Abstract
Euphausia pacifica is a good candidate for a resource of marine n-3 PUFA. However, few reports exist of the lipid and fatty acid composition of E. pacifica. To examine the potential of E. pacifica as a resource of marine n-3 PUFA, we analyzed E. pacifica oil. We extracted lipids from E. pacifica harvested from the Pacific Ocean near Sanriku, Japan. Lipid classes of E. pacifica oil were analyzed by TLC-FID and the fatty acid composition of the oil was analyzed by GC/MS. Free fatty acids and hydroxy-fatty acids were analyzed by LC/QTOFMS. The lipid content of E. pacifica ranged from 1.30% to 3.57%. The ratios of triacylglycerols, phosphatidylcholine, phosphatidylethanolamine and free fatty acids in E. pacifica lipids were 5.3–23.0%, 32.6–53.4%, 8.5–25.4% and 2.5–7.0%, respectively. The content of n-3 PUFA in E. pacifica lipids was 38.6–46.5%. We also showed that E. pacifica contains unusual fatty acids and derivatives: C16-PUFAs (9,12-hexadecadienoic acid, 6,9,12-hexadecatrienoic acid and 6,9,12,15-hexadecatetraenoic acid) and hydroxy-PUFAs (8-HETE and 10-HDoHE). E. pacifica is a good resource of marine n-3 PUFA. Moreover, E. pacifica can provide C16-PUFA and hydroxy-PUFAs.
Similar content being viewed by others
Introduction
The common term ‘krill’ refers to Euphausiids, a group of small crustaceans that comprises over 80 species. Euphausiids are widespread in oceans worldwide and are dominant organisms in zooplankton populations. Euphausiids are important links in the marine food chain because they are omnivorous, representing the first trophic level. Euphausia pacifica (Pacific krill) is the most common krill species in the North Pacific Ocean. E. pacifica is also found across the Bering Sea, through the southern part of the Sea of Okhotsk and the Sea of Japan, extending southwards to around 30 °N. The southern limit for E. pacifica is at the 9.5 °C isotherm at a depth of 200 m1. E. pacifica is one of the few commercially harvested Euphausiids; however, more than 30,000 tons are harvested every year in Japan alone.
Epidemiological and clinical studies have shown various health benefits from the consumption of marine n-3 polyunsaturated fatty acids (PUFAs), which include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)2,3,4. The effects of marine n-3 PUFAs on various risk factors of cardiovascular disease are in particularly well documented4. Reports on these health benefits have led to an increase in demand for products containing marine n-3 PUFAs. At present, the main source of marine n-3 PUFAs is fish oil. However, fish oil is a restricted resource and therefore other sources of marine n-3 PUFAs are required. In terms of n-3 marine PUFA content and biomass, krill oil is one of the most suitable resources of marine n-3 PUFAs5,6,7. Krill oil is not only an adequate substitute for fish oil, it also contains substances not found in fish oil, such as the phospholipid form of n-3 PUFA and the antioxidant astaxanthin. Because the phospholipid form of n-3 PUFA is incorporated into plasma faster than the triglyceride form, krill oil increases omega-3 index at a lower dose in humans5, 6. At the present, krill oil usually refers only to Antarctic krill (Euphausia superba) oil; however, it has been reported that Pacific krill oil contains a large amount of n-3 PUFA8 and is therefore a good candidate for a resource of marine n-3 PUFA.
