Abstract
Water stress and hypersensitive response (WHy) domain is typically found as a component of atypical late embryogenesis abundant (LEA) proteins closely associated with resistance to multiple stresses in numerous organisms. Several putative LEA proteins have been identified in Deinococcus bacteria; however their precise function remains unclear. This work reports the characterization of a Deinococcus-specific gene encoding a novel WHy domain-containing hydrophobic LEA5C protein (named DrwH) in D. radiodurans R1. The expression of the drwH gene was induced by oxidative and salinity stresses. Inactivation of this gene resulted in increased sensitivity to oxidative and salinity stresses as well as reduced activities of antioxidant enzymes. The WHy domain of the DrwH protein differs structurally from that of a previously studied bacterial LEA5C protein, dWHy1, identified as a gene product from an Antarctic desert soil metagenome library. Further analysis indicated that in E. coli, the function of DrwH is related to oxidative stress tolerance, whereas dWHy1 is associated with freezing-thawing stress tolerance. Under oxidative stress induced by H2O2, DrwH protected the enzymatic activities of malate dehydrogenase (MDH) and lactate dehydrogenase (LDH). These findings provide new insight into the evolutionary and survival strategies of Deinococcus bacteria under extreme environmental conditions.
Similar content being viewed by others
Introduction
Bacteria of the genus Deinococcus are among the most radiation-resistant organisms found on Earth. In particular, the Deinococcus radiodurans strain R1 was the first identified deinobacteria exhibiting remarkable resistance to a variety of stresses, including gamma radiation, UV light, desiccation, heat, and oxidation1,2,3. Resistance to ionizing radiation observed in Deinococcus may results from its ability to resist to extreme desiccation2. It has been postulated that these bacteria possessed specialized genetic systems for DNA repair and response to stresses to account for their extreme resistance3,4,5. Other hypotheses proposed that the extreme resistance of Deinococcus was resulting from horizontal transfer of genes. The analysis of the D. radiodurans genome revealed the existence of at least 15 genes likely transferred horizontally from unexpected sources, such as eukaryotes and viruses3. Moreover, there is increasing evidence that genes coding for the proteins involved in the radiation-desiccation resistance properties of Deinococcus bacteria might originate from other extremophile bacteria after horizontal transfer6,7,8,9,10. It is particularly noteworthy that three Deinococcus genes (designated dr1372, dr0105 and dr1172) encode homologs of plant desiccation-related late embryogenesis abundant (LEA) proteins3, 7.
LEA proteins, originally found in plants, are a group of heterogeneous proteins accumulating under various stress conditions like drought, salinity, extreme temperature and oxidative stress11,12,13,14,15,16,17. Subsequently several genes encoding putative LEA proteins have been found in bacteria18, 19, such as Bacillus subtilis 20 and D. radiodurans 3, 21. The LEA protein families have diverse structures and functions. Generally, typical LEA proteins are characterized by repeat motifs, structural disorder and high hydrophilicity in their native forms17, 22, 23. In contrast to the typical LEA proteins, the LEA proteins from the group 5 display relatively high hydrophobicity but lack significant signature motifs and are thus considered atypical LEA protein24, 25. In particular, the LEA5C proteins, found in plants, contain a Water stress and Hypersensitive response (WHy) domain, suggesting that they are probably functionally different from typical LEA proteins16, 26, 27. There is only one report on the functional characterization of a bacterial protein containing a WHy domain, dWHy128. The structural gene coding for this protein was identified from an Antarctic desert soil metagenome library and it was found that dWHy1 displayed in vivo protection against cold and freeze damage28.
D. radiodurans has been extensively used as a model bacterium to study the resistance to irradiation and desiccation, but the underlying mechanisms of its extreme resistance are still poorly understood. Several putative LEA proteins have been found in Deinococcus bacteria, but their role has not been experimentally investigated. We report here the characterization of the drwH gene, a Deinococcus-specific LEA5C gene from D. radiodurans R1. The drwH gene encodes a novel hydrophobic protein, named DrwH, containing a novel WHy domain. Expression of the drwH gene was significantly up-regulated in response to oxidative and salinity stresses and its expression was under control of the global regulator IrrE via unknown mechanisms. The drwH mutant displayed increased sensitivity to oxidative and salinity stresses as well as reduced activities of antioxidant enzymes. Secondary structure prediction and enzyme protection assays of the WHy domain from the D. radiodurans DrwH and the bacterial dWHy1 from an Antarctic desert soil metagenome library revealed the evolutionary and functional diversity of the LEA gene family in bacteria under extreme environmental conditions. These results indicated that DrwH protects enzymatic activity from damage caused by oxidative stress, probably contributing to the extreme tolerances of D. radiodurans.
