Abstract
Chronic intermittent hypoxia (IH) contributes to obstructive sleep apnea (OSA)-related cardiovascular diseases through increasing oxidative stress. It has been widely recognized that estradiol decreases the risk for cardiovascular disease, but the estrogen replacement therapy is limited for its side effects. Thioredoxin (Trx) and its endogenous inhibitor, thioredoxin-interacting protein (Txnip), are associated with the protective effect of estradiol in some conditions. In this study, we aimed to explore whether estradiol could protect against IH-induced vascular injury, and the possible effect of Trx-1/Txnip in this process. Forty-eight adult female C57/BL6J mice were randomly divided into 4 groups, ovariectomy combined with IH group, sham operation combined with IH group, IH group and the control group. The mice treated with IH for 8 hrs/day, and 28 days. IH induced the injury of aorta, and ovariectomized mice were more prone to the IH-induced aortic injury, with higher level of oxidative stress. In vitro, estradiol increased Trx-1 level, but decreased the level of Txnip and oxidative stress in human umbilical vein endothelial cells (HUVECs) treated with IH for 16 hrs. Knock-down of Txnip by specific siRNA rescued oxidative stress and apoptosis. In conclusion, estradiol protects against IH-induced vascular injury, partially through the regulation of Trx-1/Txnip pathway.
Similar content being viewed by others
Introduction
Characterized by repetitive episodes of upper airway collapse during sleep, obstructive sleep apnea (OSA) affects about 4% of men and 2% of women1. Chronic intermittent hypoxia (IH) caused by repetitive upper airway collapse contributes to systemic complications including cardiovascular diseases (CVD)2, 3. As the first-choice treatment of OSA, continuous positive airway pressure (CPAP) decreases the risk for CVD4. However, poor compliance with CPAP therapy is quite a common clinical problem. Thus, the novel prevention and treatment strategies for CVD in OSA patients should be concerned. Series of clinical evidences support the protective effect of estrogen against CVD5, 6. A prospective survey on 121,964 female nurses favored the postmenopausal estrogen use to reduce the risk for severe CVD5. However, long-term estrogen replacement therapy increases the risk for endometrial cancer and breast cancer6. Therefore, uncovering the underlying mechanisms of cardiovascular protection of estrogen will be helpful for the development of new therapeutic targets for the CVD complication in OSA patients.
The increased oxidative stress is the main mechanism of OSA associated CVD. The oxidative stress injury is caused by the imbalance between oxidation and reduction system, with excessive reactive oxygen species (ROS). Trx and Txnip play an essential role in the regulation of oxidative stress level. Trx-1 mainly exists in cytoplasm, with anti-oxidative effect mediated by the dithiol-disulfide exchange in the active site -Cys-Gly-Pro-Cys-. As an endogenous inhibitor of Trx, Txnip has been found to modulate the oxidative stress through binding to the active center of Trx.
Previous study indicated that estrogen protects against CVD through preventing oxidative stress-associated endothelial cell apoptosis7. It was also shown that the protective effect of estrogen was associated with the Trx-1 and Txnip8, 9. Thus, it’s reasonable to infer that estrogen could protect against CVD through Trx-1/Txnip pathway in OSA patients. Herein, we established the IH mouse and cell models to explore the role of estrogen on IH-induced vascular injury, and its association with Trx-1/Txnip.
Results
Ovariectomy promoted the IH-induced vascular injury
The ovariectomy was conducted to induce the deficiency of estrogen in female adult mice. The derangement, enlargement and proliferation of smooth muscle cells, and the disrupted aortic endothelium were observed in mice treated with IH alone (Fig. 1B and C) or combined with ovariectomy (Fig. 1D). Additionally, the thickening of intima, an early sign of atherosclerosis, presented in mice treated with both chronic IH and ovariectomy (Fig. 1D).
