of the trials and patients are shown in Table 1. One trial17 compared BP-BES with paclitaxel-eluting stents (PES, Taxus Liberté/Express, Boston Scientific, Natick, MA, USA), three trials13,16,19 compared BP-BES with sirolimus-eluting stents (SES, Cypher Select, Cordis Corporation, Bridgewater, New Jersey, USA), and the remaining trial20 compared BP-BES with everolimus-eluting stents (EES, XIENCE V, Abbott Vascular, Santa Clara, CA; PROMUS, Boston Scientific, Inc., Natick, MA) or zotarolimus-eluting stents (ZES, RESOLUTE Integrity, Medtronic Vascular, Minneapolis, MN).
All of the trials included five years of clinical follow-up data. The incidences of clinical outcomes at five years of follow-up are shown in Table 2. Dual antiplatelet therapy was administered to all patients for at least six or twelve months after discharge. Aspirin and clopidogrel were used in the majority of patients13,16,17,19; however, prasugrel or ticagrelor were also used20. Diabetes prevalence among the included trials ranged from 15.7–65.4%. The number of PCI patients with acute coronary syndrome ranged from 6.7%–100.0%.
The pooled ORs and 95%CIs of the comparisons of the main outcomes between DP-BES and BP-DES are shown in Table 3. Three outcomes were significantly different: MACE, TLR and ST; the results of death, MI and TVR were not significantly different.
Clinical Endpoints
As seen in Figure 2, a total of 886 patients (18.9%) had MACE. In the first-generation durable polymer drug-eluting stents (1st DP-DES) group, the use of BP-BES significantly reduced the risk of MACE compared to the- 1st DP-DES (20.3% versus 23.7%; OR [95% CI] = 0.80 [0.68, 0.95], P = 0.01). In the second-generation durable polymer drug-eluting stents (2nd DP-DES) group, there was no significant difference in the risk of MACE between BP-BES and 2nd DP-DES (12.5% versus 12.9%; OR [95% CI] = 0.96 [0.68, 1.37], P = 0.83). Finally, BP-BES significantly reduced the risk of MACE compared to DP-DES (18.8% versus 19.0%; OR [95% CI] = 0.83 [0.71, 0.97], P = 0.02; I2 = 0%, Pheterogeneity = 0.56). No heterogeneity was found across the included trials.
As seen in Figure 3, 366 patients (7.8%) underwent repeat revascularization of the target lesion. In the 1st DP-DES group, the use of BP-BES significantly reduced the risk of TLR compared to 1st DP-DES (9.1% versus 12.1%; OR [95% CI] = 0.73 [0.58, 0.92], P = 0.008). In the 2nd DP-DES group, there was no significant difference in the risk of TLR between BP-BES and 2nd DP-DES (2.7% versus 2.0%; OR [95% CI] = 1.34 [0.63, 2.83], P = 0.44). Finally, BP-BES was superior to DP-DES in reducing the risk of TLR (7.9% versus 7.7%; OR [95% CI] = 0.77 [0.62–0.96], P = 0.02; I2 = 45%, Pheterogeneity = 0.12). No heterogeneity was found across the included trials.
Definite/probable ST was observed in a total of 155 patients (3.3%), as presented in Fig. 4. In the 1st DP-DES group, the use of BP-BES significantly reduced the risk of ST compared to 1st DP-DES (2.9% versus 5.3%; OR [95% CI] = 0.57 [0.39, 0.82], P = 0.002). In the 2nd DP-DES group, there was no significant difference in the risk of ST between BP-BES and 2nd DP-DES (1.6% versus 1.9%; OR [95% CI] = 0.87 [0.35, 2.16], P = 0.76). Finally, BP-BES significantly reduced the risk of ST compared to DP-DES (2.6% versus 3.8%; OR [95% CI] = 0.60 [0.43–0.84], P = 0.003; I2 = 27%, Pheterogeneity = 0.24). No heterogeneity was found across the included trials.
