Abstract
The mortality prediction models for the general diabetic population have been well established, but the corresponding elderly-specific model is still lacking. This study aims to develop a mortality prediction model for the elderly with diabetes. The data used for model establishment were derived from the nationwide adult health screening program in Taiwan in 2007–2010, from which we applied a 10-fold cross-validation method for model construction and internal validation. The external validation was tested on the MJ health screening database collected in 2004–2007. Multivariable Cox proportional hazards models were used to predict five-year mortality for diabetic patients ≥65 years. A total of 220,832 older subjects with diabetes were selected for model construction, of whom 23,241 (10.5%) died by the end of follow-up (December 31, 2011). The significant predictors retained in the final model included age, gender, smoking status, body mass index (BMI), fasting glucose, systolic and diastolic blood pressure, leukocyte count, liver and renal function, total cholesterol, hemoglobin, albumin, and uric acid. The Harrell’s C in the development, internal-, and external-validation datasets were 0.737, 0.746, and 0.685, respectively. We established an easy-to-use point-based model that could accurately predict five-year mortality risk in older adults with diabetes.
Similar content being viewed by others
Introduction
Population aging is the most important mainspring of the escalating growth of diabetes prevalence throughout the world1. According to the report from the US Centers for Disease Control and Prevention, the prevalence of diabetes in older Americans (≥65 years) was 25.9% of in 20122, which was much higher than those at age 20–44 (4.1%) and 45–64 (16.2%). Similarly, aging has had a significant impact on the rise of diabetes in Asian populations3. In addition to being susceptible to micro- and macro-vascular complications, older adults with diabetes are prone to develop premature death or hypoglycemia4,5. The Emerging Risk Factors Collaboration disclosed that the elderly people with diabetes were associated with substantial premature mortality from vascular, cancers, renal, liver, digestive diseases and infection which incurred about 4–5 years of life loss6. However, this vulnerable group receives less attention by empirical studies. In renowned guidelines, most healthcare standards proposed for older adults with diabetes were based on expert consensus7. Furthermore, some recommended goals for blood pressure or glycemic control derived from large-scale randomized control trials8,9 may be inappropriate to apply to older patients, who often suffer from physically dysfunction or multiple comorbidities. To improve diabetes care for the elderly, we are in urgent need of developing an evidence-based clinical guidance for risk stratification and therapeutic purpose. In recent years, some prediction models have been separately established to estimate the mortality risk for older people10,11,12 and diabetic patients13,14,15. Moreover, a one-year mortality prediction model was constructed for diabetic patients with dialysis commencement16. To our knowledge, there is still lack of a prediction model that can identify high-risk groups and can prevent premature mortality for an older population with diabetes. Therefore, we incorporated factors that may influence health outcomes for these patients, including glucose and blood pressure control, metabolic syndrome, renal and liver function, and nutritional and inflammatory status, to form a thorough set of predictors and then developed a reliable and validated risk score system to predict five-year mortality of diabetic patients age 65 or older.
Results
Table 1 shows the demographic characteristics as well as the clinical and biochemical measures of the older subjects with diabetes in the development, internal-, and external-validation datasets. There were no significant differences between subjects in the internal-validation and development datasets. Compared to the subjects in the development dataset, those in the external-validation dataset were younger, more likely to be male, more likely to smoke, and had higher levels of the biochemical measures such as fasting blood glucose, systolic blood pressure (SBP), total cholesterol (TCHOL), albumin (Alb), hemoglobin (Hb), estimated glomerular filtration rate (eGFR), glutamic pyruvic transaminase (GPT), and uric acid (UA).
As shown in Table 2, the overall mortality rate of the subjects in the development and internal validation datasets were similar (3.38–3.48 deaths per 100 person-years), but higher than that in the external validation dataset (1.38 per 100 person-years). The lower mortality rates in the external validation dataset were even more obvious in males, and those older than 75.
