Abstract
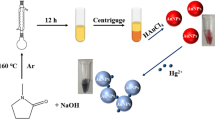
In this study, we report a novel application of valine-capped gold nanoparticles for colorimetric and visual detection of lead ions. The –COO− group of the hydrophobic valine molecules present efficient electrostatic repulsion resulting in generation of stable, well-dispersed and size-controlled GNPs. The GNPs were highly selective for Pb2+ ions and showed visible colour change in the assay mixture on addition of solution containing lead ions. Interestingly, a decrease in the intensity of original SPR peak at 530 nm was observed, with concomitant appearance of a new peak at longer wavelength due to agglomerated GNPs. The free –COO− groups on GNP surface interacted with Pb2+ and ion-dependent chelation mechanism lead to cross-linking of particles and subsequent agglomeration. Binding of Pb2+ ions and valine-capped GNPs occur in a stochiometric ratio of 1:2. The GNPs displayed colorimetric sensing in the range of 0 to 100 ppm concentration with a very high selectivity towards lead even in the presence of other metal ions. The minimum detection limit (MDL) for Pb2+ was 30.5 µM. We anticipate that these valine-capped GNPs may be employed for lead detection in polluted water/wastewater through a cost-efficient, one-step assay protocol as it does not require additional functionalization with specific ligand molecules.
Similar content being viewed by others
Introduction
Lead (Pb) is considered as a major and dangerous ubiquitous heavy metal pollutant and is associated with serious health problems when present in the aquatic system and human body1. In the last decade, a number of anthropogenic and industrial activities such as smelting, use of pesticides, battery production, landfill leaching have significantly contributed towards Pb contamination in the environment2. Pb2+ is a bio-accumulative and potent neurotoxin that cause potential damage to normal cellular functions, affects signal transduction, gene expression and calcium-mediated cellular processes3,4,5. The maximum acceptable limit of Pb2+ is 0.05–0.10 mg/L in waste water; however, Pb2+ concentration has been reported to reach to 250 mg/L in industrial waste water6 and is becoming a major environmental concern. Owing to this, there is a huge demand for developing a rapid assay method that would allow detection of Pb2+ ions.
A number of analytical techniques like atomic absorption spectroscopy, mass spectroscopy, fluorescence, electrochemical and DNAzyme based methods are being currently used for determining Pb2+ ion7,8,9,10,11. However, the development of a simple and rapid colorimetric method for Pb2+ detection could circumvent the use of expensive and tedious analytical instruments. Gold nanoparticles (GNPs) have been widely investigated for their potential ability to detect Pb2+ ions. They allow colorimetric detection of metal ions because of their intriguing optical properties. Depending on the size, shape and aggregation state, GNPs show distinct colour and concomitant shifting of the absorption spectra12. The detection of metal ions using gold nanoparticles is fundamentally based on the aggregation of GNPs induced by binding of metal ions to GNP surface, resulting in corresponding chromatic change in colour and shifting of plasmon peaks. Based on this principle, GNPs functionalized calixarene13, pentapeptide cysteine-alanine-leucine-asparagine-asparagine(CALNN)14 and DNAzyme based methods15, 16 have been used for detection of Pb2+ ions. Although these aforesaid assay methods have high sensitivity and selectivity for Pb2+ detection, they are tedious and time consuming. These require additional steps of modification of the GNP surface with Pb2+ specific functionalizing ligand after the synthesis.
In the present work, we developed a rapid, sensitive and label-free colorimetric method for detection of Pb2+ ions using as-synthesized GNPs. The strategy is based on selective metal induced agglomeration of the monodispersed GNPs leading to a change in colour of colloidal GNP solution and changes in absorption spectra. On adding Pb2+ ions to the GNP solution, Pb2+ binds to the free –COO− groups of valine capped GNPs with high affinity resulting in destabilization of negative charges on GNP surface. Electrostatic destabilization leads to agglomeration of individual particles resulting in change in colour of colloidal solution as well as surface plasmon resonance (SPR) peak. Detection of Pb2+ ions could be comprehended within few minutes, with a minimum detection limit (MDL) of 20 ppm. The approach involves visual detection without the use of electrolytes or transducers. Hence, the method allows rapid and cost effective colorimetric detection of Pb2+ ions. Furthermore, in comparison to other colorimetric approaches reported, the present method avoids the use of additional functionalizing agent. The method, thus presents the advantage of using as-synthesized GNPs, without the requirement of two step tedious procedure of modification/ functionalization of GNP surface. The optical sensor developed herein presents a cost-efficient yet rapid tool for Pb2+ monitoring and holds potential applicability as next generation colorimetric tools for Pb2+ detection.