As shown in Fig. 5, a total of 388 patients (8.3%) died. BP-BES showed no superiority in reducing the risk of death compared to 1st DP-DES (9.7% versus 10.6%; OR [95% CI] = 0.92 [0.73, 1.16], P = 0.49) or the 2nd DP-DES (3.5% versus 4.8%; OR [95% CI] = 0.72 [0.39, 1.33], P = 0.29). There was no significant difference in the risk of death between BP-BES and DP-DES (8.5% versus 8.1%; OR [95% CI] = 0.89 [0.72, 1.11], P = 0.30; I2 = 0%, Pheterogeneity = 0.70).
As shown in Fig. 6, a total of 348 patients (7.2%) had an MI. BP-BES showed no superiority in reducing the risk of MI compared to 1st DP-DES (7.8% versus 9.2%; OR [95% CI] = 0.85 [0.66, 1.09], P = 0.19) or 2nd DP-DES (5.4% versus 5.3%; OR [95% CI] = 1.03 [0.61, 1.73], P = 0.91). Finally, there was no significant difference in the risk of death between BP-BES or DP-DES (7.3% versus 7.5%; OR [95% CI] = 0.88 [0.70, 1.10], P = 0.27; I2 = 0%, Pheterogeneity = 0.70).
As shown in Fig. 7, a total of 461 patients (9.8%) required TVR. BP-BES showed no superiority in reducing the risk of MI compared to 1st DP-DES (11.0% versus 13.3%; OR [95% CI] = 0.84 [0.67, 1.04], P = 0.11) or 2nd DP-DES (6.0% versus 4.9%; OR [95% CI] = 1.22 [0.74, 2.03], P = 0.43). Finally, there was no significant difference in the risk of death between BP-BES and DP-DES (10.0% versus 9.6%; OR [95% CI] = 0.89 [0.73, 1.08], P = 0.23; I2 = 0%, Pheterogeneity = 0.68).
Sensitivity analysis
A sensitivity analysis was performed by sequentially omitting 1 individual at a time to reflect the influence of each study on the overall meta-analysis. No heterogeneity was observed in the polymorphism (Fig. 8); thus, the results of our meta-analysis were stable.
Discussion
Comparisons between BP-BES and DP-DES in terms of prognosis after PCI have attracted a great deal of attention. Many studies have been performed at different time points based on six outcomes (MACE, death, MI, TVR, TLR, ST), especially at one year21,22,23,24 or three years25 of follow-up. However, no significant evidence from one or three years of follow-up have supported the superiority of BP-BES or DP-DES. Our meta-analysis first compared the outcomes at five years of follow-up between BP-BES and DP-DES in terms of prognosis after PCI. The most inspiring finding of our meta-analysis was that BP-BES can significantly reduce the risk of MACE, TLR and ST without benefits on death, MI, or TVR.
MACE, as a composite endpoint of death; MI; and coronary revascularization were not unified in our study or in previous studies. In our study, at five years of follow-up, BP-BES demonstrated superiority in reducing the risk of MACE compared to 1st DP-DES (OR [95% CI] = 0.80 [0.68, 0.95], P = 0.01) and were not inferior to 2nd DP-DES (OR [95% CI] = 0.96 [0.68, 1.37], P = 0.83). Ultimately, BP-BES had a lower risk of MACE compared to DP-DES (OR 0.83, 95% CI = 0.71, 0.97), whereas the results of one and three years of follow-up demonstrated that there was no difference between BP-BES and DP-DES, as reported by Zhang et al. and Sakurai et al.
The difference between outcomes may attributed to the different time point of follow-up. The potential clinical advantage of the BP-BES might be expected to emerge once the biodegradable polymer has dissolved, and this may have occurred 9 months after implantation14,15. Thus, it was not surprising that BP-BES did not reduce the risk of MACE at one or even three years of follow-up. Moreover, only three trials were included at three years of follow-up; more trials with larger populations of patients are needed to support the conclusion. Five trials were included in our study; four13,16,19,20 of them demonstrated that BP-BES were associated with a lower risk of MACE than DP-DES. However, of these four trials, the results of three of the trials were not statistically significant (Serruys et al. OR [95% CI] = 0.81 [0.65, 1.02]; Jaguszewski et al. OR [95% CI] = 0.96 [0.68, 1.37]; Grundeken et al. OR [95% CI] = 0.85 [0.50, 1.46]). Finally, the results showed that BP-BES were superior in reducing the risk of MACE compared to DP-DES, and no heterogeneity was found across the included trials (I2 = 0%, Pheterogeneity = 0.56).