Recently, we identified 5-, 8-, 9-, 12-, and 18-hydroxyeicosapentaenoic acid (HEPEs) as ligands of peroxisome proliferator-activated receptors (PPARs) in dried E. pacifica 9. Next, we examined whether HEPEs were naturally contained in E. pacifica or produced by the drying process, and found that fresh E. pacifica or frozen E. pacifica stored at temperatures lower than −30 °C contained only 8-HEPE. Thus, 8-HEPE is the only HEPE contained naturally in E. pacifica, and other HEPEs (5-, 9-, 12-, and 18-HEPE) are by-products of the drying process. We also found that 8-HEPE reduces plasma and hepatic triglycerides in high-fat diet-induced obese mice10. Saito et al. reported that C16-polyunsaturated fatty acids (C16:2, C16:3, C16:4) are contained in E. pacifica 8. 8-HEPE and C16-polyunsaturated fatty acid are rare lipids and have scarcely been reported in other marine animals. These could suggest that krill have a unique system of lipid metabolism. However, the lipid metabolism in krill remains obscure. In this study, to evaluate E. pacifica oil as a resource of marine n-3 PUFA and to determine the fatty acid and hydroxy-fatty acid compositions in E. pacifica, we analyzed E. pacifica lipids and thereby identified C16-polyunsaturated- and hydroxy-fatty acids.
Results
Lipid content and lipid classes of E. pacifica
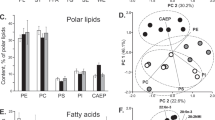
We extracted lipid from E. pacifica, E. superba, Balanus rostratus Hoek, and Marsupenaeus japonicus, and analyzed lipid class composition. The lipid content and lipid class composition of each species are summarized in Table 1. The lipid content of E. pacifica ranged from 1.30% to 3.57%. The E. pacifica lipids contained mainly triacylglycerol (TG, 5.3–23.0%), diacylglycerol (DG, 5.2–15.2%), phosphatidylcholine (PC, 32.6–53.4%), phosphatidylethanolamine (PE, 8.5–25.4%) and free fatty acids (FA, 2.5–7.0%). The lipid content in E. pacifica increased from February to April, as the lipid content increased, the TG composition in E. pacifica lipid also rose. The lipid content was higher in E. superba than in E. pacifica; especially, the amount of TG was high in E. superba.
Fatty acid composition in E. pacifica lipid
The fatty acid composition of lipid from E. pacifica, E. superba, Balanus rostratus Hoek, and Marsupenaeus japonicus is summarized in Table 2. Palmitic acid, EPA and DHA were found to be the major fatty acids, these three fatty acid together accounted for more than 60% of the fatty acid composition of E. pacifica lipid. From February 26 to April 24 in 2017, the relative composition of palmitic acid and palmitoleic acid increased, whereas that of DHA decreased. In E. pacifica lipid, the population of marine n-3 PUFA was more than 35%, which was higher than that in E. superba lipid, Balanus rostratus Hoek lipid, and Marsupenaeus japonicus lipid.
Free fatty acids in E. pacifica
Saito et al. reported that E. pacifica oil contained five saturated fatty acids, nine monounsaturated fatty acids and thirteen polyunsaturated fatty acids. To examine the fatty acids contained in E. pacifica, we analyzed fatty acids by LC/QTOFMS. We detected four saturated fatty acids, two monounsaturated fatty acids and twelve polyunsaturated fatty acids as free fatty acids in E. pacifica. The carbon lengths of the fatty acids detected in E. pacifica were C12, C14, C16, C18, C20 and C22. In C12 and C14 fatty acids, only saturated fatty acid was detected. In C16 fatty acid, five types of fatty acid, each with different degrees of unsaturation, were detected (Fig. 1). Palmitic acid (C16:0) and palmitoleic acid (C16:1(9)) were identified using standards. To identify the C16:2, C16:3 and C16:4 fatty acids predicted by exact mass, we purified these fatty acids from E. pacifica. The methylated fatty acids were then analyzed by GC/MS. We searched for the detected ion fragment patterns in the NIST library and identified the C16:2, C16:3 and C16:4 fatty acids as 9,12-hexadecadienoic acid (C16:2 (6,9)) (Fig. 2), 6,9,12-hexadecatrienoic acid (C16:3 (6,9,12)) (Fig. 3) and 6,9,12,15-hexadecatetraenoic acid (C16:4 (6, 9, 12, 15)), respectively (Fig. 4). In C18 fatty acid, six types of fatty acid were detected. Stearic acid (C18:0), oleic acid (C18:1 (9)), linoleic acid (C18:2 (9,12)), alpha-linoleic acid (C18:3 (9, 12, 15)) and stearidonic acid (C18:4 (6, 9, 12, 15)) were identified using standards. The other fatty acid predicted to be C18:5 by exact mass was purified and analyzed using GC/MS. However, we did not find any compound with the same ion fragment pattern in the NIST library. The compound with the most similar ion fragment pattern in the NIST library was DHA. In C20 and C22 fatty acids, four types of fatty acid dihomo-gamma linoleic acid (C20:3 (8,11,14)), arachidonic acid (C20:4 (5,8,11,14)), EPA (C20:5 (5,8,11,14,17)), and DHA (C22:6 (4,7,10,13,16,19)) were detected.
