Abstract
We have previously shown that normobaric hyperoxia may benefit peri-lesional brain and white matter following traumatic brain injury (TBI). This study examined the impact of brief exposure to hyperoxia using diffusion tensor imaging (DTI) to identify axonal injury distant from contusions. Fourteen patients with acute moderate/severe TBI underwent baseline DTI and following one hour of 80% oxygen. Thirty-two controls underwent DTI, with 6 undergoing imaging following graded exposure to oxygen. Visible lesions were excluded and data compared with controls. We used the 99% prediction interval (PI) for zero change from historical control reproducibility measurements to demonstrate significant change following hyperoxia. Following hyperoxia DTI was unchanged in controls. In patients following hyperoxia, mean diffusivity (MD) was unchanged despite baseline values lower than controls (p < 0.05), and fractional anisotropy (FA) was lower within the left uncinate fasciculus, right caudate and occipital regions (p < 0.05). 16% of white and 14% of mixed cortical and grey matter patient regions showed FA decreases greater than the 99% PI for zero change. The mechanistic basis for some findings are unclear, but suggest that a short period of normobaric hyperoxia is not beneficial in this context. Confirmation following a longer period of hyperoxia is required.
Similar content being viewed by others
Introduction
While normobaric hyperoxia (NH) has been used to increase brain tissue oxygen partial pressure (BptO2) following traumatic brain injury (TBI) it is not routine therapy. Reductions in BtpO2 are associated with worse outcome1,2, and interventions aimed at optimising oxygen delivery have shown benefit1,3,4. 15O positron emission tomography (15O PET) has been used to show that NH can improve oxygen utilisation in “at-risk” regions of metabolically compromised tissue in pericontusional and white matter regions4. In addition, evidence obtained using diffusion tensor imaging (DTI) show how cytotoxic oedema within a rim of pericontusional tissue can be ameliorated with a short NH intervention5.
These results are in conflict with evidence demonstrating increases in microdialysis glutamate6, and studies showing an association between arterial hyperoxia and poor outcome following severe TBI7. These highlight the potential deleterious effects on pulmonary function and worsening neuronal injury due to oxidative stress8,9. Given this background, it is clear that further study of the regional effects of normobaric hyperoxia across the injured brain is warranted to ensure it is used appropriately.
Diffusion tensor imaging has been used to demonstrate evidence of traumatic axonal injury following TBI even when conventional imaging appears normal10. Imaging findings are dynamic and potentially reversible11,12, suggesting that DTI could be used as a biomarker of the effectiveness of therapeutic interventions in TBI. In this study we aimed to address the impact of NH distant from visible contusions using novel data from a cohort of subjects previously included within a publication by Veenith et al.5. Such data should help inform the design and conduct of any future clinical trial of this intervention in TBI.
Results
Effect of graduated oxygen therapy on diffusion tensor imaging in healthy volunteers
The DTI data at each level of inspired oxygen are displayed in Tables 1 and 2 for white and mixed cortical and deep grey matter regions respectively. As expected, there were significant differences between the brain regions for fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) (p < 0.001 for all comparisons using ANOVA with Bonferoni correction). While the DTI parameters were variable across the different brain regions there were no significant changes in FA, AD, RD and MD with an increase in the fraction of inspired oxygen (FiO2) within white matter (p = 0.82, 0.87, 0.70 and 0.68 respectively, analysis of variance (ANOVA)) and mixed cortical and deep grey matter regions of interest (ROIs) (0.66, 0.32, 0.47 and 0.40 respectively, ANOVA).
Diffusion tensor imaging in patients and healthy volunteers
Patient characteristics are shown in Table 3. For the 14 patients and 32 healthy volunteers there was no significant difference in age (p = 0.48, Mann-Whitney U test). The baseline ROI data for healthy volunteers and normoxic patients from predominantly white matter, and mixed cortical and deep grey matter are summarised in Tables 4 and 5 respectively. These demonstrate that baseline patient data show lower FA, MD, AD and RD values than healthy volunteers in a variety of normal appearing white and mixed cortical and deep grey matter regions (p < 0.05, unpaired t tests with Bonferroni correction).
Impact of hyperoxia in patients
The ROI data in patients at normoxia and following hyperoxia for white matter and mixed cortical and deep grey matter are shown in Tables 6 and 7 respectively. These demonstrate that there were no changes in AD and MD. Within white matter FA was lower and RD higher within the left uncinate fasciculus (p < 0.05, paired t tests with Bonferroni correction). Within mixed cortical and deep grey matter FA was significantly lower following hyperoxia within the right caudate and occipital regions (p < 0.05, paired t tests with Bonferroni correction).