The estimated relative risks of mortality are shown in Table 3. The hazard ratio (HR) is 1.07 for every one-year increment in age. Males and ever smokers were more likely to die (HR = 1.70 and 1.13, respectively). Some measured biomarkers demonstrated a U-shaped effect on risk of death. For example, HR was greater than 1 for fasting blood glucose of being either <70 mg/dL or ≥140 mg/dL. Similar harmful effects could be seen at both extreme levels of TCHOL (<150 or ≥240 mg/dL), eGFR (<60 or ≥100 mL/min/1.73 m2), and UA (<3.5 or ≥7 mg/dL). The mortality risk would be increased for those with body mass index (BMI) < 24 kg/m2, SBP < 110 mmHg, diastolic blood pressure (DBP) ≥80 mmHg, Hb < 14 g/dL, Alb < 4 g/dL, white blood cell count (WBC) ≥ 8,200/µL, or GPT ≥ 40 U/L. If an older adult who has diabetes as well as albumin < 3.5 g/dL, the HR would be increased by 3.64 fold.
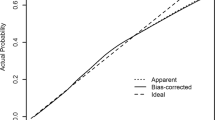
The risk-score point system is also listed in Table 3. One risk point was added to every five-year increment in age. Two risk points were added to male subjects. Similarly, two risk points were added to those who had fasting blood glucose ≥200 mg/dL, Alb = 3.5–3.9 g/dL, BMI = 18.5–21.9, eGFR < 30 mL/min/1.73 m2, WBC ≥ 10,000/µL, or GPT ≥ 120 U/L. Three additional risk points were assigned to those with BMI < 18.5 kg/m2 or Hb < 12 g/dL. As shown in Fig. 1, the 5-year estimated mortality rate for those with point totals equal to 5, 10, and 14 were 0.130, 0.552, and 0.962, respectively. The Harrell’s C of the development and of the internal- and external-validation datasets were 0.737, 0.746, and 0.685, respectively.
Discussion
We established a risk-point system to predict five-year mortality for older adults with diabetes. Physicians and public health workers can apply this handy tool to more accurately identify high-risk groups for individualized counselling or treatment. All 14 parameters used in this risk-point system are essential clinical indicators to monitor older adults with diabetes. In many clinical settings, these data are also collected in annual health check-ups for the elderly. To overcome the capricious nature of diabetes prognosis in older population, this easy-to-use risk-prediction algorithm can serve as a desk reference in daily practice.
Several risk engines have been developed for diabetes patients, targeted at predicting specific disease outcomes such as cardiovascular diseases17,18,19,20, stroke21, and end-stage renal disease22. However, few studies have been conducted to assess overall mortality risk for diabetes patients. Wells et al. used a type 2 diabetes cohort in the Cleveland Clinic to develop a six-year mortality risk-prediction tool with a C-statistic of 0.75223. The uniqueness of this model was the incorporation of anti-diabetes drugs in its development; however, there were two drawbacks in the Well’s risk model. First, the cohort used in the Well’s study was limited only to those “who were prescribed a single one of the four most common types of oral hypoglycemic agents”. By excluding those who used insulin, the Well’s model may not be generalizable to those who had long diabetic duration or severer conditions. Second, there was lack of external validation for their proposed model. Another risk engine, developed by the Hong Kong Diabetes Registry, was to assess five-year mortality risk for Chinese patients with diabetes13. The strength of this model was its good discriminatory ability in prediction of five-year mortality risk (C-statistic = 0.845 in the all-cause mortality model). However, the mean age of their study subjects was 57–58 years, indicating that this model may be less accurate in applying to older patients. Moreover, some important indicators of diabetes care, including control of blood pressure and glycemic status, were not included in their model, and the generalizability of this model has not been externally validated yet. In order to supplement the weaknesses of the aforementioned studies, the current study, using common clinical biomarkers to predict mortality risk for older adults with diabetes, surpassed the general geriatric mortality models10,11,12 which seldom had sufficient biomedical profiles applied in their developed models.