Results
DrwH is a novel hydrophobic LEA5C protein containing a WHy domain
Analysis of the D. radiodurans genome revealed the presence of a 495-bp open reading frame (ORF), dr1372, coding for a putative LEA protein. Phylogenetic analysis indicated that the DR1372 protein and its homologs only found in Deinococcus genomes are clustered into a small subgroup distinct from that of plants and archaea (see Supplementary Fig. S1). DR1372 is rich in hydrophobic residues, such as Leu (13.4%), Ala (11.6%), Val (11.0%), and Pro (9.1%), while lacking Cys and Trp residues. As shown by hydropathy plots29, the grand average hydropathy (GRAVY) value for DR1372 was predicted to be 0.220, suggesting a relatively high level of overall hydrophobicity (Fig. 1A and Supplementary Table S1). Furthermore, bioinformatics analysis revealed the presence of a signal peptide located at the N-terminal end and a WHy domain with an invariant triplet “NPN” motif from the 36th to 136th residues (see Supplementary Fig. S2). The DR1372 protein disorder predictions suggested a small propensity to be an order protein using Cspritz software (Fig. 1B). The predicted hydropathy and ordered characteristics of the truncated DR1372 protein without the signal peptide showed a very high degree of similarity to the intact DR1372 protein (Fig. 1C,D). These results, together with the phylogenetic tree of DR1372 and related protein sequences (see Supplementary Fig. S1), indicate that DR1372 belongs to the LEA5C group. The other LEA5C characterized protein of bacterial origin, dWHy128, also containing a WHy domain, cluster in the Pseudomonas genus subgroup (see Supplementary Fig. S1). A total of 27 Deinococcus proteins had a relatively high similarity (57.2–79.3%) with DR1372. No DR1372 homolog was found in any other bacterial species. In addition, DR1372 had very low similarity (<30%) with the LEA proteins previously identified (see Supplementary Figs S1 and S2). Because we did not identify homologs in any other bacterial species by a BLAST analysis and did not find any match to the LEA families in the Pfam database, DR1372 is likely a novel Deinococcus-specific LEA5C protein containing a WHy domain. We therefore renamed this protein DrwH.
Hydropathy and disorder prediction of intact and truncated DrwH proteins without putative signal peptide. (A) Hydropathic index plot of the deduced DrwH amino acid sequence analyzed using the Kyte–Doolittle algorithm. Regions with a hydropathy score above zero are hydrophobic. (B) Prediction of DrwH disordered regions using Cspritz. Regions with scores above the threshold line are considered to be disordered. (C) Hydropathic index plot of the deduced amino acid sequence of truncated DrwH analyzed using the Kyte–Doolittle algorithm. (D) Prediction of truncated DrwH disordered regions using Cspritz.
The drwH gene is significantly induced by oxidative and salinity stresses
Bacterial LEA proteins are thought to be involved in generalized stress responses. To test the function of the drwH gene, the expression pattern of the drwH gene was examined after exposure to various stresses similar to those encountered under extreme environments for Deinococcus. Cultures of D. radiodurans R1 were subjected to oxidative stress (induced by H2O2), osmotic stress (induced by NaCl and D-sorbitol), heat and cold shocks. Total RNA was isolated and the drwH expression was detected by quantitative real-time PCR (qRT-PCR) analysis. As shown in Fig. 2A, drwH expression was significantly up-regulated in response to oxidative and salinity stresses. After 30 min treatment with 50 mM H2O2, drwH expression was induced by nearly 2.5-fold. In addition, a 2.8-fold increase in response to salt stress was observed. No significant expression changes were detected after treatment with 0.5 M D-sorbitol and different temperature treatments. D. radiodurans genome encodes only one predicted σ70 factor (RpoD/SigA, DR0916), which thus plays a major role in transcription of growth-related genes30. Sequence analysis of the drwH promoter region revealed a presence of a putative typical −35/−10 consensus sequence (TTGAGA-N20-TTTAAT) (Fig. 2C), suggesting that it may be a target site recognized by the σ70 factor. Previous studies have shown that IrrE is a global regulator involved in salt tolerance31, 32. To determine whether drwH is regulated by IrrE, we checked the expression levels of drwH in the D. radiodurans ΔirrE mutant strain previously constructed33. As shown in Fig. 2B, the expression of drwH was down-regulated by 2-fold in the ΔirrE mutant background under salt stress, whereas no significant difference was observed under oxidative stress. These results suggest that the expression of the drwH gene is significantly induced by oxidative and salinity stresses and under the control of IrrE via unknown mechanisms during salinity stress.
Transcriptional analysis of drwH in D. radiodurans. (A) Relative expression level of drwH in response to various stresses in D. radiodurans. Total RNA of D. radiodurans was extracted after exposure to 50 mM H2O2 (30 min), 0.3 M NaCl (2 h), 50 °C (2 h), 15 °C (2 h), and 0.5 M D-sorbitol (2 h). Different letters indicate significant differences (P < 0.05). (B) drwH transcription in D. radiodurans WT and ΔirrE mutant strains under oxidative and salt conditions. Error bars represent the standard error of the mean of 3 independent experiments. The asterisk indicates a significant difference, which was calculated with Student’s t-test (*P < 0.05, NS: no significant difference). (C) Nucleotide sequence of the putative promoter region of drwH. The open boxes represent the putative σ70-dependent promoter. The start and termination codons of the drwH gene are indicated in red bold letters. The 300-bp region containing a predicted WHy domain is highlighted in blue.