The percentage of white and mixed cortical and deep grey matter ROIs in patients and healthy volunteers exposed to hyperoxia showing a change in DTI parameters following NH that was greater than the overall population and regional 99% prediction intervals (PIs) for zero change are summarised in Figs 1 and 2 respectively. In healthy volunteers these changes are shown from baseline air to 100% oxygen. Using the overall population 99% PI significant decreases in FA were found within 16% of white matter ROIs from 9/14 patients and in 14% of mixed cortical and deep grey matter ROIs from 8/14 patients. Changes in the other DTI parameters were less frequent; some regions showed significant decreases in AD and MD while RD was generally unchanged. Supplementary Tables S1 and S2 provide a detailed list of which patients and regions showed significant change for white and mixed cortical and deep grey matter regions respectively. Decreases in FA were found across the whole brain in many different brain regions. Supplementary Tables S3 and S4 provide the equivalent data for the 6 healthy volunteers who underwent graded exposure to oxygen. The results using the individual ROI reproducibility data were similar and demonstrate significant FA decreases in 9% of white and 19% of mixed cortical and deep grey matter ROIs respectively (see Figs 1 and 2). Supplementary Tables S5 and S6 provide a detailed list of the regions showing significant change for white and mixed cortical and deep grey matter in patients, while S7 and S8 show the equivalent for healthy volunteers.
Impact of hyperoxia in patients. Fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) within atlas regions of interest (ROI) applied in normalised space for 14 patients using “lesion free” brain by exclusion of lesion core and contusion tissue. Data displayed are the percentage number of white (white) and mixed cortical and deep grey matter (grey) ROIs showing a change greater than the overall population (left panel) and individual regional (right panel) 99% prediction interval (PI) for zero change. The total number of regions in this cohort was 320 and 223 for white matter and mixed cortical and deep grey matter respectively.
Impact of hyperoxia in healthy volunteers. Fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) within atlas regions of interest (ROI) applied in normalised space for 6 healthy volunteers. Data displayed are the percentage number of white (white) and mixed cortical and deep grey matter (grey) ROIs showing a change greater than the overall population (left panel) and individual regional (right panel) 99% prediction interval (PI) for zero change. The total number of regions in this cohort was 138 and 96 for white matter and mixed cortical and deep grey matter respectively.
Discussion
In this study we used DTI to examine whether an increase in the fraction of inspired oxygen had any beneficial effects within deep grey and mixed cortical, and white matter regions distant from visible contusions following TBI. Baseline patient data showed evidence of traumatic injury with lower MD and FA in several regions compared with healthy volunteers, consistent with cytotoxic oedema and axonal injury respectively. Exposure to a brief period of NH had no effect on healthy volunteers, and did not ameliorate these findings in patients with some regions showing further FA decreases following intervention. Using published reproducibility data from a historical cohort of 26 healthy volunteers we demonstrated that 16% of white matter and 14% of mixed cortical and deep grey matter regions in patients showed a reduction in FA more than the expected population 99% PI for zero change. The mechanistic basis for some of the DTI findings are unclear, but imply that a short period of NH has no beneficial impact within brain that appears normal using conventional structural imaging. To confirm these findings and investigate further will require a longer duration of hyperoxia with serial DTI and conventional MRI in comparison with clinical outcome.
Monitoring of focal tissue oxygen and brain metabolism using microdialysis has shown that hyperoxia can correct derangements4,13,14 and may be associated with improved outcome1,15. Further, a 15O PET study suggested that improvements in metabolism with hyperoxia may be particularly relevant within brain regions with physiology consistent with the greatest risk of infarction4. We have also used DTI to demonstrate contusion expansion within a rim of low MD consistent with cytotoxic oedema that surrounds a region of high MD (vasogenic oedema)11, and normobaric hyperoxia can increase MD values towards normal within this perilesional rim5. Both these imaging studies demonstrate how a short period of exposure to normobaric hyperoxia (~60 minutes) can result in potential benefit. Such findings suggest improvements in oxygen delivery that may relate to evidence of microvascular injury16 within the ‘traumatic penumbra’ and are consistent with post mortem studies showing microvascular occlusion and perivascular oedema associated with selective neuronal loss post TBI17,18. Increased brain oxygen levels may overcome diffusion barriers to oxygen delivery5 or improve mitochondrial function where low oxygen tension allows nitric oxide to competitively inhibit cytochrome oxidase19. Mitochondrial dysfunction has been shown in ex vivo clinical and experimental TBI studies20, and mitochondrial function can be preserved using hyperoxia21. Other studies demonstrate that hyperoxia has neuroprotective and anti-inflammatory effects within the injured brain22.