Prevention of hypoglycemia is key to optimizing diabetes management for the elderly7. Our risk estimate for those with fasting glycemic level <70 mg/dL also concurred with this point. In this study, fasting glucose ≥140 mg/dL was considered as a risk of mortality. Although we adjusted the risk score up to 2 for those with fasting glucose ≥200 mg/dL, due to data availability, our proposed risk point system was not fine-tuned according to the elders’ physical function and health status as recommended in the ADA guidelines for older adults7.
For cardiovascular risk prevention, all evidence supports treatment for the elderly with hypertension24. Our study showed that uncontrolled high blood pressure (indicated by DBP ≥ 80 mmHg) is a mortality risk, which is lower than the target (90 mmHg) set by the ADA guideline7 but supported by the HOT study25. Our study also cautioned about potential harm caused by hypotension (indicated by SBP < 110 mmHg), which was usually not precisely emphasized in most diabetes care guidelines but in accord with some RCT and cohort studies. The ACCORD study9 did not show protective effects except for stroke prevention for those reducing their SBP < 120 mmHg and a British cohort study26 actually demonstrated those with blood pressure < 110/75 mmHg were associated with poor health outcome.
High leukocyte count, long recognized as an indicator of chronic inflammation, has been shown associated with cardiovascular disease27,28, cancer, and mortality29. We also found high WBC count (≥8,200/μL) was a mortality risk for the elderly. Furthermore, severe chronic inflammation (WBC count ≥ 10,000/μL, risk score = 2), anemia (hemoglobin < 12 g/dL, risk score = 3) and malnutrition (albumin < 3.5 g/dL, risk score = 4; BMI < 18.5 kg/m2, risk score = 3), which are often involved in the intricate mechanisms of sarcopenia and frailty30, would incur the highest mortality risk for the investigated cohort. In addition to the expected effect of age, our results showed that nutrition status is sensitive and crucial to survivorship in the elderly, whose mortality would be increased by even only a mild decline of hemoglobin (<14 g/dL) and serum albumin level (<4 g/dL). The risk of BMI < 23 kg/m2 in the elderly has also been shown in many studies31. However, different from some previous research, obesity was not considered as a risk score in the current study due to the fact that our study subjects with BMI > 35 kg/m2 were only 1.14%.
If GPT = 40 U/L is considered as the upper limit of normal (ULN)32, we found those with elevated GPT but with ULN of less than three would have an aggravated mortality risk (by increasing 1 risk score), whereas the corresponding risk would be doubled for those with GPT > 3 ULN (by increasing 2 risk scores). Elevated GPT, involved in liver dysfunction, was also shown as a dose-response mortality risk in an American community study33. Liver enzyme activity should be regularly monitored for the diabetic elderly in Southeast Asia, a region with a high prevalence of viral hepatitis and hepatocellular carcinoma34. Some studies have indicated that extremely low GPT — possibly associated with frailty, malnutrition, or hepatic aging process — may also be a mortality risk for the elderly35. However, perhaps due to an insufficient sample size for this group (our study subjects with GPT < 5 U/L were 0.05%), this inverse association was not identified in this study.
Our results revealed a U-shaped relationship between mortality and some biochemical indicators — including total cholesterol, uric acid, and eGFR — in the elderly with diabetes. Similar to other studies36,37,38, low cholesterol level (<150 mg/dL), probably related to chronic disease or malnutrition, was identified as a risk factor for all-cause mortality in our study. Moreover, we also recommend that older adults should keep TCHOL below 240 mg/dL to prevent premature mortality. In accordance with other studies39,40, a moderate uric acid level was also suggested in this study to balance its dynamic function of antioxidant properties and endothelial integrity for the elderly. Chronic hyperuricemia would stimulate the renin-angiotensin system and inhibit release of endothelial nitric oxide, contributing to vasoconstriction and atherosclerosis, then possibly increase blood pressure and cause renal and cardiovascular disease for the elderly41,42. Poor protein intake, hypo-osmolality, underlying diseases and medication may have contributed to the development of lower uric acid level43. In line with the traditional guideline44 in which eGFR < 60 ml/min/1.73 m2 was considered as a cutoff point to define chronic kidney disease, this study confirmed that deterioration of renal function in the elderly was also a mortality risk, especially for those with eGFR < 30 ml/min/1.73 m2. Furthermore, our results revealed that extremely high eGFR (>100 ml/min/1.73 m2) was a hazardous indicator to older people. The U-shaped relationship between eGFR and mortality has also been observed in an Asian population45 and a multinational cohort in the Chronic Kidney Disease Prognosis Consortium46.