Deletion of drwH decreases abiotic stress tolerances of D. radiodurans
The drwH gene, which was found to be significantly up-regulated in response to abiotic stresses (Fig. 2), is most likely involved in the extreme stress tolerance phenotype of D. radiodurans. To assay this hypothesis, a drwH deletion mutant strain (ΔdrwH) was constructed using a kanamycin-resistance-gene (nptII) replacement strategy (see Supplementary Fig. S3)34. To examine whether the drwH gene deletion has an effect on the expression of the flanking genes (dr1371 and dr1373), we compared the expression levels of dr1371 and dr1373 in D. radiodurans WT and ΔdrwH mutant by qRT-PCR. We found that expression of dr1371 and dr1373 was up-regulated by ~5 fold and 2 fold in the ΔdrwH mutant, respectively. This observation suggests that the deletion somehow affected the flanking gene expression, possibly due to the global effect of the drwH gene deletion. The phenotype of the mutant strain is due to the lack of the DrwH protein and consequent changes in the transcription of affected genes, including the upregulation of the expression of dr1371 and dr1373. Then we performed stress tolerance assays as indicated in Fig. 3. The survival growth curves showed that the ΔdrwH mutant strain was more susceptible to increasing H2O2 concentrations than D. radiodurans wild-type (WT) strain (Fig. 3A). Furthermore, the absence of drwH caused a significant decrease in the survival rates of the ΔdrwH mutant strain when the cells were treated with NaCl (ranged from 0 to 5 M). The rate of survival, 5 hours after a 4 M NaCl shock treatment, was only of 5.2% for the ΔdrwH mutant strain while it was of 48.5% for D. radiodurans WT under the same treatment (Fig. 3B). TGY plate assays for oxidative and salinity stresses led to similar findings as shown in Supplementary Fig. S4. In general, LEA proteins accumulate during late embryogenesis in seeds, but it is also expressed in vegetative tissues and its expression can be induced by various abiotic stresses such as desiccation, salinity, extreme temperatures and oxidative stress18, 22, 23, 25. Unexpectedly, the ΔdrwH mutant response to desiccation at 5% humidity of up to 60 days was similar to that of WT (Fig. 3C). These results suggested that the in vivo function of DrwH is related to both oxidative and salinity stress tolerances, but not to desiccation stress.
Survival curves for D. radiodurans WT and ΔdrwH mutant following exposure to H2O2 (A), NaCl (B), and desiccation (C) treatments. Different dilutions of these cells were plated on TGY agar plates and incubated at 30 °C for 3 days before colonies were enumerated. The survival rate was expressed as the percentage of the number of colonies in the treated samples compared with those in untreated controls. All experiments were performed three times and are represented as mean ± standard deviation.
DrwH is required for the expression and activity of antioxidant enzymes
Various detoxifying enzymes, which serve to protect cells from the toxic effects of reactive oxygen species (ROS) generated by oxidative stress, were proposed to contribute to the D. radiodurans extreme resistance phenotype35. We thus assessed the effect of drwH deletion on the expression and activity of ROS scavenging enzymes, such as catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD). Consistent with their oxidative sensitive phenotypes, the ΔdrwH mutant strain showed lower expression and activities of the three ROS scavenging enzymes than did the WT strain after treatment with 50 mM H2O2 for 30 min (Fig. 4A). The CAT, POD and SOD activities showed decreases of 3-, 3.4-, and 1.8-fold, respectively. In addition, two genes (dr1998 and drA0259) encoding CAT, two genes (drA0145 and drA0301) encoding POD, and four genes (dr1279, dr1546, dr0644 and drA0202) encoding SOD were significantly down-regulated in the ΔdrwH mutant strain under oxidative stress, consistent with the decreased activities of the antioxidant enzymes (Fig. 4B). Together, these results suggested that DrwH is required for expression and activity of ROS scavenging enzymes and likely contributes to the extreme tolerances of D. radiodurans.
Expression and catalytic activities of ROS scavenging enzymes in D. radiodruans WT and ΔdrwH mutant strains under oxidative stress. (A) Effects of the drwH deletion on the enzyme activities after treatment with 50 mM H2O2 for 30 min. The asterisk indicates a significant difference, which was calculated with Student’s t-test (*P < 0.05, NS: no significant difference). (B) Effects of drwH deletion on the expression of antioxidant enzyme genes after treatment with 50 mM H2O2 for 30 min. All experiments were performed three times and are represented as mean ± standard deviation. Different letters indicate significant differences (P < 0.05).
Structural and functional analysis of the WHy domain
To date, only two bacterial water hypersensitivity-like proteins, DrwH from D. radiodurans and dWHy1 from an Antarctic desert soil metagenome library, have been experimentally characterized. The sequence similarity of the WHy domains from DrwH and dWHy1 was compared using ClustalX2 software, and they shared only 33.3% sequence similarity (Fig. 5A). Furthermore, the secondary structure prediction indicated that the WHy domain of DrwH structurally differs from that of dWHy1 (Table 1). Since the WHy domain from DrwH showed low sequence similarity to dWHy1, we hypothesized that the DrwH protein may have a different function from dWHy1. Bioinformatics analysis suggested the presence of a predicted signal peptide in both DrwH and dWHy1. Anderson et al. (2015) have previously shown that the survival rates were significantly higher under stress conditions for the E. coli expressing the complete wild-type dWHy1 protein, and even higher when the truncated dWHy1 protein without the predicted signal peptide was expressed28. This is consistent with our observation that expression of the complete wild-type DrwH protein significantly decreased growth of recombinant E. coli, regardless of the presence or absence of H2O2 (see Supplementary Fig. S5). Since expression of wild-type protein with the predicted signal peptide affected E. coli growth, we constructed the truncated protein with an intact WHy domain but without the predicted signal peptide and performed in vivo stress tolerance assays or in vitro enzyme protection assays.