Whilst these changes are most evident within perilesional regions pathophysiological derangements are also evident in regions distant from visible injury based on conventional structural imaging12,23,24. Several PET studies have shown evidence of ischaemia and other metabolic derangements within brain that may initially appear structurally normal16,23,25,26 but ultimately demonstrates late atrophy, and is associated with poor outcome27. Further, benefit shown with normobaric hyperoxia in the 15O PET study by Nortje et al.4 within brain demonstrating physiology consistent with the greatest risk of infarction included normal appearing white matter. Studies using DTI are particularly relevant in this regard since evidence of cytotoxic oedema and traumatic axonal injury are often identified using this technique when conventional structural imaging appears normal12,28. Our findings were consistent with these data. Despite the exclusion of visible contusions and other areas of brain injury, the patient regional baseline data demonstrated significant DTI abnormalities consistent with cytotoxic oedema and axonal injury in comparison with healthy controls. Such regions were the focus of this study, and our expectation was that we may see amelioration of cytotoxic oedema and other DTI signal changes in brain distant from contusions following hyperoxia secondary to an improvement in oxygen delivery and/or mitochondrial function. It is important to acknowledge that any change must be sustained if it is to result in improved neuronal survival and better functional outcome for patients, but it is likely that this will require a much longer period of exposure to NH. However, we wished to demonstrate whether it was possible to use DTI as a biomarker of the trajectory of such injury or its recovery in the assessment of therapeutic interventions such as hyperoxia. Previous imaging studies have limited exposure to NH to one hour4,5, and have conducted repeat imaging within a single session in which changes in other physiological and patient related factors can be minimised. There are also concerns regarding excessive exposure to NH since it can result in atelectasis and pulmonary injury, increased oxidative stress and potential harm in critically ill patients. In this context a further preliminary study of the impact of NH on the injured brain was warranted.
Experimental and clinical ischaemia following middle cerebral artery occlusion results in early evidence of cytotoxic oedema with a reduction in MD29, and while AD and RD are typically reduced, it has been hypothesised that oligodendrite swelling can compress the axoplasm and result in a greater decrease in RD than AD within white matter30. This may explain why acute ischaemia can result in an initial increase in white matter FA if imaging is conducted within 4.5 hours of acute stroke30,31. Later, loss of cellular integrity results in large decreases in white matter FA30. These finding are relevant to ischaemic stroke, but were hyperoxia to improve oxygen delivery and attenuate cytotoxic oedema following TBI, MD should increase towards normal and, in theory, an increase in RD that was greater than AD could result in an initial reduction in white matter FA. While we did find evidence of low MD in TBI patients at baseline consistent with cytotoxic oedema we did not see evidence of an increase in MD towards normal within white or grey matter regions following exposure to hyperoxia. This suggests that the intervention was ineffective, or that a longer period of hyperoxia was needed to demonstrate any effect. In addition, the lack of evidence for a reversal of cytotoxic oedema cannot provide explanation for our finding of a reduction in FA within white or grey matter.
We exposed healthy volunteers to oxygen therapy since oxygen has a known paramagnetic effect and could have resulted in systematic changes to our DTI findings32. We saw no relationship between a step increase in administered oxygen and any of the DTI parameters. Healthy volunteers received oxygen via a venturi mask, in comparison with TBI patients who received fixed concentrations of inspired oxygen via a closed ventilatory circuit as they had been intubated and ventilated as part of routine clinical care. The Venturi mask provides a means of reliably titrating the FiO2 in spontaneously breathing subjects33, and while arterial blood gases were not monitored in healthy volunteers each step increase in delivered oxygen will have resulted in higher PaO2. Following 15 minutes of breathing 60% oxygen volunteers underwent ~45 minutes of imaging (DTI and whole brain proton spectroscopy) whilst continuing to breath 60% oxygen. Then, following an additional 15 minutes breathing 100% oxygen imaging was repeated for the last time. So, by the final DTI sequence subjects had been breathing an increased fraction of inspired oxygen for over 60 minutes. At this stage the PaO2 of the healthy volunteers would have been at least as high as that achieved in patients5.