Experimental Section
Materials
Hydrogen tetrachloroaurate (HAuCl4.3H2O) was purchased from Sigma-Aldrich Co. (USA). All other chemicals used in the study were purchased from SRL, India. All the chemicals were of analytical grade and used as received. Standard stock solutions of metals were prepared in ultrapure Milli-Q water and stored at room temperature for further use.
Preparation of gold nanoparticles
The working concentration of valine in the study was 0.1 M, which was prepared by appropriately diluting the stock solution in MilliQ water and adjusting the pH of the solution with dilute NaOH. 100 ml of 0.1 M valine solution was boiled with continuous stirring. HAuCl4.3H2O (stock solution of 100 mM) was added to the boiling valine solution such that the final concentration of gold salt was 1 mM. The solution was boiled with continuous stirring till a change in colour of the solution from slight yellow to intense violet was observed. The solution was then cooled and aliquots of the solution were sealed and stored at room temperature and refrigerated conditions, to investigate the effect of aging on stability of synthesized GNPs.
To analyze the effect of pH of valine solution, the GNP synthesis was conducted with valine solution of different initial pH (in range from 6 to 10), prior to boiling. The pH that favoured stable synthesis of GNPs was chosen as the optimized pH for subsequent experiments. All other experimental analysis were conducted using colloidal GNP solution prepared using this method, without any further modifications. Effect of stability of synthesized valine-capped GNP was analyzed by adding NaOH at different concentration to the synthesized GNP solution and recording the corresponding change in absorption spectra.
Characterization of gold nanoparticles
UV-Vis spectra in the range of 200–800 nm were recorded using CECIL UV-Vis spectrophotometer. Dynamic Light Scattering (DLS) analysis was performed using a Dawn Heleos II (Wyatt) system operating at 658 nm wavelength, at a scattering angle of 90°. The values of hydrodynamic radius (Rh) reported in the paper are the mean of four independent measurements. Transmission electron microscopy (TEM) analysis was done using a FEI, TECNAI-G2 TEM microscope, 20-TWIN operating at 200 kV equipped with a GATAN CCD camera. Sample for TEM analysis was prepared by coating a drop of GNP solution on a carbon coated copper grid followed by drying.
Detection of metal ions by synthesized gold nanoparticles
The valine capped GNPs were investigated for their ability to detect heavy metal ions that are generally present in waste water as pollutant. The heavy metal ions used in the study were Cu2+, Ba2+, Hg2+, Cr3+, Pb2+, Zn2+, Cd2+, Sb3+, As3+ and Ni2+. For metal detection study, synthesized GNPs were treated with 100 ppm of respective metal solutions in the ratio of 1:2 respectively and changes in the optical property was recorded at regular intervals.
The assay solution showed significant changes when treated with Pb2+ ion and hence we further analyzed the sensitivity of the GNPs over a range of Pb2+ ion concentration. UV-Vis Absorbance spectroscopy and DLS analysis of the treated solution were performed. Metal interference was studied by simultaneously treating GNPs with 100 ppm of Pb2+ ions and 100 ppm of different metal ions (Cu2+, Ba2+, Hg2+, Cr3+, Zn2+, Cd2+, Sb3+, As3+, Ni2+) separately. All detection experiments were conducted in three separate batches with triplicates in each batch to ensure reproducibility.