Ovariectomy promoted the IH-induced aortic injury (HE 100×). The morphology of aorta arch by HE staining presented as derangement, enlargement and proliferation of smooth muscle cells and the disrupted endothelium in mice treated with IH alone (B and C) or combined with the estradiol deficiency after ovariectomy (D). The early sign of atherosclerosis, the thickening of intima, was observed in mice with the combined treatment of IH and estradiol deficiency (D). The morphology of aorta in control group was normal (A). IH: intermittent hypoxia. ↙ Endothelial cell; Δ Vascular smooth muscle.
Ovariectomy increased the intima-media thickness (IMT)
IMT is a subclinical biomarker of CVD. We found that the chronic IH treatment increased the IMT of aorta, when compared with the control. The combined treatment of ovariectomy and IH presented increased IMT than IH treatment alone (Fig. 2). These results indicated that estradiol efficiency caused by ovariectomy promoted IH-induced vascular injury.
Ovariectomy increased the IMT of aorta in mice under IH. The chronic IH treatment (IH group) increased the IMT of aorta than that in control group (n = 6, *P < 0.01). In addition, the IH and ovariectomy treatments (OVI group) further significantly increased the IMT, in contrast to the mice treated with IH and sham-operation (n = 6, *P < 0.01). IMT: intima-medial thickness. IH: intermittent hypoxia.
Ovariectomy increased the level of plasma malondialdehyde (MDA)
MDA is generally considered as a biomarker of oxidative stress level, and Superoxide Dismutase (SOD) is one of the most important anti-oxidation substances. We found higher level of MDA in plasma of mice treated with IH, and much more significant in IH mice further treated with ovariectomy (Fig. 3A). Besides, the total SOD activity was significantly lower in mice under chronic IH than the control. No further difference was found in SOD activity caused by ovariectomy (Fig. 3B).
Ovariectomy increased the MDA level under IH model. Compared with control (CON group), higher levels of MDA in plasma and lower level of SOD activity in aorta tissue were induced by IH (IH group) in mice (n = 12, *P < 0.01, A and B, respectively). Besides, the MDA level increased more significantly in mice treated with IH and ovariectomy than in that only treated with IH (n = 12, *P < 0.01, A). MDA: malondialdehyde; SOD: superoxide dismutase.
Estradiol rescued cell apoptosis and oxidative stress in vitro
After 16 hrs of IH exposure, the apoptotic rate of HUVECs increased significantly (Fig. 4A). The apoptosis of HUVECs under IH was completely reduced by estradiol (Fig. 4A). The MDA level in supernatants of HUVECs increased significantly, while the activity of total SOD decreased after IH treatment for 16hrs (Fig. 4B). Estradiol partially rescued the IH-associated increased oxidative stress, as supported by the decreased MDA level and increased activity of SOD (Fig. 4C).
Estradiol reduced the IH-induced cell apoptosis and oxidative stress in vitro. IH significantly increased the apoptotic rate of HVUECs and the MDA level in supernatants, which were completely rescued by estradiol supplement (n = 5, *P < 0.01, A and B, respectively). (C) Besides, estradiol significantly increased the SOD activity which is reduced by the IH treatment (n = 5, *P < 0.01). MDA: malondialdehyde; SOD: superoxide dismutase. E2: estradiol.
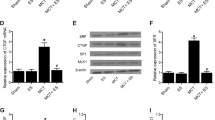
Estradiol increased Trx-1 and decreased Txnip expressions in vitro
The IH induced the expression of Trx-1 mRNA and protein levels, and estradiol further elevated the expression of Trx-1 (Fig. 5A and C). The mRNA and protein levels of Txnip were down-regulated by IH, and estradiol further decreased Txnip expression (Fig. 5B and D).
Estradiol increased Trx-1 and reduced Txnip expressions. (A) The mRNA and protein levels of Trx-1 increased significantly in HUVECs after 16hrs of IH, which were further elevated by estradiol supplement (n = 5, # P < 0.05 and *P < 0.01). (B) IH treatment inhibited the mRNA and protein expressions of Txnip in HUVECs, which were more significant combined with estradiol treatment (n = 5, # P < 0.05 and *P < 0.01).