The handy algorithm demonstrated in this study is the first mortality predication model for the elderly with diabetes. The strengths of this study include its high discriminative ability, its robustness (confirmed by both internal- and external-validation processes), and development of a simple point system that would be easy to use in clinical applications. However, some inherent limitations should be acknowledged. First, because no detailed medical records could be linked to the investigated datasets, we did not include a full list of comorbidity status in our models; however, the comprehensive biochemical profiles used in model development may be able to reveal most clinical information from our study subjects. In addition, because self-reported hypertension and hyperlipidemia were available in the development dataset, we have conducted subgroup analyses for those with hypertension and hyperlipidemia. The estimated risk point system for these two subgroups was shown in the Supplementary Table, in which most of the risk score was similar to that shown in the original model. A moderate weight (+1 or −1) might have to be adjusted for some categories, but the changes have been kept in the same direction, indicating the robustness of our prediction model. Second, health behaviors such as physical activity and drinking pattern were not fully included in our proposed models due to lack of availability; however, we incorporated the most important behavior factor, smoking status, as a covariate in the models. Third, because the Adult Health Screening Program is an ongoing government-sponsored national program, the laboratory data analysis is done by individual medical facilities and cannot be centralized. To ensure the quality of the laboratories, the sponsoring agent, the Health Promotion Administration, accredits the involved laboratories every three years. We thus believe the assay quality of those eligible laboratories is standardized and acceptable. Fourth, it may incur concerns about the moderately lower, although still acceptable, Harrell’s C statistic (0.685) in the external-validation dataset. We acknowledge that it is common to use the data collected in the same period or in the later period to validate the newly established model, but because of the data availability, we had to use the dataset collected in an earlier period for external validation. In addition, our data showed the subjects in the external-validation dataset had significantly better metabolic profiles and lower mortality rates compared to those in the development dataset (Tables 1 and 2). We believe no any datasets in the real world can be found to be completely identical to the development dataset. An acceptable Harrell’s C derived from a distinct dataset may just reveal our model’s robustness and generalizability to Chinese population. Fifth, the available dataset could not allow us to categorize the elderly based on their health status and functional capacity, so the purpose of our calculated risk point system is to estimate mortality risk for the diabetic elders, but we cannot use it to fine-tune the individualized diabetic care plan as suggested by the ADA guideline7. Finally, the current results, derived from older Chinese adults with diabetes, may not be directly generalizable to other ethnic groups or younger diabetic patients.
In conclusion, we have developed a simple point system that uses common clinical measures and biochemical profiles in clinical settings to predict five-year mortality risk for the elderly with diabetes. Facing the challenges of a rapid elevation in the aging population and diabetes prevalence, health providers are recommended to take this point system into account to better predict outcomes and to refine treatment strategies for their older diabetic patients.
Methods
Study Cohorts
The data used in this study were collected from the Taiwan’s Adult Health Screening Program (AHSP) from 2007 to 201047. The AHSP is a government-sponsored nationwide annual health check-up program for Taiwanese citizens ≥40 years. Information recorded in the AHSP database includes demographics, smoking status, common biochemical measures of blood pressure, lipid, fasting glucose, and kidney and liver function. Diabetes was defined as self-reported or fasting blood glucose ≥126 mg/dL. After excluding those who were younger than 65 years, were not diabetic patients, had missing data, or had an extreme level in the collected biomarkers (the highest and lowest 1%), we selected 245,370 study subjects for further analysis. Every study subject selected in this study was followed up until December 31, 2011 (up to 5 years).