Extract chromatogram of free fatty acids contained in E. pacifica. Fatty acids in E. pacifica oil were analyzed by LC/QTOFMS. Fatty acids were predicted form the extracted ionized compounds by exact mass and molecular formula, and compounds were confirmed by using fatty acid standards. The compounds identified by using standards are shown with their molecular formula and structure. The compounds that were not identified by standards are shown with molecular formula only.
Structure analysis of 9,12-hexadecadienoic acid. We purified and analyzed the compound predicted as a C16H28O2 fatty acid. The exact mass and molecular formula of the compound were identified by using LC/QTOFMS. Fatty acid structure was identified by GC/MS. (A) Mass to charge (m/z) acquisition by LC/QTOFMS. (B) Mass spectra acquisition by GC/MS. (C) The result to access mass spectra for NIST2.0 library.
The quantities of free fatty acids detected in E. pacifica are shown in Table 3. The major free fatty acids in E. pacifica were palmitic acid, oleic acid, EPA, and DHA. The content of palmitoleic acid, oleic acid, arachidonic acid and EPA in E. pacifica was high in samples collected on April 24 2017. In a comparison of E. pacifica with E. superba, the palmitoleic acid and arachidonic acid content was clearly higher in E. pacifica, whereas the palmitic acid was higher in E. superba than in E. pacifica. As compared with Balanus rostratus Hoek and Marsupenaeus japonicus, the EPA and DHA content was much higher in E. pacifica.
Hydroxy-fatty acids in E. pacifica
We identified 8-HEPE as a PPAR ligand from E. pacifica in a previous study. In this study, to examine whether E. pacifica contains other hydroxy-fatty acids, we analyzed E. pacifica extracts by LC/QTOFMS. We searched for hydroxy-fatty acids in E. pacifica extracts by exact mass and estimated molecular formulas and found the potential compounds HETE (m/z = 319.228, RT = 8.6) and HDoTE (m/z = 343.228, RT = 8.3) (Fig. 5A–D). To identity these compounds, we analyzed the MS/MS fragments and compared them with HETEs and HDoTE standards (Fig. 5E–H). As a result, we determined that both 8-HETE and 10-HDoHE are present in E. pacifica. The quantities of hydroxy-fatty acids and 8-HEPE in E. pacifica are summarized in Table 4. The content of 8-HEPE gradually increased from February to April. In E. pacifica, the 8-HEPE content was higher than that of 8-HETE and 10-HDoHE. Furthermore, the 8-HEPE content was much higher in E. pacifica than in E. superba, Balanus rostratus Hoek or Marsupenaeus japonicus
8-HEPE, 8-HETE and 10-HDoHE share a common hydroxyl at the n-12 carbon position. To examine whether 8-HEPE is produced from EPA by enzymatic oxidation, we incubated fresh or boiled E. pacifica protein with EPA and determined the amount of 8-HEPE (Fig. 6). We found that 8-HEPE was produced by incubating E. pacifica protein with EPA, and the amount of 8-HEPE produced was significantly decreased by heating E. pacifica protein at 95 °C for 5 min.