Result and Discussion
Synthesis and stability of gold nanoparticles
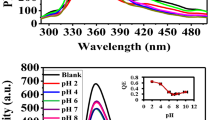
We used valine, a hydrophobic amino acid, for the synthesis of GNPs. Valine acted as both reducing and stabilizing agent in the synthesis of highly stable GNPs. Colloidal GNPs were synthesized using valine solution of different pH, ranging from 6 to 10. Figure 1a shows the absorption spectra of valine capped GNPs synthesized at different pH after an hour of formation. UV-visible spectra showed significant difference in pattern. The SPR peak at pH 8 was narrowest compared to other pH. Furthermore, broadening and red shift of peaks were observed at higher pH, which is evidence of agglomeration of GNPs at highly basic pH17. Agglomeration of particles above pH 9 may be because of the weak interaction between –NH of valine and Au° at alkaline pH18. The Rh values obtained from DLS analysis was in concurrence with the UV-Vis spectral data (Fig. 1b). The GNPs synthesized at pH 8 were smallest in size with average Rh of 22.69 ± 2.64 nm. Those synthesized at other pH were larger in size, as indicated by the red shift of SPR peak in UV Vis spectra.
Stability is an important concern in nanoparticle based applications. We therefore analyzed the stability of GNPs synthesized at pH 6 to 10. It was observed that except for particles synthesized at pH 8, GNP synthesized at all other pH precipitated within 24 hours of synthesis. This demonstrated high stability of valine-capped GNPs synthesized at pH 8.
Further study on the stability of valine-capped GNPs showed that GNPs were more stable under refrigerated condition compared to at room temperature (RT) (Fig. 1c and d). The refrigerated valine-capped GNP solution was stable for several weeks and retained the initial colour of colloidal GNP, whereas the aliquot stored at RT showed precipitation after 2 weeks of storage.
Kinetics of valine-capped gold nanoparticle synthesis
The valine-capped GNPs synthesized at pH 8 were most stable and of smallest size. Hence all further experiments were conducted at that particular pH (pH 8).
Based on the experimental results, we have put forth an analytical kinetic model for GNP synthesis using valine. Analysis suggested that the synthesis process follows an autocatalytic reaction model. Autocatalysis is characterized by the sigmoid nature of the kinetics graph, obtained by plotting the absorption intensity of SPR peak at 530 nm as a function of progressing reaction time (Fig. 2a). Sigmoid plot implies that a slow nucleation phase is followed by a rapid growth phase. During the initial nucleation stage, seed particles are formed which with progressing reaction time favour rapid reduction and particle growth19. Measurement of Rh of synthesized particles at consecutive time intervals also confirmed the autocatalytic mode of reaction (Fig. 2b). This was evident by a gradual increase in particle size with increasing time. The Rh of particles was seen to increase from 35.73 ± 1.69 nm to 73.55 ± 2 nm in the first 50 minutes of reaction and remained stable thereafter, indicating efficient capping by the valine molecules.
A quantitative measurement of the reaction rate of valine-capped GNPs synthesis was determined by plotting ln[a/(1 − a)] versus time (where a = [OD(t)/OD(∞)]; OD(t) and OD(∞) are the optical density/absorbance at time t and ∞ respectively)20, 21. The plot gives a straight line curve, the slope of which is the rate constant (kobs) of the reaction. In the present case kobs value of 0.048/min was obtained (Inset in Fig. 2a).
Mechanism behind valine-capped gold nanoparticle synthesis
On addition of HAuCl4 to valine solution, redox reaction occurs reducing Au ions to Au, which subsequently acts as seed particles and assists in further nucleation and growth of particles. Valine acts as the sole reducing and stabilizing agent. The amine groups of valine molecule coordinates with Au ions via weak covalent linkage as reported22. The formation of –NH-Au0 bond renders electrostatic stabilization to the synthesized particles. Additionally, the presence of free negatively charged carbonyl (–COO−) on GNP surface, provides electrostatic repulsion, stabilizing the particles and preventing particle aggregation in aqueous system.