Knock-down of Txnip decreased IH-induced cell apoptosis and oxidative stress
In order to investigate the effect of Txnip on IH-induced vascular injury, we knocked down Txnip by specific siRNA. It was found that apoptotic rate decreased significantly when treated with Txnip knockdown or estradiol alone and further decreased under treatment of Txnip knockdown and estradiol (Fig. 6A and B). Knock-down of Txnip reduced MDA level and elevated SOD activity, similar to effects of estradiol (Fig. 6C and D). However, the level of MDA and activity of SOD showed no further reduction under treatment of Txnip knockdown combined with estradiol.
Knock-down of Txnip suppressed the apoptosis of HUVECs more significant than E2, while had no difference in oxidative stress. Knock-down of Txnip significantly suppressed the IH-induced apoptosis of HUVECs, and further reduced apoptosis combined with estradiol treatment (n = 5, # P < 0.05 and *P < 0.01, (A and B). The level of MDA decreased (B) and the SOD activity (C) increased when treated with knock-down of Txnip or estradiol (n = 5, # P < 0.05). While, no difference was found in the levels of MDA and SOD between knock-down of Txnip and estradiol treatment (n = 5, ns P > 0.05, (C and D), implying effects of estradiol partially through inhibition of Txnip. siT: siTxnip; siC: cont-siRNA.
Discussion
OSA is considered as an independent risk factor for CVD10. Our study found that the IH, the main characteristic of OSA, increased IMT and contributed to the injury of endothelial cells and proliferation of vessel smooth muscle cells. In vitro, we further confirmed that estradiol treatment reduced the apoptosis of HUVECs.
To explore the effect of estradiol on vascular injury, the ovariectomy was performed to induce the deficiency of estrogen in adult female mice, as reported in previous study11. The increased IMT and vascular injury, regarded as subclinical markers for atherosclerosis, are positive correlated with the increased risk for OSA-related CVD12,13,14,15. These results suggested that estradiol prevented the IH-induced vascular injury, which was consistent with the previous studies16, 17. Estrogen protects against vascular injury and atherosclerosis mainly through directly binding to estrogen receptor-alpha (ERα). In human research, increased level of ERα in the vasculature of pre-menopausal women correlated with low atherosclerosis incidence rates18. The protective role of estrogen against vascular injury diminished after knocking down of ERα in ovariectomized female mice19. In addition, membrane-bound G protein-coupled estrogen receptor (GPER-1) is found to be a regulator of endothelial inflammation, which could also be a potential therapeutic target against atherosclerosis20. Although the effects of estrogen on ovariectomized female mice or the male mice have not been further explored in our study, the cardiovascular effect of estradiol are both seen in male and ovariectomized female animals in previous study21. The estradiol was also found in human to regulate the vascular function in male22. This may be explained by the widely existence of ERs in vascular cells both in female and male.
Further, our results also showed increased MDA level and decreased SOD activity under IH treatment. Besides, estradiol deficiency increased MDA level, which was rescued by estradiol in vitro. MDA is one of the most important products of lipid peroxides, further oxidizing low density lipoprotein (LDL), enhancing the atherosclerosis. Estradiol has been reported to prevent the oxidation of LDL, the disruption of the endothelial barrier initiated by vascular accumulation of modified LDL23 and inhibit macrophage uptake of acetylated LDL24. Although the molecular mechanism of vascular responses to estradiol has not been established well, some evidences show that estradiol protects against vascular injury through reducing oxidative stress25, 26. SOD is an enzyme catalyzing superoxide anion into H2O2 and eliminates the ROS. Estradiol has been found to inhibit NADPH oxidase expression27 and stimulate the expressions of the anti-oxidation substances, such as manganese SOD28 to reduce oxidative stress. It was reported that SOD activity of heart homogenates decreased over time in rats treated with IH29, which is consistent with our finding. Thus, the increased oxidative stress level might be associated with the IH-induced vascular injury, and estradiol is effective in preventing this process.