A lower risk of ST at a very long time (>one year, as defined by the Academic Research Consortium26) was observed in the BP-BES group21,27, which agreed with our study results. At five years of follow-up, BP-BES demonstrated superiority in reducing the risk of ST compared to 1st DP-DES (OR [95% CI] = 0.57 [0.39, 0.82], P = 0.002) and were not inferior to 2nd DP-DES (OR [95% CI] = 0.87 [0.35, 2.16], P = 0.76). Ultimately, BP-BES significantly reduced the risk of definite/probable ST (2.6% versus 3.8%; OR [95% CI] = 0.60 [0.43–0.84], P = 0.003). The results from each trial that was included in our study were not statistically significant (Serruys et al. OR [95% CI] = 0.69 [0.43, 1.10]13, Zhang et al. OR [95% CI] = 0.61 [0.31, 1.18]16, Chevalier et al. OR [95% CI] = 0.06 [0.00, 1.06]17, Grundeken et al. OR [95% CI] = 0.13 [0.02, 1.06]19, Jaguszewski et al. OR [95% CI] = 0.87 [0.35, 2.16]20), but all of them showed that BP-BES were associated with a lower risk of ST than DP-DES, so we archived an inspiring result. Incomplete endothelialization and the inflammatory response caused by the persistence of a durable polymer play important roles in very late ST6,10. Drug-eluting stents gradually release drugs from polymer coatings that are applied to the stent surface, which prolongs the time of completely endothelialization. Three to four months are required for complete endothelialization with BMS28,29, whereas with DES, more time is required6. At the same time, the level of endothelial coverage in BP- BES was comparable to that of BMS at four weeks, with no significant increase in inflammatory reactions up to 15 months30. Moreover, compared with DP-DES, BP-BES contains a biodegradable polymer that gradually dissolves into water and carbon dioxide, which are associated with a lower risk of inflammatory responses in animal studies31. Thus, these observations may explain why the BP-BES may be associated with a lower risk of very late ST and a better long-term outcome in our meta-analysis.
In our study, BP-BES demonstrated superiority in reducing the risk of TLR compared to 1st DP-DES (OR [95% CI] = 0.73 [0.58, 0.92], P = 0.008) and were not inferior to 2nd DP-DES (OR [95% CI] = 1.34 [0.63, 2.83], P = 0.44). Ultimately, BP-BES had a lower risk of TLR than DP-DES (OR [95% CI] = 0.77 [0.62–0.96]). However, a previous meta-analyses showed no difference between BP-BES and DP-DES at one and three years of follow-up22,25,27,32. The major reasons for this difference were as follows: first, the data in our included studies was collected at five years, which was longer than the above studies. Second, PES represented a weak competitor in comparison with SES and EES33,34,35. The number of PES in our meta-analysis was 2.7% (125/4687), which is higher than in the above studies (1.4% [125/9114]22, 1.0% [125/12090]32, and 0% [0/8436]25), which may contribute to the better result.
Based on our study we found no differences in mortality, MI, or TVR between BP-BES and DP-DES, but the insufficient sample size may contribute to this discrepancy. However, based on the current studies, we found that BP-BES could reduce the risk of MACE, ST and TLR compared to DP-DES. These results had statistical significance and no heterogeneity was found. Many elements may have resulted in this discrepancy, including the lack of an adequate sample size, differences between stents, experience of operators, presence of complications after PCI, seriousness of lesion, and so on.