Further, stability of valine-capped GNPs with increasing alkalinity of solution was determined by addition of increasing amount of NaOH solution. It was observed that the supplementation of high amount of NaOH to the already synthesized colloidal GNP solution, had no significant effect on the SPR peak of valine-capped GNPs (Inset in Fig. 3). This is because the addition of more –OH− ions to synthesized GNPs above of pI of valine, (i.e. 6) have no effect on the surface charge of the biomolecule and they still remain negatively charged. This is in accordance with the results of Kumar et al., that suggests that valine capped GNPs possess a net negative charge on their surface beyond pH 623. This confirms that GNP bind to valine molecule via –NH group and are stabilized by the –COO− groups. The synthesized GNPs carry a net negative charge on their surface. Zeta potential analysis provided further information about high stability of valine capped GNP. The valine-capped GNPs reported in this work were highly stable and even variation of pH after GNP synthesis has no effect on their size, shape and aggregation state (Inset in Fig. 3). A schematic representation of the mechanism of reduction and stabilization of GNPs by valine is exemplified in Fig. 3.
Selective colorimetric detection of metal ions
The use of GNPs as optical sensors is generally based on determination of changes in the SPR peak positions and colour of the assay mixture. The change occurs due of the target-analyte induced agglomeration/aggregation of nanoparticles. This effect relies on the selectivity of the functionalizing ligand/capping molecule attached to the GNP surface. In the current study, valine molecules attach to GNP surface through the –NH group. The free –COO− groups present a net negative charge on the surface of nanoparticles, stabilizing the GNP particles. Addition of metal ion (present in polluted water sample) may induce the cross-linking of GNPs by the target metal ion by coordinate bonding leading to destabilization of GNP and hence agglomeration. If the reaction is selective to only one metal ion this may form basis of metal detection.
With this in mind, we treated the valine capped GNPs with 100 ppm of different metal ions (Cu2+, Ba2+, Hg2+, Cr3+, Pb2+, Zn2+, Cd2+, Sb3+, As3+, Ni2+) individually and recorded the absorption spectra of the assay solution. We observed that when GNPs were treated with Pb2+ ion solution, an immediate change in colour of the assay solution from the original violet colour to blue occured, while all other metal assay solutions retained the original colour of colloidal GNP (Fig. 4a). The absorption spectra also showed evident changes in the SPR peak of Pb2+ assay solution. The plasmon peak of colloidal GNP was originally situated at 530 nm. Except for Pb2+, the SPR peak of all other metal treated assay solution showed a negligible change and remained at the same position as that of untreated colloidal GNP. However, in case of Pb2+ assay solution, a new absorption peak appeared at higher wavelength region (778 nm) along with decrease in original SPR peak at 530 nm. The typical change in colour and absorption spectra of treated GNP solution are shown in Fig. 4b. Quantitative analysis of detection was performed by comparing the absorption ratio at 778 and 530 nm (A 778/A 530). This ratio represents the degree of aggregation, a representative of the relative quantity of aggregated and dispersed nanoparticles in the solution24. Comparative quantitative analysis of the tested metal assay solution suggested an apparently high absorption ratio for Pb2+ suggesting the maximum aggregation state, while all other metals had an absorption ratio similar to that of untreated GNP (Fig. 4b).
(a) Absorption spectra depicting the selectivity of valine- GNPs for Pb2+ ions. (b) Quantitaive analysis of selectivity of valine- GNPs. (c) Graph showing the absorption ratio obtained by treating valine- GNP with a comixture of 100 ppm of Pb2+ ion and 100 ppm of respective metal ion. (d) TEM images of valine- GNPs (1, 2) before treatment with Pb2+ ions (3, 4) after treatment with Pb2+ ions.
We also analyzed possible interference of other metal ions that may be present in the polluted water sample during Pb2+ ion detection using valine capped GNP. Of all the metal ions (Cu2+, Ba2+, Hg2+, Cr3+, Zn2+, Cd2+, Sb3+, As3+, Ni2+) tested in the study, only Cr3+, As3+ and Hg2+ affected the sensitivity of Pb2+ ion detection by valine capped GNPs (Fig. 4c ). Higher absorption ratio (A778/A530) was observed in case of the three metals (Cr3+, As3+, Hg2+) compared to original valine capped GNP. This indicates that their co-presence with Pb2+ ions triggers the aggregation of GNPs and induces a change in colour from violet to blue. But from Fig. 4b it is clear that in absence of Pb2+, the interfering ions like Cr3+, As3+ and Hg2+ do not react with GNP. Thus aggregation occurs only if Pb2+ is present in assay solution. Other tested ions did not interfere in the detection of Pb2+ and retained the original colour and SPR peak of colloidal GNPs. Thus, the valine-capped GNPs are capable of detecting Pb2+ ions selectively in presence of other metal ions also.