In patients, we looked for regions where changes in DTI were greater than the 99% PI for zero change using published data from 26 historical healthy volunteers who underwent DTI on up to 4 occasions within two imaging sessions34. Both patients and volunteers underwent scanning within the Wolfson Brain Imaging Centre (WBIC) using the same scanner, software version and scanner sequences. Since patients underwent baseline and post intervention imaging during the same session the expected variability in patients, who were also sedated and paralysed during imaging, is likely to be at least as good as that found in awake spontaneously breathing healthy volunteers who underwent repeat DTI during two sessions separated by up to six months34,35.
Patients suffered a TBI and the presence of brain lesions will produce errors in spatial processing, particularly were non-linear algorithms are used to co-register and transform data to a standard template. The ROI template was eroded by a single voxel to limit problems resulting from co-registration, normalisation and partial volume errors. Visible areas of injury were manually delineated in native space, and subsequently, a normalised binary mask of the lesions was used to exclude this volume of brain tissue from the individualised standard ROI template of each patient. All registered datasets were reviewed to ensure that the spatial processing had not resulting in significant errors, and no subjects were excluded on this basis. While these concerns may lead to an over estimate of the difference between regional DTI values in patients compared to healthy volunteers, it is important to emphasise that the focus of this study was to compare change following NH within individual subjects during the same imaging session. There were no structural differences between the baseline and post NH datasets, and therefore, any small errors in registration and normalisation would have been replicated in both datasets. Patients were sedated, paralysed and ventilated throughout imaging sessions as part of routine care. This would have prevented movement artefact and helped optimise data collection, processing and subsequent analyses. Under these circumstances small changes within individual ROIs that relate to problems with spatial processing would be unlikely to introduce systematic errors between baseline and post NH intervention imaging within individual subjects. While it is possible that the DTI changes we found occurred purely by chance, we cannot ignore the fact that over 10% of all patient regions showed a fall in FA greater than the 99% PI for zero change following NH.
While the significance of a fall in FA following a brief exposure to hyperoxia is unknown it still represents some detectable and reversible change in the local tissue environment that did not occur in healthy volunteers exposed to a similar intervention. Given the concern regarding the use of hyperoxia it would be important to exclude the possibility, however small, that this could represent some early evidence of axonal injury within white matter resulting from oxidative stress. In chronic TBI a reduction of FA within white matter is consistent with axonal injury, with the extent of changes dependent on the time since ictus12. Interestingly, late cortical FA increases can also occur and may relate to scarring post mild TBI36. Clearly, it would be important to undertake serial MRI with DTI to understand how these DTI parameters evolve within both grey and white matter following exposure to longer periods of NH. At the very least these findings demonstrate how such measurements could be used to assess the impact of a longer duration of therapeutic NH and should be compared with evidence of late tissue fate based on structural MR and clinical outcome. Finally, since AD and RD are parameters that relate to the orientation of white matter fibres the small changes we found within mixed cortical and deep grey matter following hyperoxia are of little consequence.
Patients underwent imaging between days 1 – 9 post injury, and DTI changes may reflect different trajectories within individual subjects with resolution of oedema mixed with loss of tissue integrity within established lesions. Despite this concern, there was no significant interaction between FA changes following hyperoxia and the time since injury (p = 0.59, ANOVA). All patients in this cohort sustained TBI severe enough to require intensive care management of raised intracranial pressure, and had comparable imaging patterns of injury (Table 3). Despite this, outcome was variable and it is possible that the changes in DTI parameters seen may reflect individual variability within this small cohort. However, reductions in FA were seen in over half the patients and in the majority of brain regions with no clear relationship to injury type or eventual outcome. Nevertheless, definitive statements concerning the significance of DTI changes would require data from a larger cohort of patients showing evidence of sustained reductions in FA associated with poor functional outcome in comparison with a control arm before it could be concluded that they were indicative of axonal injury37. Sequential imaging could be used as a biomarker of the trajectory of such injury or its recovery in the assessment of therapeutic interventions such as hyperoxia12.
Previous clinical studies have suggested that the use of high partial pressures of oxygen may be beneficial1,3, but there may be a relatively narrow margin of safety9. We limited the maximum FiO2 in this interventional study to 0.8 to minimise direct side effects such as alveolar atelectasis and pulmonary injury. Clinical studies show little evidence of increased oxidative stress when therapy is applied in a controlled manner within the first three days post injury3,5,38. We show how changes in DTI can be detected in patients following NH based on reproducibility data from a historical group of healthy volunteers. The pathophysiological basis and significance of any fall in FA following exposure to NH remains unknown, particularly following such a brief intervention. Nevertheless, any potentially adverse effect should be considered and further studies should incorporate serial DTI to help determine how and when this intervention should be used within a precision medicine approach to optimise the beneficial impact on patient outcome. Such data could be invaluable in the design of any future clinical trial since studies to date do not provide definitive evidence of an improvement in clinical outcome3.