8-HEPE is produced from EPA by an enzymatic reaction. BSA, E.pacifica protein or boiled E pacifica protein (50 µg) was incubated with 2 nmol of EPA at 20 °C for 1 hour. The concentration of 8-HEPE produced was measured by LC/QTOFMS. Values are the mean and s.d. of five individual experiments. ** indicates p < 0.01.
Discussion
In this study, we showed that the oil content of E. pacifica was 1.30–3.57%, approximately half of which consisted of phosphatidylcholine and phosphatidylethanolamine. Palmitic acid, EPA, and DHA were the major fatty acids that compromised the E. pacifica oil. The content of n-3 PUFAs was 38.6–46.5% (Table 2). In previous reports, the lipid content of E. pacifica caught near the Ogasawara Coast, Miyagi, and Hokkaido was determined as 0.59–1.75%, 3.34–6.41%, and 1.1–3.2%, respectively, on a wet weight basis8, 11. In each area, the lipid content of E. pacifica was high in spring (March – May) as compared with any other season. In spring, phospholipids accounted for more than 40% of the lipid content of E pacifica, and the content of n-3 PUFA was more than 29%. These results indicate that E. pacifica lipid composition has remained stable for at least the past 20 years, and that E. pacifica caught in spring is a good resource of the phospholipid form of n-3 PUFA.
It has been shown in a previous study that E. pacifica contains C16-PUFAs (C16:2 n-4, C16:3 n-4 and C16:4 n-1)8, however, chemical structure of these C16-PUFAs were not identified. In this study, we showed that the C16-PUFAs contained in E. pacifica were 9,12-hexadecadienoic acid, 6,9,12-hexadecatrienoic acid and 6,9,12,15-hexadecatetraenoic acid (Figs 2–4). C16-PUFAs are unusual fatty acids and are not present in E. superba or Euphausia crystallorophias 12. 9,12-Hexadecadienoic acid and 6,9,12-hexadecatrienoic have been reported in Phaeodactylum ticornutum 13 and Acanthameba castellanii 14. 6,9,12,15-Hexadecatetraenoic acid has been reported only in marine phytoplankton12 and Acanthameba castellanii 14. It is not certain whether the C16-PUFAs are synthesized in E. pacifica or derived from phytoplankton. This question will be answered when the E. pacifica genome is decoded.
We previously reported that E. pacifica contains 8-HEPE9. In this study, we demonstrated that E. pacifica also contains 8-HETE and 10-HDoTE (Fig. 5). Prior to our previous study9, hydroxy-PUFAs had not been reported in krill. However, prostanoids, oxylipins, cyclooxygenase and lipoxigenase had been reported in crustaceans, and some biological functions have been predicted15,16,17,18. Our present results indicate that E. pacifica has a lipoxygenase that can oxidize the n-12 carbon of EPA, AA, and DHA (Fig. 6). The 8-HEPE contents in E. pacifica was increased from February to April (Table 3); thus, it seems that 8-HEPE might contribute to E. pacifica growth and maturation. Identification of the E. pacifica lipoxygenase and biochemical analysis will clarify the biological function of hydroxy-PUFAs. In addition, the identification of 8-HEPE synthetic pathways in E. pacifica might provide a way to produce 8-HEPE artificially.
Conclusions
To meet the continuing increasing demand for marine n-3 PUFA globally, E.pacifica might represent a new resource of the phospholipid form of n-3 PUFA. Moreover, E. pacifica can provide unusual fatty acids, C16-PUFA and hydroxy-PUFAs that cannot be obtained from other resources.
Materials and Methods
Materials
E. pacifica was purchased from Kawashu Inc. (Iwate, Japan) and Kokuyou Inc. (Iwate, Japan), and was originally harvested from the Pacific Ocean near Iwate, Japan. E. superba was purchased from HiromatsuKyu Fishery Co.,Ltd (Fukuoka, Japan). Balanus rostratus Hoek was purchased directly from a fisherman culture in Ofunato, Iwate, Japan. Marsupenaeus japonicus was purchased from a local fisherman in Aichi, Japan. Fatty acids, HEPEs, hydroxyeicosatetraenoic acids (HETEs) and hydroxy docasahexaenoic acids (HDoHEs) were purchased from Cayman Chemical (Ann Arbor, MI). Dipalmitoyl Phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, glyceryl tripalmitate, DL-a-palmitin and 1-palmitoyl-sn-glycero-3-phosphocoline were purchased from Sigma-Aldrich (St. Louis, MO).