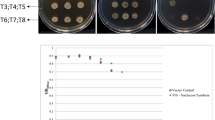
Structural and functional analysis of the WHy-domains from DrwH and dWHy1. (A) Primary structure analysis of the DrwH protein, and the alignment between the WHy-domains from DrwH and dWHy1. (B) Survival phenotype plate assay of the E. coli wild-type strain BL21 and recombinant strains expressing Dr-WHy (truncated DrwH, BL21-1), dW-WHy (truncated dWHy1, BL21-2), and an empty vector (BL21-0) as a control, respectively, after treatment with 15 mM H2O2 for 10 min and two freezing-thawing cycles. (C) and (D) Protective effect of Dr-WHy on MDH and LDH activities after H2O2 treatment. The His-tagged Dr-WHy (truncated-DrwH) protein was used for the in vitro protection assays. Each column represents an average of three independent experiments, and error bars represent standard deviation. The statistical difference was tested by Student’s t-test (P < 0.05). Different letters indicate significant differences.
To test this, we constructed a truncated-DrwH (Dr-WHy) strain BL21-1 and a truncated-dWHy1 (dW-WHy) strain BL21-2, with intact WHy domains but without the predicted signal peptides, and examined their growth under abiotic stresses. After freezing-thawing stress treatment, BL21-2 (dW-WHy) displayed significantly enhanced tolerance to freezing-thawing stress, whereas E. coli wild-type BL21 and BL21-1 (Dr-WHy) strains did not (Fig. 5B). This result is consistent with the previous observation that expression of dWHy1 in E. coli significantly protected the recombinant host against cold and freezing stresses28. Following treatment with 15 mM H2O2 exposure for 10 min, overexpression of Dr-WHy significantly enhanced oxidative resistance of E. coli, whereas dW-WHy had no effect (Fig. 5B). These results suggest that the in vivo function of DrwH is related to oxidative stress tolerance, whereas dWHy1 is associated with freezing-thawing stress tolerance. Collectively, secondary structure prediction and in vivo stress tolerance assays of the WHy domains from DrwH and dWHy1 revealed the evolutionary and functional diversity of the bacterial LEA family under different extreme environments.
To further evaluate the potential protective effect of the WHy domain, we carried out in vitro assays to test the effects of Dr-WHy on the enzymatic activities of malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) following exposure to oxidative stress induced by H2O2. Bovine serum albumin (BSA) and potassium phosphate buffer were used as positive and negative controls, respectively. As shown in Fig. 5C, MDH retained only 10% residual activity following exposure to 1.0 or 2.0 mM H2O2. However, the addition of Dr-WHy efficiently prevented inactivation, with the samples retaining 30–50% of their initial activity. Similar protective effects of Dr-WHy were also observed when LDH was subjected to oxidative treatment (Fig. 5D). BSA is a well-known cryoprotectant protein, which also had protective effects on MDH and LDH but it was much less efficient than Dr-WHy. The in vitro results suggested that the intact WHy domain efficiently protects MDH and LDH activities from inactivation caused by oxidative stress.
Discussion
Until now, the complete genome sequencing has been established for 23 Deinococcus spp., including D. radiodurans R1 isolated from canned meat spoiled following exposure to X-rays36, D. geothermalis DSM11300 from a hot spring8, D. actinosclerus BM2 from soil of a rocky hillside37, D. maricopensis LB-34 from a soil sample from the Sonoran Desert in Arizona38, D. deserti VCD115 from the Sahara surface sands, an extreme and nutrient-poor environment39, D. peraridilitoris KR-200 from a sample of arid soil collected from a coastal desert in Chile40, and D. gobiensis I-0 from the cold Gobi desert9. These species can survive when exposed to extreme conditions, such as acute irradiations, cycles of extreme temperatures, extreme desiccation and numerous oxidizing agents, known to cause protein damage lethal to most organisms on Earth. Therefore, Deinococcus bacteria should possess an extraordinary adaptive strategy to withstand environmental extremes. Comparative genomic and transcriptome analyses indicated that Deinococcus bacteria contain a surprisingly large number of horizontally acquired genes, some of which are induced by various abiotic stresses, suggesting that their extreme resistance phenotype may be attributable to still unknown genes and pathways6, 8,9,10. In this study, we functionally characterized a novel bacterial WHy domain-containing hydrophobic LEA5C protein, DrwH, which is induced by oxidative and salinity stresses. Since DrwH is specific to the genus Deinococcus and can protect various intracellular enzymes as shown by in vivo stress tolerance assays or in vitro enzyme protection assays (Figs 4 and 5), it is implicated in the extreme tolerance of Deinococcus bacteria. Based on the results presented here and previous literature reports, we proposed a working model for DrwH in D. radiodurans, which tentatively illustrates its ability to effectively protect activities of various intracellular enzymes from damage caused by oxidative stress (see Supplementary Fig. S7).
The DrwH protein is a novel Deinococcus-specific LEA5C protein with a relatively high level of overall hydrophobicity, a certain degree of cytotoxicity to the growth of E. coli and D. radiodurans, and a pleiotropic role in D. radiodurans physiology. These characteristics have generated confusion and difficulties in studying the function of the drwH gene, particularly in complementation experiments. We constructed a complementation strain using the wild-type drwH gene with its endogenous promoter and performed complementation assays under oxidative stress (see Supplementary Fig. S8). We observed that the complementation strain grew almost similar to the wild type and mutant strains under normal growth conditions. However, plasmid-mediated complementation resulted in an obviously decreased sensitivity to hydrogen peroxide, and the complementation strain was significantly more sensitive than the wild type and mutant strains (see Supplementary Fig. S8B). On the other hand, we compare expression level of the drwH gene between the wild type and complementation strains. Unexpectedly, although the drwH gene possesses its endogenous promoter in the complementation strain, its transcriptional level is much higher than that of D. radiodurans WT following exposure to 80 mM H2O2. As observed in Supplementary Fig. S5, expression of the wild-type DrwH protein significantly decreased growth of recombinant E. coli, suggesting the cytotoxicity of the DrwH protein to the cell growth. We thus suspect that the overexpression of drwH in the plasmid-mediated complementation strain could result in reduced growth under oxidative stress, but it requires further investigation.