Prior TBI studies have suggested that an increase in the fraction of inspired oxygen can improve cerebral metabolism within perilesional and normal appearing white matter4,39, and using DTI, result in benefit within the rim of cytotoxic oedema found around brain contusions5. Using DTI we showed evidence of cytotoxic oedema and traumatic axonal injury distant from visible lesions with no improvement following the short-term administration of normobaric hyperoxia. To confirm these findings and investigate further will require a longer duration of hyperoxia with serial DTI and conventional MRI in comparison with clinical outcome.
Methods
Ethical approval was obtained from the Cambridgeshire Research Ethics Committee (reference numbers 97/290 and 02/293), and assent from next-of-kin with later written informed consent, where appropriate, obtained in all cases in accordance with the Declaration of Helsinki.
Subjects
Patients
Fourteen adult patients (12 males and 2 females) with median (range) age 33 (21 – 70) years with TBI were recruited from the Neurosciences Critical Care Unit (NCCU), Addenbrooke’s Hospital, Cambridge, UK between 2010 and 2012. Patients presented with median (range) post resuscitation Glasgow Coma Score (GCS) of 7 (3–14), but all subsequently had a GCS <8 requiring sedation and ventilation for control of intracranial pressure (ICP) (Table 3). Patients were recruited between day 1 and day 9 post injury and underwent imaging whilst sedated and ventilated. Patients with previous TBI, other neurological disease, or contraindication to magnetic resonance imaging (MRI) were excluded. All patients were managed by protocol driven care; which included sedation, paralysis and ventilation to ensure that intracranial pressure (ICP) <20 mmHg and cerebral perfusion pressure >60 mmHg were maintained4. Physiological stability was meticulously ensured during imaging through the titration of fluids and vasoactive agents and the presence of a critical care physician and nurse. Patients who received surgical intervention (CSF drainage or decompressive craniectomy) or second-tier medical therapies (barbiturate coma or moderate hypothermia (33–35 °C)) before imaging are specified in Table 3.
Based on previous imaging studies we acquired baseline DTI at a partial pressure of oxygen (PaO2) of approximately 75–90 mmHg (10–12 kPa) and then increased the FiO2 to a maximum of 0.8 in order to achieve a PaO2 of approximately 225–260 mmHg (30–35 kPa). Following a 60-minute period to allow the impact of higher PaO2 (and by inference, brain pO2) levels on cerebral metabolism, repeat DTI was obtained within the same imaging session without moving the patient.
Controls
A total of 32 healthy volunteers (19 females and 13 males) with a median (range) age of 34 (22–52) years underwent DTI breathing room air. Six of these volunteers were exposed to graded oxygen therapy (60% and 100% inspired oxygen) delivered via a venturi mask (Flexicare Medical Limited, Mid Glamorgan, Wales) and underwent repeat DTI within the same imaging session. Diffusion tensor imaging and whole brain proton spectroscopy were obtained at each level of inspired oxygen (21%, 60% and 100%) following an equilibration period of 15 minutes. The baseline data obtained breathing room air in all 32 subjects, and the graded oxygen therapy data in the six healthy volunteers, are presented in the results section of this manuscript. As part of a previously published study, 26 of these volunteers underwent DTI on up to 4 occasions within two imaging sessions separated by a maximum of six months. The reproducibility data from this historical cohort have been published, and are used in the subsequent analyses34.
Imaging
All subjects were scanned using a 3 T Siemens Verio MRI scanner (Siemens AG, Erlangen, Germany) within the WBIC, University of Cambridge. During the study period there were no major changes or upgrades to the scanner or software. Sequences included a 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE), fluid attenuated inversion recovery (FLAIR), gradient echo (GE), susceptibility weighted (SWI), dual spin echo (proton density/T2-weighted) and whole brain proton spectroscopy (26 minutes). The DTI data were acquired over 13:50 minutes using 63 non-collinear directions, b = 1000 s/mm2 with one volume acquired without diffusion weighting (b = 0), echo time (TE) 106ms, repetition time (TR) 11700ms, 63 slices, field of view 192mm × 92mm, and 2 × 2 × 2mm3 isotropic voxels. All imaging was reviewed by a specialist clinical neuroradiologist.