As a key determinant of cellular sulfhydryl redox homeostasis, Trx has been found to be involved in a variety of diseases. Higher level of Trx-1 was observed in patients with high risk factors for CVD30. In our study, higher level of Trx-1 expression was induced by IH, consistent with previous finding of increased Trx level in OSA patients31. As a negative regulator of Trx, Txnip increased the susceptibility of cell to oxidative stress, resulting in higher rate of cell apoptosis32, 33. The inhibition of Txnip may prevent from cardiac injury, through promoting the antioxidant and anti-apoptosis effects of Trx-134. It was found lower level of Trx and higher level of Txnip in male than in female mice under IH35. In addition, estradiol acted as an antioxidant substance by increasing the expression of Trx and decreasing the expression of Txnip in the uterus of mice9. Yuan et al. also found the increased expression level of Trx in ovariectomized female mice treated with estradiol36. Thus, the increased Trx-1 and decreased Txnip levels might be regulated by estradiol to prevent IH-induced vascular injury.
During the past decade, Txnip has been studied as a potential pharmacotherapeutic target in cardiovascular disease37. In this study, Txnip was knocked down in HUVECs to explore the effect of Txnip on oxidative stress and apoptosis. The results indicated lower MDA level and higher total SOD activity level were achieved by knocking down of Txnip, similar to effects of estradiol treatment. This result suggested estradiol could decrease the oxidative stress through reducing the expression of Txnip. Txnip has been found to induce apoptosis via disrupting the Trx-ASK1 complex, which further actives the ASK1-c-jun-N-terminal kinase (JNK) and p38MAP kinase pathway38. Besides, it also induces the release of pro-inflammatory mediators such as TNF-α or IL-1β, and results in cell cycle arrest37. In view of this, Txnip might be a key regulator during IH-induced vascular injury, and it needs to be further studied.
Limitations should be mentioned in this study. First of all, these findings are lack of studies in male or in ovariectomized female mice treated with estradiol. Secondly, analysis of subgroups of SOD is essential to distinguish the source from mitochondria or cytoplasm. Finally, estradiol receptor mechanisms involved in the regulation of Trx-1/Txnip and oxidative stress need to be explored further.
In summary, the decreased oxidative stress level and apoptosis rate are associated with protection effect of estradiol on vascular injury induced by IH, involving the regulation of Trx-1 and Txnip in this process. Additionally, knock-down of Txnip significantly reduced the cell apoptosis rate, which is partially associated with estradiol. Thus, our study implies that estradiol protects against IH-induced vascular injury, partially through the regulation of Trx-1/Txnip pathway.
Material and Methods
Animal model
The animal study was approved by the Committee for Research and Ethics of Ruijin Hospital, affiliated to Shanghai Jiao Tong University School of Medicine in accordance with NIH guidelines. A total of 48 adult female C57/BL6J mice (aged 6–8 weeks, obtained from Lake Hayes Laboratory Animal Co., Ltd. Shanghai, China) were randomly divided into 4 groups: the ovariectomized IH group (OVI group), the sham-operation IH group (SOI group), IH group and the control group (CON group). Mice in the former 3 groups were exposed to IH (5% O2 for 10 s followed by 21% O2, 40 cycles/h) and mice in CON group were exposed to intermittent air. These mice were exposed to IH or air for 8 hrs/d, and 28 days. Mice in OVI group underwent bilateral ovariectomy under 1.5% isoflurane inhalational anesthesia as described previously39. The SOI group underwent the sham-operation. At day of 29, all mice were euthanatized by cervical dislocation40.
Hematoxylin-eosin staining and IMT measurement of aorta arch
Hematoxylin-eosin (HE) staining of aorta arch tissue followed the methods as described before41. IMT of aorta arch was measured by the image analysis software (Image-Pro Plus6.0, Media Cybernetics, USA). The IMT was measured in two vascular cross sections in 6 samples of each group.
Measurements of SOD activity and MDA
The oxidative stress associated biomarkers include MDA and the activity of SOD. SOD activities in the arterial tissues and in the supernatants of HUVECs, and the levels of MDA in plasma of mice and in the supernatants of HUVECs were measured using SOD assay kit and MDA assay kit (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China) according to the manufacturer’s protocols.