Generally, the function of LEA family proteins can be explained by two molecular mechanisms, namely the chaperone-like action41 and the molecular shield activity42. In some instances, LEA proteins function as a chaperone-like molecular shield reducing aggregation of target proteins, suggesting that both mechanisms may not be mutually exclusive41. The protective capacity of the typical hydrophilic LEA proteins is due to their ability to bind to interaction partners accompanied by a folding transition, such as disorder-to-a mostly α-helix conformation upon drying43, 44. Similarly, chaperone-like activities have been reported for MtPM25, an atypical LEA protein that dissociates cold and desiccation-aggregated proteins24. This chaperone-like activity could be explained by the hydrophobic nature of MtPM25 together with the high water sorption capacity of the disordered conformation, which both facilitate or induce transient hydrophobic interactions with exposed surfaces of the aggregates, as suggested by Haaning et al.45. To date, no precise function has been attributed to the drwH gene. On the basis of the protective action of the hydrophobic LEA proteins as discussed above, DrwH might stabilize proteins by a chaperone-like mechanism involving transient hydrophobic interactions; however, this possibility has not yet been examined experimentally.
The WHy domain is widespread among plants, and also detectable in some bacteria and a few euryarchaeota species26, 27. This sporadic occurrence can be explained by horizontal gene transfer from plants to bacteria. The first lateral transfer probably took place between plants and proteobacteria and the second one between plants and archaea after the duplication of the plant domain and the divergence between Hin1 and LEA26. This hypothesis is supported by the observation that the position of the D. radiodurans sequence in the Why domain tree does not reflect the usual phylogenetic relationships within bacteria26. Deinococcus bacteria are known to be prone to horizontal transfers3, 6,7,8,9,10. DrwH is a novel WHy domain-containing hydrophobic LEA5C protein exclusively present in the genus Deinococcus. This raises interesting questions concerning the evolution of putative LEA proteins within Deinococcus bacteria. Comparative genomic analysis showed that the gene cluster containing six genes (from dr1369 to dr1374) in the D. radiodurans genome is interrupted by additional coding sequences in other Deinococcus strains, and is accompanied by gene rearrangement and gene truncation (see Supplementary Fig. S9). As expected, the gene subcluster containing the dr1370, dr1371 and drwH genes displayed a high degree of similarity in all Deinococcus strains. This high conservation, combined with the rareness of the LEA genes in bacteria, suggests the possibility of acquisition by horizontal transfer. Furthermore, structural and functional properties of the WHy domains from DrwH and dWHy1 are schematically shown in Fig. 5, reflecting the divergent evolution due to different niches and selection pressures. In contrast to highly host-adapted pathogens and symbionts undergoing genome reduction, Deinococcus bacteria continually expand their genomic repertoires through many nearly simultaneous gene transfer events, leading to a highly adapted extremophile, which may contribute to their extreme tolerances. The results presented here provide new insight into the adaptive evolution and survival strategies of Deinococcus bacteria under extreme environmental conditions. Further investigation of the function and action of bacterial LEA proteins during abiotic stresses will be required to elucidate the molecular mechanisms underlying the extreme resistance of D. radiodurans in more detail.
Materials and Methods
Bacterial strains, plasmids and media
Strains and plasmids used in this study are described in Supplementary Table S2. D. radiodurans WT and its derivatives were cultured aerobically at 30 °C in TGY medium (1% tryptone, 0.5% yeast extract, and 0.1% glucose) in the presence of antibiotics as required. E. coli strains were grown in Luria-Bertani (LB) broth at 37 °C with appropriate antibiotics. Solid media contained 1.5% agar.
qRT-PCR for gene expression
qRT-PCR was performed as previously described46. Primer pairs used for qRT-PCR are listed in Supplementary Table S3. The PCR reactions were carried out with an AB 7500 Real Time PCR System according to the manufacturer’s recommendations. See Supplementary Materials and Methods for more details.
Construction of the drwH deletion mutant
The drwH deletion mutant was constructed using double crossover recombination of a kanamycin resistance cassette into the genome as described previously (see Supplementary Fig. S3)34. Primers were designed based on the full sequence of the drwH gene and are listed in Supplementary Table S3. Amplification of a 749-bp DNA fragment upstream of the drwH using the primer set P1/P2, a 1,007-bp kanamycin-resistance gene (nptII) from the plasmid pKatAPH3 using primes P3/P4, and a 594-bp DNA fragment downstream of the drwH using the set P5/P6 was performed. The three amplified products were used as templates for the overlap reaction as described previously47, and the resulting PCR fragment (2,268-bp) was directly transformed into D. radiodurans strain R1. Then, colonies resistant to kanamycin (20 μg/mL) were selected. The absence of the drwH gene in the mutant was verified by PCR and DNA sequencing (see Supplementary Fig. S3). When using the primer pair P7/P8, a band corresponding to the migration of a 346-bp molecule was detected in the WT strain; however, it was not detected when the analysis was performed with DNA extracted from the deletion mutant. In addition, PCR products with the primer pair P9/10 were sequenced; the results verified that the nptII gene was inserted into the right position and replaced the drwH gene. The resulting drwH deletion mutant, named R1-01 (ΔdrwH), was used for further study.