Lipid extraction and the analysis of lipid classes
Lipids were extracted from E. pacifica, E. superba, Balanus rostratus Hoek, and Marsupenaeus japonicus according to the Bligh-Dyer method19. Lipid classes were analyzed by silica gel thin-layer chromatography (TLC). First, 5 µg of lipid extract was applied to a Chromarod (LSI Medience, Tokyo, Japan). Next, chloroform/methanol/water (42:24:2.5) was drawn 5 cm up the Chromarod. After drying the Chromarod, hexane/diethyl ether (50:30) was drawn 10 cm up the Chromarod. Lipids were detected and measured by TLC-FID (LSI Medience). Dipalmitoyl Phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, glyceryl tripalmitate, DL-a-palmitin and 1-palmitoyl-sn-glycero-3-phosphocoline were used as lipid standards.
Gas chromatography/mass spectrometry (GC/MS)
Triacylglycerol (TAG) and phospholipid (PL) were esterified by incubation with methanol containing 5% hydrochloric acid at 50 °C for 30 min. Analysis of the fatty acid methyl esters was performed on a gas chromatography (Agilent Technologies 6890 N)/mass spectrometry (Agilent Technologies 5975B) system using with a DB-23 gas chromatography column (60 m × 0.25 mm i.d. and 0.15-µm film thickness [Agilent technologies]). Helium (carrier gas) was passed through the column at a constant linear velocity of 40.0 mL/min, and the split ratio was 50. The initial oven temperature was maintained at 50 °C for 1 min, increased to 175 °C at a rate of 25 °C/min, increased to 230 °C at a rate of 4 °C/min, and then maintained for 5 min. The temperatures of the inlet, interface, and ion source were 250 °C, 280 °C and 230 °C, respectively. Electron impact (EI, 70 eV) was used as the ionization mode.
MS data were analyzed with Agilent GC/MSD ChemStation software. MS fragment data were searched for in NIST atomic spectra database 2.0.
Liquid chromatography/hybrid quadrupole time of flight mass spectrometry (LC/QTOFMS)
HEPEs, HETEs and HDoTEs were separated on an InertSustain ODS-3 column (2.0 mm dia. × 250 mm; GL Science Inc.) with gradient elution (water containing 10 mM ammonium acetate/acetonitrile, 55/45 to 5/95 in 25 min) at a flow rate of 0.2 mL/min. The temperature of the column was kept at 40 °C. The compounds were identified and quantified by LC/QTOFMS (Agilent Technologies) using Agilent Mass Hunter Workstation Software (Agilent Technologies). The velocity of the drying gas was 10 L/min. The temperature of the gas was 325 °C. The voltages of Vcap, fragmentor, and skimmer were 3500, 125 and 65 V, respectively. The pressure of the nebulizer was 30 psig.
E. pacifica protein extraction and enzyme reaction
E. pacifica was homogenized in a 3-fold higher volume of Tris-HCl buffer (pH 7.4) on ice and centrifuged at 20,000 g for 10 min at 4 °C. Next, E. pacifica protein in the liquid layer was concentrated by Amicon Ultra 10 K (Merck Millipore). The concentration of E. pacifica protein was measured by Coomassie Plus Reagent (Thermo Scientific), and then 50 µg of E. pacifica protein and 2 nmol EPA were incubated in 20 µL of Tris-HCl (pH7.4) buffer at 20 °C for 1 hour. After the enzymatic reaction, 60 µL of acetonitrile containing 1% of formic acid was added to the samples and mixed. Next, the samples were centrifuged at 20,000 g for 10 min at room temperature. The 8-HEPE concentration of the liquid layer was measured by LC/QTOFMS.