Sensitivity of gold nanoparticles towards lead ions
For practical applicability, it’s essential to determine the sensitivity and minimum detection limit (MDL) of the sensing system. Therefore, to quantitatively establish the dynamic detection concentration of Pb2+ ions by valine capped GNPs, experiments were performed using varying concentration of Pb2+ ions. With increasing concentration of Pb2+ ions, the intensity of original absorbance of GNP at 530 nm decreased, along with the appearance of new absorbance peak at higher wavelength range (780 nm). Furthermore, red shifting of the new longitudinal SPR peak was observed with increasing Pb2+ ion concentration. Distinct change in colour of colloidal GNP solution from purple, violet to blue was seen with progressively increasing concentration of Pb2+ ions. The variation in visual change in colour was consistent with the absorption spectroscopy (Fig. 5a). Interaction with Pb2+ ions leads to the formation of GNP agglomerates that results in a decrease in concentration of free mono-dispersed colloidal GNP. This results in redistribution of spectral intensity to longer wavelengths with decrease in absorption at 530 nm. Quantitative representation of agglomeration obtained by plotting Pb2+ concentration versus A778/A530 ratio showed a straight line graph with a very good curve fitting (R2 = 0.98) in the range of 1 to 100 ppm Pb2+ concentration.
The synthesized GNPs were diluted and then treated with Pb2+ ions to analyze the effect of dilution on detection sensitivity. It was observed that the diluted GNP solution showed a lower detection limit compared to that of original GNPs (Fig. 5b). Dilution results in well dispersed GNP particles with lower concentration. Hence, addition of even small amount of Pb2+ ions is enough to aggregate all the available GNPs. Gunupuru et al.13 reported detection of Pb2+ ions with a MDL of 10 ppm using calixarene functionalized GNPs, while in the present study the as-synthesized GNPs were capable of efficiently detecting Pb2+ ions with a MDL of 10 ppm using 1:3 diluted solution of GNPs.
TEM analysis of valine capped GNPs before and after treatment with Pb2+ ions clearly suggested the aggregation of synthesized particles. TEM micrographs of synthesized GNPs show individual separated particles (Fig. 4d (1 and 2)). The dispersed particles signify an efficient electrostatic barrier between the particles offered by the negative –COO− groups that present an electrostatic repulsion against the vanderwaals attraction between the GNPs25, thus preventing agglomeration. On the other hand, TEM images of GNPs treated with 100 ppm of Pb2+ ions, after 5 minutes of incubation showed significant aggregation of particles (Fig. 4d (3 and 4). It is attributed that addition of Pb2+ to GNPs results in a change in colour of solution as well as distinct changes in the absorption spectra of the assay solution. Addition of Pb2+ ions to GNP results in chelating interaction of valine carboxylate group. Saunders et al. (2011) while studying the X-ray crystallography of Pb-Val molecule reported that Pb interacts with two valine carboxylate units and a water molecule with high affinity resulting in the chelation of Pb2+. As already stated the synthesized GNPs are electrostatically stabilized by the –COO− functional groups of valine, hence interaction of Pb2+ ions with –COO− groups leads to the destabilization of net negative charge on GNP surface consequently leading to agglomeration of particles. This forms the basis of high selective and sensitive colorimetric detection of Pb2+ ions by valine capped GNPs. The mechanism of interaction of Pb2+ ions with valine capped GNPs is illustrated in Fig. 5c.