The construction of truncated Dr-WHy and dW-WHy proteins
The 306-bp DNA fragment of the drwH gene with the intact WHy domain but without the predicted signal peptide, whose product is designated Dr-WHy, was amplified from genomic DNA of D. radiodurans using primers Dr-WHy-F (CGGGATCCATGCTCACGAGTTTCAGTTT) with a BamHI site and Dr-WHy-R (CCCAAGCTTTCACGTCAGAGTTCCGTCCA) with a HindIII site. Similarly, a 306-bp DNA fragmentof the dwhy1 gene with the intact WHy domain but without the predicted signal peptide, whose product is designated dW-WHy, was amplified from a synthetic recombinant plasmid PUC57-dwhy1 using the primers dW-WHy-F (CGGGATCCATGCTCGTTGATGTTGAACT) with a BamHI site and dW-WHy-R (CCCAAGCTTTTAAGTTTTTACCTCTGCAT) with a HindIII site. Two PCR products were digested with BamHI/HindIII and cloned into the pET28a vector. The resulting plasmids were transformed into the applicable E. coli host strain BL21 (DE3) to generate the truncated-DrwH strain BL21-1 and the truncated-dWHy1 strain BL21-2, respectively. The E. coli strain carrying the empty vector pET28a was designated BL21-0 as a control.
Abiotic stress-resistance assays
The cells were treated with various concentrations of H2O2 and NaCl, different periods of desiccation, and two cycles of freezing and thawing as previously described2, 28, 48. Different dilutions of these cells were plated on agar plates and incubated at 30 °C (D. radiodurans) or 37 °C (E. coli) for 1-3 days before colonies were observed and enumerated. The survival rate was expressed as the percentage of the number of colonies in the treated samples compared with those in the untreated controls. All experiments were performed three times, and values are shown as the mean ± standard deviation. See Supplementary Materials and Methods for more details.
Activity measurement of major antioxidant enzymes
The D. radiodurans WT and ΔdrwH mutant cells (OD600≈0.6, treated with 50 mM H2O2 or not) were harvested by centrifugation, washed and resuspended with sterile PBS (pH 7.0), and disrupted on ice with an ultrasonicator. The debris was removed by centrifugation at 13,000 g at 4 °C for 20 min. Protein concentrations of the supernatants were determined by the Bradford method using BSA as the standard. The CAT activity was measured using spectrophotometric assays and calculated by monitoring the decrease in absorbance at 240 nm resulting from the disappearance of H2O2 as previously described49. The SOD activity was determined according to method of Beauchamp & Fridovich50, in which one unit of SOD was defined as the amount required to inhibit the photoreduction of nitroblue tetrazolium by 50%. The POD activity was measured according to method described by Omran51, in which one unit of POD was defined as the amount that oxidized 1 mol of diaminobenzidine tetrahydrochloride as a substrate. All experiments were repeated at least three times independently.
MDH and LDH enzymatic activity measurement under oxidative stress in vitro
The protective effects of the purified intact recombinant Dr-WHy (truncated-DrwH) on MDH and LDH against oxidative stress were assayed as described previously52, 53 with some modifications. The enzymes and corresponding protein were diluted in 50 mM potassium phosphate buffer (pH 7.2) (MDH) or 25 mM Tris-HCl stabilizer solution (pH 7.5) (LDH) to a final concentration of 1.0 mg/mL following the manufacturer’s recommendations. Two concentrations of H2O2 (1.0 and 2.0 mM) were added to the enzyme mixture and incubated at room temperature for 1 hour. MDH and LDH activities were monitored as the rate of decrease in absorbance at 340 nm for 1 min due to the conversion of NADH into NAD+ at 25 °C. The rate determined for the untreated samples was considered to be 100% in all graphs. See extra details in Supplementary Materials and Methods.
References
Anderson, A. W., Nordan, H. C., Cain, R. F., Parrish, G. & Duggan, D. Studies on a radio-resistant Micrococcus. 1. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol. 10, 575–578 (1956).
Mattimore, V. & Battista, J. R. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178, 633–637, doi:10.1128/jb.178.3.633-637.1996 (1996).
Makarova, K. S. et al. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. R. 65, 44–79, doi:10.1128/MMBR.65.1.44-79.2001 (2001).
Tanaka, M. et al. Analysis of Deinococcus radiodurans’s transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 168, 21–33, doi:10.1534/genetics.104.029249 (2004).
Kriško, A., Smole, Z., Debret, G., Nikolić, N. & Radman, M. Unstructured hydrophilic sequences in prokaryotic proteomes correlate with dehydration tolerance and host association. J. Mol. Biol. 402, 775–782, doi:10.1016/j.jmb.2010.08.012 (2010).
Liu, Y. et al. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc. Natl. Acad. Sci. USA 100, 4191–4196, doi:10.1073/pnas.0630387100 (2003).
Omelchenko, M. V. et al. Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: divergent routes of adaptation to thermophily and radiation resistance. BMC Evol. Biol. 5, 57, doi:10.1186/1471-2148-5-57 (2005).