Image processing
Fractional anisotropy, MD and AD maps were created using the Oxford Centre for functional MRI of the brain FSL Diffusion Toolbox40, while RD values were calculated as the mean of the second and third eigenvalues. To aid coregistration, the skull and extracranial soft tissue were stripped from the MPRAGE image using the Brain Extraction Tool of FSL41. The diffusion weighted data were normalized to the Montreal Neurological Institute 152 (MNI152) template using the non-linear vtkCISG normalized mutual information algorithm42. Using the same non-linear algorithm the T1 weighted images were coregistered to the MNI152 template and each subject’s b = 0 image subsequently coregistered to the individual T1 weighted image. The transformation matrix normalizing the MPRAGE was then applied to the b = 0 image. All coregistered and normalized images were visually checked to ensure that they were aligned.
Region of interest analysis
Lesions were defined in native FLAIR space by a single author (JG) using patient FLAIR, MPRAGE, GE and SWI images. Lesion core was identified as a region of mixed signal intensity consistent with haemorrhage and necrotic tissue and contusion as an area of high signal on FLAIR (Fig. 3). The ROIs were drawn using Analyze 8.5 (Analyze Direct, Lenexa, KS, USA). FLAIR images were coregistered to T1 space using SPM8, and the coregistration matrix applied to the individual lesion ROIs.
Regions of interest from the Harvard Oxford subcortical and MNI structural probabilistic atlases available within FSL were applied in normalised space43. The ROI template was modified by erosion of a single voxel using the fslmaths tool within FSL to improve spatial localisation and reduce the impact of coregistration, normalisation and partial volume errors. In patients, these analyses were performed on “lesion free” brain by exclusion of lesion core and contusion tissue following transformation of the lesion ROI to normalised space (Fig. 4). While this resulted in the removal of a few regions were a lesion covered the entire ROI, normal appearing tissue from within the remaining volume of brain of each region was retained for subsequent analyses. The FA, MD, AD and RD values for the different ROIs were calculated using in-house software using Matlab (Mathworks, Natick, USA).
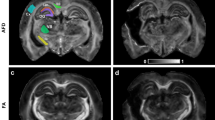
Individualised template regions of interest. Standard T1 weighted magnetic resonance image and patient fluid attenuated inversion recovery (FLAIR), with fractional anisotropy (FA) at baseline normoxia and following hyperoxia; all images are displayed in Montreal Neurological Institute 152 (MNI152) space. The region of interest (ROI) template for this subject (subject 1) has been individualised by the exclusion of lesion core and contusion tissue. On the FLAIR image slice shown lesions can be seen within the right frontal and temporal cortex, white matter, right caudate and right thalamus. On this axial slice regions shown include frontal left (Front_L), frontal right (Front_R), temporal left (Temp_L), temporal right (Temp_R), parietal left (Par_L), parietal right (Par_R), occipital left (Occip_L), occipital right (Occip_R), anterior corpus callosum (ACC), posterior corpus callosum (PCC), caudate left (Caud_L) and thalamus left (Thal_L).
Impact of hyperoxia
Using published DTI reproducibility data from the historical cohort of 26 volunteers included in this manuscript34, we assessed the significance of changes in DTI parameters following NH. Based on the standard deviation (SD) of DTI measurements the overall population 99% PIs for zero change (based on three SD values) were 9.6 × 10−5, 9.6 × 10−5 and 2.5 × 10−4 mm2/second for AD, RD and MD respectively, and 3.6 × 10−2 for FA34. We calculated the percentage of ROIs in patients with increases or decreases in DTI parameters greater than the overall population 99% PI for zero change. Since measurements of reproducibility can vary depending on the brain region examined34 we also used an estimate of the regional 99% prediction interval for zero change calculated for each ROI. As this is based on the four independent measurements obtained for each ROI in isolation, we must be more cautious. For a t distribution with 3 degrees of freedom this should be based on 5.8 SD values, and this estimate was used for each individual ROI value in calculating the regional 99% prediction interval for zero change.