Most of the earlier studies on colorimetric sensing of Pb+2 ions, reported the use of Pb-specific ligands for the detection process. In the present study, as-synthesized valine-GNPs showed the potential of Pb2+ ion detection. The method involves a rapid, one-step method for selective optical sensing of Pb2+ ion, even in presence of other major toxic heavy metal ions present in waste water. However development of an assay kit for such detection in environmental sample requires optimization of many parameters and several challenges need to be resolved. One of the major challenges is interference with sample matrix. Apart from the metal ions tested in the present study many other common ions including Cl, K, Na, Mg, P, Ca, Fe, sulphate, nitrate, phosphate, carbonate, bicarbonate ions are also found in waste water. Some organic molecules including pesticides, antibiotics, bacteria etc may also interfere in the detection process. Therefore, further studies on optimization of the optical detection of lead with respect to sensitivity in presence of these interfering matrix ions needs to be completed. These studies will assist in the development of heavy metal assay methods and kits for feasible on-site detection of lead ions in water/waste water samples with optimum efficiency and sensitivity.
Conclusion
A cost effective and label free, one-pot approach for colorimetric detection of Pb2+ ions using valine-capped GNPs has been addressed in the present work. Detection of Pb2+ was based on an ion-dependent chelation mechanism. Treatment of valine-GNPs with Pb2+ ions resulted in rapid change in colour and absorption spectra due to the agglomeration of GNPs. The Pb2+ ions bind to the free –COO− groups destabilizing the net charge on GNP surface. The synthesized GNPs showed selectivity for Pb2+ compared to other tested divalent cations. An enhancement in sensitivity of detection was observed when diluted solution of valine-GNPs was used. As the valine capped GNPs showed significant potential for detecting Pb2+ ions, the present sensing system holds tremendous potential for applications as cost effective and rapid colorimetric detection. This eliminates the functionalization step which is a major step reported in most of the other colorimetric studies.
References
Sparks, D. L. Toxic Metals in the Environment: The Role of Surfaces. Elements. 1, 193–197 (2005).
Jaroslawiecka, A. & Piotrowska-Seget, Z. Lead Resistance in Microorganisms. Microbiology. 160, 12–25 (2014).
Yoosaf, K., Ipe, B. I., Suresh, C. H. & Thomas, K. G. In situ synthesis of Metal Nanoparticles and Selective Naked Eye detection of lead ions from aqueous media. J. Phys. Chem. C. 111, 12839 (2007).
Milosevic, N. & Maier, P. Lead stimulates intracellular signalling between hepatocytes and Kupper cells. Eur. J. Pharmacol. 401, 317–328 (2000).
Barciszewska, M. Z. et al. Lead toxicity through the leadzyme. Mutat. Res. 589, 103–110 (2005).
Sag, Y., Ozer, D. & Kutsal, T. A comparative study of biosorption of lead ions (II) to Z. Ramigera and R. Arrihizus. Process Biochem. 30, 169–174 (1995).
Yan, X. P. & Ni, Z. M. A. Determination of lead by hydride generation atomic absorption spectrometry with in situ, in a zirconium coated graphite tube. J. Anal. Atom. Spectrom. 6, 483–6 (1991).
Yang, H. J. & Jiang, S. J. J. Hydride generation inductively coupled plasma mass spectrometric detection of lead compounds separated by liquid chromatography. Anal. Atom. Spectrom. 10, 963–7 (1995).
Davis, C. L., Smith, B. W. & Winefordner, J. D. A miniature glow discharge for laser excited atomic fluorescence detection of lead. J. Microchem. 52, 383–95 (1995).
Babyak, C. & Smart, R. R. Electrochemical detection of trace concentration of cadmium and lead with a boron doped diamond electrode: Effect of KCl and KNO3 Electrolytes, Interference and Measurement in River water. Electroanalysis. 16, 175–82 (2004).
Xiao, Y., Rowe, A. A. & Plaxco, K. W. Label-free electrochemical detection of DNA in blood serum via target-induced resolution of an electrode-bound DNA pseudoknot. J. Am. Chem. Soc. 129, 262–3 (2007).
Hilliard, L. R., Zhao, X. & Tan, W. Immobilization of oligonucleotides onto silica nanoparticles for DNA hybridization studies. Anal. Chim. Acta. 470, 51–56 (2002).
Gunupuru, R. et al. Colorimetric detection of Cu2+ and Pb2+ ions using calix[4]arene functionalized gold nanoparticles. J. Chem. Sci. 126(3), 627–635 (2014).
Xiao-kun, L. I. & Zhen-xin, W. Chem. Res. Chinese Universities 26(2), 194–197 (2010).