Internal- and External-Validation Datasets
We used the 10-fold cross-validation method48 to randomly allocate the selected AHSP cohort into 10 subsets in which nine subsets were chosen to develop and to fit a prediction model and the remaining subset was used to conduct the internal validation. We repeatedly tested the performance of the combination of the selected development and internal validation subsets until the weighted mean square error (MSE), which was calculated by using the inverse probability of censoring weights (IPCW)49, reached minimum among all tests. Finally, the pair with smallest MSE (n = 220,832 for the development subset and n = 24,538 for the internal-validation subset) was selected for further analysis. For the external-validation dataset, we used the MJ Health Screening database (2004–2007), a reliable epidemiological data source in Taiwan45, from which we selected 2,093 older people (≥65) with diabetes. The subjects in the external-validation dataset were followed through until December 31, 2008 (up to 5 years).
Outcome and Definition of Candidate Predictors
The primary outcome investigated in this study was five-year all-cause mortality, which was ascertained by the national death registry. The candidate predictors used for model development were those that were considered as important influencing factors related to diabetic outcomes and health in older people, including gender, age, smoking status, BMI, fasting glucose, systolic and diastolic blood pressure, total cholesterol, triglyceride (TG), hemoglobin, albumin, eGFR, white blood cell count, glutamic pyruvic transaminase (GPT), and uric acid. The investigated predictors were categorized into several levels, as illustrated in Table 2. The same variables and categories were used in the internal and external validation models.
Statistical Analysis
The categorical variables of baseline demographics and biomarkers were described as frequencies with percentages; and continuous variables were described as means with standard deviations. The points system of this study calculated for both development and validation models were based on the methods proposed by Sullivan et al.50. First, we classified age and the other continuous variables into the designated categories and then assigned a clinically relevant reference level as the reference category for each investigated covariate. Second, we assessed the regression coefficient for each category of investigated covariates by using the Cox proportional hazards model and computed distance in term of regression unit between different categories and the assigned reference category for each covariate. Third, to construct a point system, we added one point for every five-year increment in age and let 5 multiply the regression coefficient of age as a reference to transform the regression coefficient in each significant category of investigated covariates into a risk point estimate. Finally, the discrimination capabilities of the established regression models were evaluated by Harrell’s C statistics51.
We also applied a bootstrapping technique52 to evaluate overfitting issue in our prediction model. We generated 200 bootstrap samples with replacement from the original development dataset. The sample size of each bootstrap sample was kept equal to the original dataset (n = 220,832). In each bootstrap model, we evaluated its individual optimism (the difference of the model performance between the bootstrap sample and the original dataset). The average optimism of the Harrell’s C statistics for all 200 bootstrap models due to variable selection and coefficient estimation was 0.0032, indicating there was no sign of overfitting.
Multivariable Cox proportional hazards model was used to determine the independent effects of investigated covariates on all-cause mortality. The survival time of each subject was defined as the time from enrollment to death in all-causes or last follow-up. Relative risks were expressed as hazard ratio (HR) for the comparison between a specific category and the corresponding designated reference level of the investigated covariate. The proportional hazard assumption, the constant HR over time, was evaluated by comparing estimated log–log survival curves for all covariates. We also performed subgroup analyses for those with self-reported hypertension and hyperlipidemia to test robustness of our model. All analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R software (http://www.r-project.org/). These two-sided tests for all test statistics were regarded as statistical significance if p values ≤ 0.05. This study was approved by the institutional review board (IRB) of the National Health Research Institutes. Because the two de-identified databases used in this study were open to the public, the IRB agreed to waive informed consent from the scrambled study subjects. The authors performed the study in accordance with the Declaration of Helsinki.
References
Kirkman, M. S. et al. Diabetes in older adults. Diabetes Care 35, 2650–2664 (2012).
Centers for Disease Control and Prevention. National Diabetes Statistics Report Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (2016).
Jiang, Y. D., Chang, C. H., Tai, T. Y., Chen, J. F. & Chuang, L. M. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc 111, 599–604 (2012).