IH exposure and 17β-estradiol treatment in HUVECs
HUVECs (ECV304) (obtained from Blood Research Institute, Shanghai, China) were cultured in DMEM medium supplied with 10% fetal bovine serum (FBS, Gibco, USA) in a humidified incubator under 5% CO2 at 37 °C. The cells were divided into the following three groups: IH group (cells were exposed to IH), IH + E2 group [cells were exposed to IH with the treatment of 10−7mol/L 17β-estradiol (Sigma, USA)], and control (CON) group. IH exposure (1% O2 for 5 min followed by 21% O2 for 5 min, 6 cycles/h, and 16 hrs) was controlled by a self-designed computer program, while CON group was treated with intermittent air. Cells used in vitro experiments were from the same batch, and the results were repeated for five times.
Measurement of cell apoptosis
HUVECs were digested by 0.25% trypsin after removing the supernatants. Apoptosis rate was tested by flow cytometer (BD, USA) using Annexin V-FITC Apoptosis Detection Kit (BD, USA) according to manufacturer’s protocol.
RNA extraction and quantitative real-time PCR
Total RNAs were extracted from HUVECs using trizol (Gibco, USA). cDNAs were synthesized using a reverse transcriptional system. Quantitative real-time PCR (qRT-PCR) was performed using SYBR (Takara Bio, Dalian, China) according to the manufacturer’s instructions. The values were normalized to the level of β-actin. The primers used in qRT-PCR were listed as follows:
Trx-1 F: 5′- CAA GAT GGT GAA GCA GAT-3′
Trx-1 R: 5′- GTG GCT GAG AAG TCA ACT A-3′
Txnip F: 5′- CCG AGC CAG CCA ACT CAA G-3′
Txnip R: 5′- ACA CCC GCC CAT CAG GAA T-3′
β-actin F: 5′- AAG GTG ACA GCA GTC GGT T-3′
β-actin R: 5′-TGT GTG GAC TTG GGA GAG G-3′
Western blotting
HUVECs were washed with PBS, harvested by lysis buffer (Beyotime, China), and then centrifuged at 1500 × g at 4 °C for 15 mins. The concentration of total proteins was measured by bicinchoninic acid (BCA) kit (Thermo Scientific, USA). Samples (containing 30 µg proteins) were boiled in 2 × loading buffer, separated by a 12% sulfate-polyacrylamide gel (PAGE-SDS), and transferred to a polyvinylpyrrolidone difluoride (PVDF) membrane. The membranes were incubated with antibodies for Trx-1 (Trx-1 rabbit anti-human polyclonal antibody, 1:1,000 dilution; Sigma, USA), Txnip (Txnip mouse anti-human polyclonal antibody,1:500 dilution; Novus, USA), and GADPH (GADPH rabbit anti-human polyclonal antibody,1:1,000 dilution; Sigma, USA) overnight at 4 °C after blocking with phosphate saline buffer containing 0.1% (v/v) Tween and 5% (w/v) dried milk. Then the membranes were washed for 3 times and incubated with second antibodies (goat anti-rabbit IgG, 1:1,000 dilution; Sigma, USA; goat anti-mouse IgG, 1:1,000 dilution; Sigma, USA; respectively) for 1 hr at room temperature. After washing for 3 times, membranes were incubated with chemiluminescence (ECL, Thermo Scientific, USA) for 1 min before exposure to the medical X-ray films.
Transfection with siRNAs
HUVECs were seeded in 6-well plates 24 hrs before transfection. Control small interfering RNA (cont-siRNA) or specific siRNA against Txnip (siTxnip) were transfected by Lipofectamine 2000 (Invitrogen, UK). The sequence of cont-siRNA was 5′-UUC UCC GAA CGU GUC ACG UTT-3′, and the sequence of siTxnip was 5′-GCC CUU AGG AUC CUG GCU UTT-3′. Forty-eight hours after transfection, cells were exposed to IH (IH + cont-siRNA group and IH + siTxnip group), or IH with the treatment of 17β-estradiol (IH + cont-siRNA + E2 group and IH + siTxnip + E2 group). The apoptosis rate and oxidative stress level were detected as mentioned before.