Makarova, K. S. et al. Deinococcus geothermalis: the pool of extreme radiation resistance genes shrinks. PLoS One 2, e955, doi:10.1371/journal.pone.0000955 (2007).
Yuan, M. et al. Genome sequence and transcriptome analysis of the radioresistant bacterium Deinococcus gobiensis: insights into the extreme environmental adaptations. PloS One 7, e34458, doi:10.1371/journal.pone.0034458 (2012).
Im, S., Joe, M., Kim, D., Park, D. H. & Lim, S. Transcriptome analysis of salt-stressed Deinococcus radiodurans and characterization of salt-sensitive mutants. Res. Microbiol. 164, 923–932, doi:10.1016/j.resmic.2013.07.005 (2013).
Dure, L. III, Greenway, S. C. & Galau, G. A. Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry 20, 4162–4168, doi:10.1021/bi00517a033 (1981).
Wise, M. J. LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4, 52, doi:10.1186/1471-2105-4-52 (2003).
Battaglia, M., Olvera-Carrillo, Y., Garciarrubio, A., Campos, F. & Covarrubias, A. A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 148, 6–24, doi:10.1104/pp.108.120725 (2008).
Bies-Etheve, N. et al. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol. 67, 107–124, doi:10.1007/s11103-008-9304-x (2008).
Hundertmark, M. & Hincha, D. K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9, 118, doi:10.1186/1471-2164-9-118 (2008).
Hunault, G. & Jaspard, E. LEAPdb: a database for the late embryogenesis abundant proteins. BMC Genomics 11, 221, doi:10.1186/1471-2164-11-221 (2010).
Liang, Y. et al. Genome-wide identification, structural analysis and new insights into late embryogenesis abundant (LEA) gene family formation pattern in Brassica napus. Sci. Rep. 6, 24265, doi:10.1038/srep24265 (2016).
Salleh, F. M. et al. A novel function for a redox-related LEA protein (SAG21/AtLEA5) in root development and biotic stress responses. Plant, cell & environment 35, 418–429, doi:10.1111/j.1365-3040.2011.02394.x (2012).
Campos, F., Cuevas-Velazquez, C., Fares, M. A., Reyes, J. L. & Covarrubias, A. A. Group1 LEA proteins, an ancestral plant protein group, are also present in other eukaryotes, and in the archeae and bacteria domains. Mol. Genet. Genomics 288, 503–517, doi:10.1007/s00438-013-0768-2 (2013).
Stacy, R. A. & Aalen, R. B. Identification of sequence homology between the internal hydrophilic repeated motifs of Group 1 late-embryogenesis-abundant proteins in plants and hydrophilic repeats of the general stress protein GsiB of Bacillus subtilis. Planta 206, 476–478, doi:10.1007/s004250050424 (1998).
Battista, J. R., Park, M. J. & McLemore, A. E. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology 43, 133–139, doi:10.1006/cryo.2001.2357 (2001).
Liang, J. et al. JcLEA, a novel LEA-like protein from Jatropha curcas, confers a high level of tolerance to dehydration and salinity in Arabidopsis thaliana. PloS One 8, e83056, doi:10.1371/journal.pone.0083056 (2013).
Gao, J. & Lan, T. Functional characterization of the late embryogenesis abundant (LEA) protein gene family from Pinus tabuliformis (Pinaceae) in Escherichia coli. Sci. Rep. 6, 19467, doi:10.1038/srep19467 (2016).
Boucher, V. et al. MtPM25 is an atypical hydrophobic late embryogenesis-abundant protein that dissociates cold and desiccation-aggregated proteins. Plant Cell Environ. 33, 418–430, doi:10.1111/j.1365-3040.2009.02093.x (2010).
Sharma, A. et al. Ectopic Expression of an Atypical Hydrophobic Group 5 LEA Protein from Wild Peanut, Arachis diogoi Confers Abiotic Stress Tolerance in Tobacco. PloS One 11, e0150609, doi:10.1371/journal.pone.0150609 (2016).
Ciccarelli, F. D. & Bork, P. The WHy domain mediates the response to desiccation in plants and bacteria. Bioinformatics 21, 1304–1307, doi:10.1093/bioinformatics/bti221 (2005).
Jaspard, E. & Hunault, G. Comparison of amino acids physico-chemical properties and usage of late embryogenesis abundant proteins, hydrophilins and WHy domain. PloS One 9, e109570, doi:10.1371/journal.pone.0109570 (2014).
Anderson, D., Ferreras, E., Trindade, M. & Cowan, D. A novel bacterial Water Hypersensitivity-like protein shows in vivo protection against cold and freeze damage. FEMS Microbiol. Lett. 362, fnv110, doi:10.1093/femsle/fnv110 (2015).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132, doi:10.1016/0022-2836(82)90515-0 (1982).
Schmid, A. K. & Lidstrom, M. E. Involvement of two putative alternative sigma factors in stress response of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 184, 6182–6189, doi:10.1128/JB.184.22.6182-6189.2002 (2002).
Earl, A. M., Mohundro, M. M., Mian, I. S. & Battista, J. R. The IrrE protein of Deinococcus radiodurans R1 is a novel regulator of recA expression. J. Bacteriol. 184, 6216–6224, doi:10.1128/JB.184.22.6216-6224.2002 (2002).
Pan, J. et al. IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus. PLoS One 4, e4422, doi:10.1371/journal.pone.0004422 (2009).