BP-BES also had some disadvantages. In this study, after implantation of the BP-BES, 18.8% of patients presented with MACE, 7.9% of patients underwent TLR and 2.6% of patients had ST, which compromised the prognosis after PCI. Furthermore, after implantation, the biodegradable polymer gradually dissolved into water and carbon dioxide, alleviating self-perpetuating inflammation and late stent thrombosis, which in turn necessitates prolonged dual antiplatelet therapy that increases the risk of long-term bleeding events after PCI36.
There were several limitations in this meta-analysis. First, only English language articles were included in our study, which may bias the results. Second, patient heterogeneity and confounding factors might have affected the analysis. Third, significant heterogeneity was detected in some pooled analyses, which may have affected the meta-analysis results, even though we adopted the random effects model or introduced sensitivity analysis. Fourth, the number of included studies was relatively small, and the results should be interpreted with caution; further studies are needed to confirm these results.
In conclusion, BP-BES can significantly reduce the risk of MACE, TLR and ST compared with DP-DES at five years of follow-up, which indicates that BP-BES are associated with a better safety and efficiency after PCI in the long term.
Materials and Methods
Identification of Studies
PubMed, the Cochrane Library and Embase databases were thoroughly searched in September 2016 by the first two investigators to identify potential studies of BP-BES and DP-DES. The terms “Biolimus”, “Nobori”, “Biomatrix”, “BioFreedom”, and “stent” were used. Missing data (the data that we failed to identify during the electronic search) were obtained by reviewing the citations of review articles and all eligible studies.
Inclusion and Exclusion Ceriteria
Citations were screened at the title and abstract level and retrieved as full reports. The inclusion criteria were: (1) comparison of BP-BES vs DP-DES; (2) studies reporting at least one of the following outcomes: MACE, all/cardiac death, MI, TVR, TLR, ST; and (3) clinical follow-up at five years. When more than one report of the same study was retrieved, the one with the longest follow-up was included. The exclusion criteria: (1) a duplication of previous publications; (2) a comment, review or editorial; and (3) a study without data. The studies were independently selected by two investigators, according to the inclusion and exclusion criteria by screening the title, abstract and full-text. Any dispute was resolved by discussion.
Data Extraction
From each study, the following data were independently extracted by the first two investigators using a standardized form: first author’s last name, year of publication, journal, BP-BES, DP-DES, sample size, age, gender, patients with diabetes, patients with ACS, left ventricular ejection fraction (LVEF), multi-vessel disease, SYNTAS scores, numbers of stents used per patient, total stent length, and mm per patient. For data from multiple treatment groups, the approach recommended in the Cochrane handbook was adopted to avoid a unit- of –error analysis that may result from entering several comparisons into one meta-analysis, which could lead to “double-counts” of patients based on the same study. Disagreements were resolved through discussion.
Statisticals analysis
RevMan version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used for statistical analyses. We calculated the odds risk (OR) and its 95%CI (confidence interval) for the five outcomes as binary data. Heterogeneity was evaluated by the magnitude of the Chi2, corresponding P value and I2 statistic. When the I2 value was above 50%, a random effects model based on the Mantel-Haenszel (MH) or inverse variance (IV) statistical approach was selected to combine the data. If the I2 value was below 50%, a fixed effects model based on the MH or IV statistical approach was selected, and a sensitivity analysis was conducted to detect the robustness of the result.
References
Moses, J. W. et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. The New England journal of medicine 349, 1315–1323, https://doi.org/10.1056/NEJMoa035071 (2003).
Stone, G. W. et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. The New England journal of medicine 350, 221–231, https://doi.org/10.1056/NEJMoa032441 (2004).
Stone, G. W. et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama 294, 1215–1223, https://doi.org/10.1001/jama.294.10.1215 (2005).
Kirtane, A. J. et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119, 3198–3206, https://doi.org/10.1161/circulationaha.108.826479 (2009).
Virmani, R. et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109, 701–705, https://doi.org/10.1161/01.cir.0000116202.41966.d4 (2004).
Joner, M. et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. Journal of the American College of Cardiology 48, 193–202, https://doi.org/10.1016/j.jacc.2006.03.042 (2006).
Pfisterer, M. et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. Journal of the American College of Cardiology 48, 2584–2591, https://doi.org/10.1016/j.jacc.2006.10.026 (2006).