Statistical Analysis
SPSS 20.0 (SPSS, Chicago, IL, USA) software was used for data analysis. Measurement data was showed as Mean ± SEM. For the normally distributed data, one-way ANOVA was used for comparison among multiple groups and LSD post hoc test was performed for comparison between two groups. For the data that were not normal distribution, Kruskal-Wallis H rank sum test was performed for the comparisons among multiple groups. P value < 0.05 was considered as statistically significant.
References
Young, T. et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 328, 1230–1235 (1993).
Christou, K., Moulas, A., Pastaka, C. & Gourgoulianis, K. I. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 4, 225–228 (2003).
Eisele, H. et al. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxid Med Cell Longev. 2015, 608438, doi:10.1155/2015/608438 (2015).
Marin, J. M., Carrizo, S. J., Vicente, E. & Agusti, A. G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 365, 1046–1053 (2005).
Stampfer, M. J. et al. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 313, 1044–1049 (1985).
Grady, D. et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 117, 1016–1037 (1992).
Sudoh, N. et al. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation. 103, 724–729 (2001).
Satoh, M. et al. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 115, 3197–3204 (2007).
Deroo, B. J., Hewitt, S. C., Peddada, S. D. & Korach, K. S. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology. 145, 5485–5492 (2004).
Drager, L. F. et al. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 140, 534–542, doi:10.1378/chest.10-2223 (2011).
Matsubara, K. et al. Estrogen deficiency attenuates neovascularization in a murine model of hindlimb ischemia. J Surg Res. 178, 1022–1028, doi:10.1016/j.jss.2012.04.067 (2012).
Fathi, R., Haluska, B., Isbel, N., Short, L. & Marwick, T. H. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 43, 616–623 (2004).
El Solh, A. A., Akinnusi, M. E., Baddoura, F. H. & Mankowski, C. R. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med. 175, 1186–1191 (2007).
Salepci, B. et al. The effect of obstructive sleep apnea syndrome and snoring severity to intima-media thickening of carotid artery. Sleep Breath. 19, 239–246, doi:10.1007/s11325-014-1002-0 (2015).
Ciccone, M. M. et al. Is there a correlation between OSAS duration/severity and carotid intima-media thickness? Respir Med. 106, 740–746, doi:10.1016/j.rmed.2011.12.016 (2012).
Ejima, K. et al. 17beta-estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol. 140, 608–613 (1999).
Matsushima, S. et al. Application of recombinant thioredoxin1for treatment of heart disease. J Mol Cell Cardiol. 51, 570–573, doi:10.1016/j.yjmcc.2010.09.020 (2011).
Losordo, D. W., Kearney, M., Kim, E. A., Jekanowski, J. & Isner, J. M. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 89, 1501–1510 (1994).
Pare, G. et al. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 90, 1087–1092 (2002).
Chakrabarti, S. & Davidge, S. T. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS One. 7, e52357, doi:10.1371/journal.pone.0052357 (2012).
Geary, G. G., Krause, D. N. & Duckles, S. P. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 279, H511–519 (2000).
Lew, R., Komesaroff, P., Williams, M., Dawood, T. & Sudhir, K. Endogenous estrogens influence endothelial function in young men. Circ Res. 93, 1127–1133 (2003).
Gardner, G., Banka, C. L., Roberts, K. A., Mullick, A. E. & Rutledge, J. C. Modified LDL-mediated increases in endothelial layer permeability are attenuated with 17 beta-estradiol. Arterioscler Thromb Vasc Biol. 19, 854–861 (1999).
Sulistiyani, S. T. & Clair, R. W. Effect of 17 beta-estradiol on metabolism of acetylated low-density lipoprotein by THP-1 macrophages in culture. Arterioscler Thromb Vasc Biol. 17, 1691–1700 (1997).
Topçuoglu, A. et al. Effects of estrogens on oxidative protein damage in plasma and tissues in ovariectomised rats. Clin Invest Med. 32, E133–E143 (2009).
Dubey, R. K. et al. Estrogen and tamoxifen metabolites protect smooth muscle cell membrane phospholipids against peroxidation and inhibit cell growth. Circ Res. 84, 229–239 (1999).