Wei, H., Li, B., Li, J., Dong, S. & Wang, E. DNAzyme based colorimetric sensing of lead (Pb2+) using unmodified gold nanoparticle probes. Nanotechnology 19, 095501 (2008).
Liu, J. & Lu, Y. A colorimetric lead biosensor using DNAzyme directed Assembly of Gold Nanoparticles. J. Am. Chem. Soc. 125, 6642–6643 (2003).
Mayya, K. S., Patil, V. & Sastry, M. On the stability of carboxylic acid Derivatized Gold Collodial Particles: The Role of Collodial solution pH studied by optical absorption spectroscopy. Langmuir. 13(15), 3944–3947 (1997).
Sanghi, R. & Verma, P. pH dependent fungal proteins in the ‘green’ synthesis of gold nanoparticles. Adv. Mat.Lett. 1(3), 193–199 (2010).
Goswami, N., Saha, R. & Pal, S. K. Protein assisted synthesis of colloidal metal nanoparticles: exploration of key chemistry of the biomolecule. Journal of Nanoparticle Research 13(10), 5485–5495 (2011).
Huang, Z. Y., Mills, G. & Hajek, B. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. 97, 11542–11550 (1993).
Priyadarshini, E., Pradhan, N., Sukla, L. B. & Mishra, B. K. Biogenic synthesis of floral shaped nanoparticles using a novel strain. Talaromyces flavus, Annals of Microbiology 64, 1055–1063 (2014).
Leff, D. V., Brandt, L. & Heath, J. R. Synthesis and Characterization of Hydrophobic, Organically-soluble Gold Nanocrystals functionalized with primary amines. Langmuir 12, 4723 (1996).
Kumar, A. et al. Amphoterization of colloidal gold particles by capping with valine molecules and their phase transfer from water to toluene by electrostatic coordination with fatty amino acid molecules. Langmuir. 16, 9775–9783 (2000).
Zhang, J., Wang, Y., Xu, X. & Yang, X. Specially colorimetric recognition of calcium, strontium and barium ions using 2-mercaptosuccinic acid functionalized gold nanoparticles and its use in reliable detection of calcium ions in water. Analyst 136, 3865 (2011).
Grabar, K. C., Freeman, R. G., Hommer, M. B. & Natan, M. Preparation and Characterization of Au colloidal monolayers. J. Anal. Chem. 67, 735–43 (1995).
Acknowledgements
E. Priyadarshini is grateful to the Department of Science and Technology, Ministry of Science and Technology, New Delhi, India for providing fellowship under the DST-Inspire Scheme (Grant no. DST/INSPIRE Fellowship/2011/[343]).
Author information
Authors and Affiliations
Contributions
Eepsita Priyadarshini has carried out the work in the manuscript, prepared the graphs and compiled the draft manuscript. Dr. Nilotpala Pradhan has provided guidance and supervised the entire work, analyzed and corrected the draft manuscript. All the authors have finally reviewed the manuscript. The work was carried out as a part of PhD work, the fellowship for which was provided under DST- Inspire Scheme, by Department of Science & Technology, New Delhi, India.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Priyadarshini, E., Pradhan, N. Metal-induced aggregation of valine capped gold nanoparticles: An efficient and rapid approach for colorimetric detection of Pb2+ ions. Sci Rep 7, 9278 (2017). https://doi.org/10.1038/s41598-017-08847-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08847-5
This article is cited by
-
Bio-fabrication of silver nanoparticles using Commelina erecta, L.: a mechanistic approach on synthesis, optimization, antibacterial, and antioxidant potential
Bioprocess and Biosystems Engineering (2024)
-
Sensing lead ions in water: a comprehensive review on strategies and sensor materials
Analytical Sciences (2024)
-
Synthesis of amino acid-functionalized Cit-T20-GNPs and investigation of their metal ion selectivity for colorimetric sensing
Chemical Papers (2023)
-
Ultrasensitive nanodrop spectrophotometric assay of Pb(II) using green fabricated gold nanoparticles
Bulletin of Materials Science (2022)
-
A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: an alternative approach to conventional methods
Analytical and Bioanalytical Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.