Luscher, T. F. et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 115, 1051–1058, https://doi.org/10.1161/circulationaha.106.675934 (2007).
Nakazawa, G. et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118, 1138–1145, https://doi.org/10.1161/circulationaha.107.762047 (2008).
Cook, S. et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120, 391–399, https://doi.org/10.1161/circulationaha.109.854398 (2009).
Park, K. W. et al. Does “late catch-up” exist in drug-eluting stents: insights from a serial quantitative coronary angiography analysis of sirolimus versus paclitaxel-eluting stents. American heart journal 159, 446–453.e443, https://doi.org/10.1016/j.ahj.2010.01.001 (2010).
Huang, S., Bjornsti, M. A. & Houghton, P. J. Rapamycins: mechanism of action and cellular resistance. Cancer biology & therapy 2, 222–232 (2003).
Serruys, P. W. et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC. Cardiovascular interventions 6, 777–789, https://doi.org/10.1016/j.jcin.2013.04.011 (2013).
Ostojic, M. et al. First clinical comparison of Nobori -Biolimus A9 eluting stents with Cypher- Sirolimus eluting stents: Nobori Core nine months angiographic and one year clinical outcomes. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 3, 574–579 (2008).
Ostojic, M. et al. The pharmacokinetics of Biolimus A9 after elution from the Nobori stent in patients with coronary artery disease: the NOBORI PK study. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 72, 901–908, https://doi.org/10.1002/ccd.21775 (2008).
Zhang, Y. J. et al. Biolimus-eluting stent with biodegradable polymer improves clinical outcomes in patients with acute myocardial infarction. Heart (British Cardiac Society) 101, 271–278, https://doi.org/10.1136/heartjnl-2014-306359 (2015).
Chevalier, B. et al. Five-year clinical outcome of the Nobori drug-eluting coronary stent system in the treatment of patients with coronary artery disease: Final results of the NOBORI 1 trial. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 11, 549–554 (2015).
Ischinger, T. A. et al. Long-term clinical results from the all-comers LEADERS trial: 4 year follow-up data. Journal of the American College of Cardiology 58, B216 (2011).
Grundeken, M. J. et al. First generation versus second generation drug-eluting stents for the treatment of bifurcations: 5-year follow-up of the LEADERS all-comers randomized trial. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 87, E248–260, https://doi.org/10.1002/ccd.26344 (2016).
Jaguszewski, M. et al. Safety and efficacy profile of bioresorbable-polylactide-polymer-biolimus-A9-eluting stents versus durable-polymer-everolimus- and zotarolimus-eluting stents in patients with acute coronary syndrome. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions, https://doi.org/10.1002/ccd.26617 (2016).
Ye, Y. et al. Efficacy and safety of biodegradable polymer biolimus-eluting stents versus durable polymer drug-eluting stents: a meta-analysis. PloS one 8, e78667, https://doi.org/10.1371/journal.pone.0078667 (2013).
Cassese, S. et al. Clinical outcomes of patients treated with Nobori biolimus-eluting stent: Meta-analysis of randomized trials. International Journal of Cardiology 175, 484–491, https://doi.org/10.1016/j.ijcard.2014.06.014 (2014).
Danzi, G. B., Piccolo, R., Galasso, G. & Piscione, F. Nobori Biolimus-Eluting Stent vs. Permanent Polymer Drug-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention. Circulation Journal 78, 1858–1866, https://doi.org/10.1253/circj.CJ-13-1558 (2014).
Zhang, Y. J. et al. NOBORI biodegradable-polymer biolimus-eluting stent versus durable-polymer drug-eluting stents: a meta-analysis. International journal of cardiology 174, 151–153, https://doi.org/10.1016/j.ijcard.2014.03.167 (2014).
Sakurai, R., Burazor, I., Bonneau, H. N. & Kaneda, H. Long-term outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer everolimus-eluting stents: A meta-analysis of randomized controlled trials. International journal of cardiology, doi:https://doi.org/10.1016/j.ijcard.2016.07.078 (2016).