Huang, E. S. et al. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 174, 251–258 (2014).
Centers for Disease Control and Prevention Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes — United States and Puerto Rico, 1996–2007. MMWR; 59, 1361–1366 (2010).
Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364, 829–841 (2011).
American Diabetes Association 11. Older Adults. Diabetes Care; 40 Suppl 1, S99–104 (2017).
Action to Control Cardiovascular Risk in Diabetes Study Group. et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358, 2545–2559 (2008).
Cushman, W. C. et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362, 1575–15585 (2010).
Gagne, J. J., Glynn, R. J., Avorn, J., Levin, R. & Schneeweiss, S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 64, 749–759 (2011).
Rose, S. Mortality risk score prediction in an elderly population using machine learning. J Clin Epidemiol 177, 443–452 (2013).
Sharifi, F. et al. Older people’s mortality index: development of a practical model for prediction of mortality in nursing homes (Kahrizak Elderly Study). Geriatr Gerontol Int 12, 36–45 (2012).
Hong Kong Diabetes Registry. et al. Development and validation of an all-cause mortality risk score in type 2 diabetes. Arch Intern Med 168, 451–457 (2008).
De Cosmo, S. et al. Development and validation of a predicting model of all-cause mortality in patients with type 2 diabetes. Diabetes Care 36, 2830–2835 (2013).
Wu, C. X., Tan, W. S., Toh, M. P. H. S. & Heng, B. H. Derivation and validation of a risk index to predict all-cause mortality in type 2 diabetes mellitus. J Endocrinol Metab 2, 88–95 (2012).
van Diepen, M. et al. Predicting mortality in patients with diabetes starting dialysis. PloS one 9, e89744 (2014).
Pinies, J. A. et al. Development of a prediction model for fatal and non-fatal coronary heart disease and cardiovascular disease in patients with newly diagnosed type 2 diabetes mellitus: the Basque Country Prospective Complications and Mortality Study risk engine (BASCORE). Diabetologia 57, 2324–2333 (2014).
van Dieren, S. et al. External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia 54, 264–270 (2011).
van der Leeuw, J. et al. The validation of cardiovascular risk scores for patients with type 2 diabetes mellitus. Heart 101, 222–229 (2015).
Yang, X. et al. Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol 101, 596–601 (2008).
Yang, X. et al. Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 30, 65–70 (2007).
Yang, X. L. et al. End-stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia 49, 2299–2308 (2006).
Wells, B. J. et al. Predicting 6-year mortality risk in patients with type 2 diabetes. Diabetes Care 31, 2301–2306 (2008).
Mazza A. et al. Antihypertensive treatment in the elderly and very elderly: always “the lower, the better?”. Int J Hypertens 590683 (2012).
Hansson, L. et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 351, 1755–1762 (1998).
Vamos, E. P. et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ. 30(345), e5567 (2012).
Madjid, M., Awan, I., Willerson, J. T. & Casscells, S. W. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 44, 1945–1956 (2004).
Margolis, K. L. et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med 165, 500–508 (2005).
Erlinger, T. P., Muntner, P. & Helzlsouer, K. J. WBC count and the risk of cancer mortality in a national sample of U.S. adults: results from the Second National Health and Nutrition Examination Survey mortality study. Cancer Epidemiol Biomarkers Prev 13, 1052–1056 (2004).
Bauer, J. M. & Sieber, C. C. Sarcopenia and frailty: a clinician’s controversial point of view. Exp Gerontol 43, 674–678 (2008).
Kvamme, J. M. et al. Body mass index and mortality in elderly men and women: the Tromso and HUNT studies. J Epidemiol Community Health 66, 611–617 (2012).
Garcia-Monzon, C. et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol 33, 716–724 (2000).
Lee, T. H., Kim, W. R., Benson, J. T., Therneau, T. M. & Melton, L. J. 3rd Serum aminotransferase activity and mortality risk in a United States community. Hepatology 47, 880–887 (2008).
El-Serag, H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(1264–1273), e1 (2012).