Data and statistical analysis
Statistical analyses were conducted using Statview (Version 5, 1998, SAS Institute Inc., Cary, North Carolina, USA). All data are expressed and displayed as mean and SD, unless otherwise stated. Individual ROIs were treated independently, since they represented a clinically relevant method of segmenting the brain, with specific location being irrelevant to this analysis. Data were compared using unpaired, paired t-tests, ANOVA and Mann Whitney U tests. All p values are quoted after Bonferroni correction (where appropriate), and p values that remained <0.05 following multiplication by the number of tests performed were considered significant. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Beynon, C., Kiening, K. L., Orakcioglu, B., Unterberg, A. W. & Sakowitz, O. W. Brain tissue oxygen monitoring and hyperoxic treatment in patients with traumatic brain injury. J Neurotrauma 29, 2109–2123, https://doi.org/10.1089/neu.2012.2365 (2012).
Spiotta, A. M. et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg 113, 571–580, https://doi.org/10.3171/2010.1.JNS09506 (2010).
Rockswold, S. B., Rockswold, G. L., Zaun, D. A. & Liu, J. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg 118, 1317–1328, https://doi.org/10.3171/2013.2.JNS121468 (2013).
Nortje, J. et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: Preliminary findings. Crit Care Med 36, 273–281 (2008).
Veenith, T. V. et al. Use of diffusion tensor imaging to assess the impact of normobaric hyperoxia within at-risk pericontusional tissue after traumatic brain injury. J Cereb Blood Flow Metab 34, 1622–1627, https://doi.org/10.1038/jcbfm.2014.123 (2014).
Quintard, H., Patet, C., Suys, T., Marques-Vidal, P. & Oddo, M. Normobaric hyperoxia is associated with increased cerebral excitotoxicity after severe traumatic brain injury. Neurocrit Care 22, 243–250, https://doi.org/10.1007/s12028-014-0062-0 (2015).
Damiani, E. et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 18, 711, https://doi.org/10.1186/s13054-014-0711-x (2014).
Ahn, E. S., Robertson, C. L., Vereczki, V., Hoffman, G. E. & Fiskum, G. Normoxic ventilatory resuscitation following controlled cortical impact reduces peroxynitrite-mediated protein nitration in the hippocampus. J Neurosurg 108, 124–131, https://doi.org/10.3171/JNS/2008/108/01/0124 (2008).
Bitterman, H. Bench-to-bedside review: oxygen as a drug. Crit Care 13, 205, https://doi.org/10.1186/cc7151 (2009).
Newcombe, V. F. et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg 21, 340–348 (2007).
Newcombe, V. F. et al. Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J Cereb Blood Flow Metab 33, 855–862, https://doi.org/10.1038/jcbfm.2013.11 (2013).
Newcombe, V. F. et al. Dynamic Changes in White Matter Abnormalities Correlate With Late Improvement and Deterioration Following TBI: A Diffusion Tensor Imaging Study. Neurorehabilitation and neural repair, https://doi.org/10.1177/1545968315584004 (2015).
Tolias, C. M. et al. Normobaric hyperoxia–induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg 101, 435–444 (2004).
Reinert, M., Schaller, B., Widmer, H. R., Seiler, R. & Bullock, R. Influence of oxygen therapy on glucose-lactate metabolism after diffuse brain injury. J Neurosurg 101, 323–329, https://doi.org/10.3171/jns.2004.101.2.0323 (2004).
Nangunoori, R. et al. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: a systematic literature review. Neurocrit Care 17, 131–138, https://doi.org/10.1007/s12028-011-9621-9 (2012).
Veenith, T. V. et al. Pathophysiological mechanisms of cerebral ischaemia and diffusion hypoxia in traumatic brain injury. JAMA neurology (2016).
Bullock, R., Maxwell, W. L., Graham, D. I., Teasdale, G. M. & Adams, J. H. Glial swelling following human cerebral contusion: an ultrastructural study. J Neurol Neurosurg Psychiatry 54, 427–434 (1991).
Stein, S. C., Graham, D. I., Chen, X. H. & Smith, D. H. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery 54, 687–691, discussion 691 (2004).
Brown, G. C. Nitric oxide inhibition of cytochrome oxidase and mitochondrial respiration: implications for inflammatory, neurodegenerative and ischaemic pathologies. Mol Cell Biochem 174, 189–192 (1997).
Robertson, C. L. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J Bioenerg Biomembr 36, 363–368 (2004).
Zhou, Z. et al. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J Neurosurg 106, 687–694, https://doi.org/10.3171/jns.2007.106.4.687 (2007).
Palzur, E., Zaaroor, M., Vlodavsky, E., Milman, F. & Soustiel, J. F. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res 1221, 126–133, https://doi.org/10.1016/j.brainres.2008.04.078 (2008).