References
Everson, I. Krill: biology, ecology and fisheries. (John Wiley & Sons, 2008).
Balk, E. M. et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189, 19–30 (2006).
Harris, W. S. n-3 fatty acids and serum lipoproteins: human studies. Am. J. Clin. Nutr. 65, 1645S–1654S (1997).
Lichtenstein, A. H. et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American heart association nutrition committee. Circulation 114, 82–96 (2006).
Ulven, S. M. et al. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 46, 37–46 (2011).
Ramprasath, V. R., Eyal, I., Zchut, S. & Jones, P. J. H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 12, 178 (2013).
Yurko-Mauro, K. et al. Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 14, 99 (2015).
Saito, H. et al. High levels of n-3 polyunsaturated fatty acids in Euphausia pacifica and its role as a source of docosahexaenoic and eicosapentaenoic acids for higher trophic levels. Mar. Chem. 78, 9–28 (2002).
Yamada, H. et al. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J. Lipid Res. 55, 895–904 (2014).
Yamada, H. et al. 8-Hydroxyeicosapentaenoic Acid Decreases Plasma and Hepatic Triglycerides via Activation of Peroxisome Proliferator-Activated Receptor Alpha in High-Fat Diet-Induced Obese Mice. J. Lipids 2016, 7498508 (2016).
Kusumoto, N., Ando, Y., Matsukura, R. & Mukai, T. Lipid Profile of Krill Euphausia pacifica Collected in the. J. Oleo Sci. 53, 45–51 (2004).
Bottino, N. R. The fatty acids of Antarctic phytoplankton and euphausiids. Fatty acid exchange among trophic levels of the Ross Sea. Mar. Biol. 27, 197–204 (1974).
Domergue, F. et al. New insight into Phaeodactylum tricornutum fatty acid metabolism. Cloning and functional characterization of plastidial and microsomal delta12 fatty acid desaturases. Plant Physiol. 131, 1648–1660 (2003).
Sayanova, O. et al. A bifunctional Δ12,Δ15-desaturase from Acanthamoeba castellanii directs the synthesis of highly unusual n-1 series unsaturated fatty acids. J. Biol. Chem. 281, 36533–36541 (2006).
Kuo, J., Pan, B. S., Zhang, H. & German, J. B. Identification of 12-lipoxygenase in the hemolymph of tiger shrimp (Penaeus japonicus Bate). J. Agric. Food Chem. 42, 1620–1623 (1994).
Steel, D. J., Tieman, T. L., Schwartz, J. H. & Feinmark, S. J. Identification of an 8-lipoxygenase pathway in nervous tissue of Aplysia californica. J. Biol. Chem. 272, 18673–18681 (1997).
Maskrey, B. H., Taylor, G. W. & Rowley, A. F. The identification and role of a novel eicosanoid in the reproductive behaviour of barnacles (Balanus balanus). J. Exp. Biol. 209, 558–66 (2006).
Wimuttisuk, W. et al. Insights into the Prostanoid Pathway in the Ovary Development of the Penaeid Shrimp Penaeus monodon. PLoS One 8, 1–15 (2013).
BLIGH, E. G. & DYER, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–7 (1959).
Acknowledgements
This work was supported by funds from the Basic Biotechnology Project of Iwate Prefecture, Japan, Grants-in-Aid from the Sanriku Foundation, the Center for Revitalization Promotion, Japan Science and Technology Agency and the Project of the NARO Bio-oriented Technology Research Advancement Institution (the special scheme project on regional developing strategy).
Author information
Authors and Affiliations
Contributions
H.Y. designed the research; H.Y., Y.Y., S.K., M.H., N.N., S.Y., A.Y., K.T. and T.O. conducted the research; H.Y., Y.Y. and S.K. analyzed the data; H.Y. wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamada, H., Yamazaki, Y., Koike, S. et al. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica . Sci Rep 7, 9944 (2017). https://doi.org/10.1038/s41598-017-09637-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09637-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.