Cutlip, D. E. et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115, 2344–2351, https://doi.org/10.1161/CIRCULATIONAHA.106.685313 (2007).
Ye, Y. et al. Efficacy and safety of biodegradable polymer biolimus-eluting stents versus durable polymer drug-eluting stents: A meta-analysis. PloS one 8 (2013).
Farb, A., Burke, A. P., Kolodgie, F. D. & Virmani, R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 108, 1701–1706, https://doi.org/10.1161/01.cir.0000091115.05480.b0 (2003).
Grewe, P. H., Deneke, T., Machraoui, A., Barmeyer, J. & Muller, K. M. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. Journal of the American College of Cardiology 35, 157–163 (2000).
Hagiwara, H. et al. Vascular responses to a biodegradable polymer (polylactic acid) based biolimus A9-eluting stent in porcine models. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 8, 743–751, https://doi.org/10.4244/eijv8i6a114 (2012).
Pendyala, L. K. et al. Nobori stent shows less vascular inflammation and early recovery of endothelial function compared with Cypher stent. JACC. Cardiovascular interventions 5, 436–444, https://doi.org/10.1016/j.jcin.2011.11.013 (2012).
Danzi, G. B., Piccolo, R., Galasso, G. & Piscione, F. Nobori biolimus-eluting stent vs. permanent polymer drug-eluting stents in patients undergoing percutaneous coronary intervention. Circulation journal: official journal of the Japanese Circulation Society 78, 1858–1866 (2014).
Zhang, X., Xie, J., Li, G., Chen, Q. & Xu, B. Head-to-head comparison of sirolimus-eluting stents versus paclitaxel-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of 76 studies. PloS one 9, e97934, https://doi.org/10.1371/journal.pone.0097934 (2014).
Meng, M. et al. Long-term clinical outcomes of everolimus-eluting stent versus paclitaxel-eluting stent in patients undergoing percutaneous coronary interventions: a meta-analysis. BMC cardiovascular disorders 16, 34, https://doi.org/10.1186/s12872-016-0206-6 (2016).
Wang, H. B., Zeng, P., Yang, J., Yang, J. & Liu, X. W. Paclitaxel-eluting stents versus sirolimus-eluting stents in patients with diabetes mellitus undergoing percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. Internal and emergency medicine 11, 1005–1013, https://doi.org/10.1007/s11739-016-1529-0 (2016).
Piccolo, R., Nicolino, A. & Danzi, G. B. The Nobori biolimus-eluting stent: update of available evidence. Expert review of medical devices 11, 275–282, https://doi.org/10.1586/17434440.2014.894458 (2014).
Acknowledgements
The work is supported by grants from the National Natural Science Foundation of China (Nos 81170205).
Author information
Authors and Affiliations
Contributions
Pan Lu, Shuai Lu and Yuanyuan Li wrote the main manuscript text. Mengmeng Deng prepared Tables 1–3. Figures 1–8 were prepared by Shuai Lu, Pan Lu and Yuanyuan Li. All of the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, P., Lu, S., Li, Y. et al. A comparison of the main outcomes from BP-BES and DP-DES at five years of follow-up: A systematic review and meta-analysis. Sci Rep 7, 14997 (2017). https://doi.org/10.1038/s41598-017-14247-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14247-6
This article is cited by
-
Five-year clinical efficacy and safety of contemporary thin-strut biodegradable polymer versus durable polymer drug-eluting stents: a systematic review and meta-analysis of 9 randomized controlled trials
Cardiovascular Intervention and Therapeutics (2020)
-
Five-Year Outcomes of Biodegradable Polymer Drug-Eluting Stents Versus Second-Generation Durable Polymer Drug-Eluting Stents: a Meta-Analysis of Randomized Controlled Trials
Cardiovascular Drugs and Therapy (2019)
-
High Bleeding Risk Patients Treated with Very Thin-Strut Biodegradable Polymer or Thin-Strut Durable Polymer Drug-Eluting Stents in the BIO-RESORT Trial
Cardiovascular Drugs and Therapy (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.