Coles, J. P. et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab 24, 202–211 (2004).
Coles, J. P. et al. Early metabolic characteristics of lesion and nonlesion tissue after head injury. J Cereb Blood Flow Metab 29, 965–975 (2009).
Vespa, P. et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab 25, 763–774 (2005).
Hattori, N. et al. Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J Nucl Med 45, 775–783 (2004).
Xu, Y. et al. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab 30, 883–894, https://doi.org/10.1038/jcbfm.2009.263 (2010).
Kraus, M. F. et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519 (2007).
Liu, Y. et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke 38, 138–145, https://doi.org/10.1161/01.STR.0000252127.07428.9c (2007).
Puig, J. et al. Increased corticospinal tract fractional anisotropy can discriminate stroke onset within the first 4.5 hours. Stroke 44, 1162–1165, https://doi.org/10.1161/STROKEAHA.111.678110 (2013).
Bhagat, Y. A. et al. Elevations of diffusion anisotropy are associated with hyper-acute stroke: a serial imaging study. Magn Reson Imaging 26, 683–693, https://doi.org/10.1016/j.mri.2008.01.015 (2008).
Anzai, Y. et al. Paramagnetic effect of supplemental oxygen on CSF hyperintensity on fluid-attenuated inversion recovery MR images. AJNR Am J Neuroradiol 25, 274–279 (2004).
Waldau, T., Larsen, V. H. & Bonde, J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia 53, 256–263 (1998).
Veenith, T. V. et al. Inter subject variability and reproducibility of diffusion tensor imaging within and between different imaging sessions. PloS one 8, e65941, https://doi.org/10.1371/journal.pone.0065941 (2013).
Coles, J. P. et al. Intersubject variability and reproducibility of (15)O PET studies. J Cereb Blood Flow Metab (2005).
Ling, J. M., Klimaj, S., Toulouse, T. & Mayer, A. R. A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 81, 2121–2127, https://doi.org/10.1212/01.wnl.0000437302.36064.b1 (2013).
Sidaros, A. et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131, 559–572 (2008).
Puccio, A. M. et al. Effect of short periods of normobaric hyperoxia on local brain tissue oxygenation and cerebrospinal fluid oxidative stress markers in severe traumatic brain injury. J Neurotrauma 26, 1241–1249, doi:https://doi.org/10.1089/neu.2008-0624
Tisdall, M. M., Tachtsidis, I., Leung, T. S., Elwell, C. E. & Smith, M. Increase in cerebral aerobic metabolism by normobaric hyperoxia after traumatic brain injury. J Neurosurg 109, 424–432 (2008).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1), S208–219, https://doi.org/10.1016/j.neuroimage.2004.07.051 (2004).
Smith, S. M. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155, https://doi.org/10.1002/hbm.10062 (2002).
Studholme, C., Hill, D. L. & Hawkes, D. J. Automated 3-D registration of MR and CT images of the head. Med Image Anal 1, 163–175 (1996).
Lancaster, J. L. et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10, 120–131 (2000).
Acknowledgements
Dr. Veenith was supported by clinical research training fellowship from National institute of Academic Anaesthesia and Raymond Beverly Sackler studentship. VFJN is supported by a Health Foundation/Academy of Medical Sciences Clinician Scientist Fellowship. JPC was supported by Wellcome trust project grant. DKM is supported by an NIHR Senior Investigator Award. This work was supported by a Wellcome Trust Project Grant (WT093267) and Medical Research Council (UK) Program Grant (Acute brain injury: heterogeneity of mechanisms, therapeutic targets and outcome effects (G9439390 ID 65883)), the UK National Institute of Health Research Biomedical Research Centre at Cambridge, and the Technology Platform funding provided by the UK Department of Health. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.V.V. was responsible for data acquisition, and with J.P.C., jointly prepared the manuscript and undertook the data analyses. J.G. defined lesion regions on the imaging data and with E.C., V.F.J.N., J.O., S.N., V.L., M.C., M.M., G.W., and D.K.M. assisted with acquisition and data analyses. T.V.V., D.K.M. and J.P.C. conceptualised and designed the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veenith, T.V., Carter, E.L., Grossac, J. et al. Normobaric hyperoxia does not improve derangements in diffusion tensor imaging found distant from visible contusions following acute traumatic brain injury. Sci Rep 7, 12419 (2017). https://doi.org/10.1038/s41598-017-12590-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12590-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.