Liu, Z. et al. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PloS ONE 9, e91410 (2014).
Newson, R. S. et al. Association between serum cholesterol and noncardiovascular mortality in older age. J Am Geriatr Soc 59, 1779–1785 (2011).
Schatz, I. J. et al. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet 358, 351–355 (2001).
Ravnskov, U. et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open 6, e010401 (2016).
Kuo, C. F. et al. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology 52, 127–134 (2013).
Mazza, A. et al. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL). Acta Diabetol 44, 99–105 (2007).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N Engl J Med 359, 1811–1821 (2008).
Puddu, P., Puddu, G. M., Cravero, E., Vizioli, L. & Muscari, A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol 59, 235–2342 (2012).
Ogino, K. et al. Clinical significance of hypouricemia in hospitalized patients. J Med 22, 76–82 (1991).
National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3, 5–14 (2013).
Wen, C. P. et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371, 2173–2182 (2008).
Hallan, S. I. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308, 2349–2360 (2012).
Health Promotion Administration, Ministry of Health and Welfare, Taiwan, 2016. Overview of Health Promotion in Taiwan. P 64. Retrieved from http://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=6&ved=0ahUKEwj8q_aFr5rPAhUDFpQKHbzNBX0QFghGMAU&url=http%3A%2F%2Fwww.hpa.gov.tw%2FEnglish%2Ffile%2FContentFile%2F201502030333474650%2FHealth%2520Promotion%2520in%2520Taiwan.pdf&usg=AFQjCNEj7uaWfN_JLWwBGCtOZzcBT3UvcQ. (2016).
Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI 1137–1145 (1995).
Gerds, T. A. & Schumacher, M. Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom J 48, 1029–1040 (2006).
Sullivan, L. M., Massaro, J. M. & D’Agostino, R. B. Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 23, 1631–60 (2004).
Harrell, F. E. Jr., Lee, K. L. & Mark, D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15, 361–387 (1996).
Miao, Y., Cenzer, I. S., Kirby, K. A., Boscardin, W. J. Estimating Harrell’s Optimism on Predictive Indices Using Bootstrap Samples. (SAS GlobalForum 2013). Retrieved from http://support.sas.com/resources/papers/proceedings13/504-2013.pdf (2013).
Acknowledgements
The authors thank the staff and participants of the nationwide adult health screening program in Taiwan for their support and contributions and for giving us access to this rich dataset. This work was supported by the grant from the Ministry of Science and Technology (MOST 105-2314-B-400-030-MY2). We also thank the Taiwan Liver Research Foundation for assistance. The interpretation and conclusions contained herein do not represent those of the funding agency or National Health Research Institutes.
Author information
Authors and Affiliations
Contributions
C.C. Hsu. and Y.K. Chang. conceptualized and designed the study, and revised and finalized the manuscript as submitted. Y.K. Chang. drafted the manuscript. C.C. Hsu., S.J. Shin., K.D. Lin., K. Chong, F.S. Yen, H.Y. Chang, S.Y. Chuang, T.J. Hsieh and C.A. Hsiung. interpreted data, and reviewed, edited, and revised the manuscript for important intellectual content. Y.K. Chang. and L.F. Huang. managed the databases, monitored data quality and performed quantitative analyses.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, Y.K., Huang, L.F., Shin, S.J. et al. A Point-based Mortality Prediction System for Older Adults with Diabetes. Sci Rep 7, 12652 (2017). https://doi.org/10.1038/s41598-017-12751-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12751-3
This article is cited by
-
A nomogram for predicting the mortality of patients with type 2 diabetes mellitus complicated with acute kidney injury in the intensive care unit
BMC Anesthesiology (2023)
-
Development and Validation of the Life Expectancy Estimator for Older Adults with Diabetes (LEAD): the Diabetes and Aging Study
Journal of General Internal Medicine (2023)
-
Statistical modeling of health space based on metabolic stress and oxidative stress scores
BMC Public Health (2022)
-
Precision prognostics for the development of complications in diabetes
Diabetologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.