Zhang, C. et al. The Site-Directed A184S Mutation in the HTH Domain of the Global Regulator IrrE Enhances Deinococcus radiodurans R1 Tolerance to UV Radiation and MMC Shock. J. Microbiol. Biotechnol. 25, 2125–2134, doi:10.4014/jmb.1507.07008 (2015).
Sheng, D. et al. RecX is involved in antioxidant mechanisms of the radioresistant bacterium Deinococcus radiodurans. FEMS Microbiol. Lett. 244, 251–257, doi:10.1016/j.femsle.2005.01.051 (2005).
Slade, D. & Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. R. 75, 133–191, doi:10.1128/MMBR.00015-10 (2011).
White, O. et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286, 1571–1577, doi:10.1126/science.286.5444.1571 (1999).
Joo, E. S. et al. Deinococcus actinosclerus sp. nov., a novel bacterium isolated from soil of a rocky hillside. Int. J. Syst. Evol. Micr. 66, 1003–1008, doi:10.1099/ijsem.0.000825 (2016).
Pukall, R. et al. Complete genome sequence of Deinococcus maricopensis type strain (LB-34T). Stand. Genomic Sci. 4, 163–172, doi:10.4056/sigs.1633949 (2011).
De Groot, A. et al. Alliance of proteomics and genomics to unravel the specificities of Sahara bacterium Deinococcus deserti. PLoS Genet. 5, e1000434, doi:10.1371/journal.pgen.1000434 (2009).
Rainey, F. A. et al. Deinococcus peraridilitoris sp. nov., isolated from a coastal desert. Int. J. Syst. Evol. Micr. 57, 1408–1412, doi:10.1099/ijs.0.64956-0 (2007).
Kovacs, D., Kalmar, E., Torok, Z. & Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 147, 381–390, doi:10.1104/pp.108.118208 (2008).
Tunnacliffe, A. & Wise, M. J. The continuing conundrum of the LEA proteins. Naturwissenschaften 94, 791–812, doi:10.1007/s00114-007-0254-y (2007).
Fuxreiter, M., Simon, I., Friedrich, P. & Tompa, P. Preformed structural elements feature in partner recognition by intrinsically unstructured proteins. J. Mol. Biol. 338, 1015–1026, doi:10.1016/j.jmb.2004.03.017 (2004).
Patil, A. & Nakamura, H. Disordered domains and high surface charge confer hubs with the ability to interact with multiple proteins in interaction networks. FEBS Lett. 580, 2041–2045, doi:10.1016/j.febslet.2006.03.003 (2006).
Haaning, S. et al. An unusual intrinsically disordered protein from the model legume Lotus japonicus stabilizes proteins in vitro. J. Biol. Chem. 283, 31142–31152, doi:10.1074/jbc.M805024200 (2008).
Zhan, Y. et al. The novel regulatory ncRNA, NfiS, optimizes nitrogen fixation via base pairing with the nitrogenase gene nifK mRNA in Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 113, E4348-E4356, doi:10.1073/pnas.1604514113 (2016).
Shevchuk, N. A. et al. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 32, e19, doi:10.1093/nar/gnh014 (2004).
Wang, L. et al. DrRRA: a novel response regulator essential for the extreme radioresistance of Deinococcus radiodurans. Mol. Microbiol. 67, 1211–1222, doi:10.1111/j.1365-2958.2008.06113.x (2008).
Kobayashi, I. et al. Characterization of monofunctional catalase KatA from radioresistant bacterium Deinococcus radiodurans. J. Biosci. Bioeng. 101, 315–321, doi:10.1263/jbb.101.315 (2006).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287, doi:10.1016/0003-2697(71)90370-8 (1971).
Omran, R. G. Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings. Plant Physiol. 65, 407–408, doi:10.1104/pp.65.2.407 (1980).
Reyes, J. L. et al. Functional dissection of hydrophilins during in vitro freeze protection. Plant Cell Environ. 31, 1781–1790, doi:10.1111/j.1365-3040.2008.01879.x (2008).
Liu, Y. et al. ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 54, 944–959, doi:10.1093/pcp/pct047 (2013).
Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2013CB733903), National Natural Science Foundation of China (No. 31230004, 31570080, 31370126), and Ministry of Agriculture (Transgenic Program, No. 2016ZX08009003-002).
Author information
Authors and Affiliations
Contributions
S.J., J.W. and M.L. conceived and designed the experiments. S.J., X.L., Y.L., C.G. and X.W. performed the experiments. S.J., M.L. and J.W. contributed to the writing of the manuscript. S.J., J.W., X.L., J.H., S.P., L.L., Z.Z. and M.L. contributed to the collection and analysis of data. All authors (S.J., J.W., X.L., Y.L., C.G., L.Z., J.H., X.W., D.X., G.A.E., S.F., H.Z., Y.C., S.P., M.C., W.Z., L.L., Z.Z., K.Z., X.L., Y.Y. and M.L.) discussed the results, commented and approved the version of final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, S., Wang, J., Liu, X. et al. DrwH, a novel WHy domain-containing hydrophobic LEA5C protein from Deinococcus radiodurans, protects enzymatic activity under oxidative stress. Sci Rep 7, 9281 (2017). https://doi.org/10.1038/s41598-017-09541-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09541-2
This article is cited by
-
Evaluation of a bacterial group 1 LEA protein as an enzyme protectant from stress-induced inactivation
Applied Microbiology and Biotechnology (2022)
-
Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.