Wagner, A. H., Schroete, M. R. & Hecker, M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 15, 2121–2130 (2001).
Stirone, C., Duckles, S. P., Krause, D. N. & Procaccio, V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 68, 959–965 (2005).
Zhou, W. et al. Effects of various degrees of oxidative stress induced by intermittent hypoxia in rat myocardial tissues. Respirology. 17, 821–829, doi:10.1111/j.1440-1843.2012.02157.x (2012).
Miwa, K. et al. Serum thioredoxin and alpha- tocopherol concentrations in patients with major risk factors. Circ J. 69, 291–294 (2005).
Takahashi, K. et al. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxid Redox Signal. 10, 715–726, doi:10.1089/ars.2007.1949. (2008).
Junn, E. et al. Vitamin D3 upregulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 164, 6287–6295 (2000).
Patwari, P., Higgins, L. J., Chutkow, W. A., Yoshioka, J. & Lee, R. T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 281, 21884–21891 (2006).
Wang, Y., De Keulenaer, G. W. & Lee, R. T. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 277, 26496–26500 (2002).
Li, Q. Y. et al. Chronic intermittent hypoxia induces thioredoxin system changes in a gender-specific fashion in mice. Am J Med Sci. 343, 458–461, doi:10.1097/MAJ.0b013e318235b03e (2012).
Yuan, L. et al. 17β-Estradiol alters oxidative stress response protein expression and oxidative damage in the uterus. Mol Cell Endocrinol. 382, 218–226, doi:10.1016/j.mce.2013.09.023 (2014).
Chong, C. R. et al. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther. 28, 347–360, doi:10.1007/s10557-014-6538-5 (2014).
Yu, Y. et al. Overexpression of thioredoxin-binding protein 2 increases oxidation sensitivity and apoptosis in human lens epithelial cells. Free Radic Biol Med. 57, 92–104, doi:10.1016/j.freeradbiomed.2012.12.022 (2013).
Buys, E. S. et al. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res. 79, 179–186, doi:10.1093/cvr/cvn068 (2008).
Roustan, A. et al. Evaluating methods of mouse euthanasia on the oocyte quality: cervical dislocation versus isoflurane inhalation. Lab Ani. 46, 167–169, doi:10.1258/la.2012.011115 (2012).
Zheng, L. et al. A model of spontaneous mouse mammary tumor for human estrogen receptor- and progesterone receptor-negative breast cancer. Int J Oncol. 45, 2241–2249, doi:10.3892/ijo.2014.2657 (2014).
Acknowledgements
This research was supported by National Natural Science Foundation of China Grants 81070068, 81270148, and 81570082. We also would like to thank the colleagues in Orthopedics Department of Ruijin Hospital, who assisted in animal feeding.
Author information
Authors and Affiliations
Contributions
The study was designed by Xiao Fei Lan, Xiu Juan Zhang, Hua Jun Xu, and Qing Yun Li. The experiments were performed by Xiao Fei Lan, Xiu Juan Zhang, Qiong Wang, Hua Jun Xu, and Li Na Zhou. Data were analyzed by Xiao Fei Lan, Xiu Juan Zhang, Ying Ni Lin, Li Na Zhou, and Qing Yun Li. The manuscript was written, edited and revised by Xiao Fei Lan, Xiu Juan Zhang, Ying Ni Lin, Pei Li Chen, and Qing Yun Li. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, X.F., Zhang, X.J., Lin, Y.N. et al. Estradiol Regulates Txnip and Prevents Intermittent Hypoxia-Induced Vascular Injury. Sci Rep 7, 10318 (2017). https://doi.org/10.1038/s41598-017-10442-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10442-7
This article is cited by
-
Prospect of thioredoxin as a possibly effective tool to combat OSAHS
Sleep and Breathing (2023)
-
Same total normal forms sperm counts of males from Lhasa and Shanghai, China
Environmental Science and Pollution Research (2022)
-
Beneficial effects of estrogens in obstructive sleep apnea hypopnea syndrome
Sleep and Breathing (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.