Abstract
Hypertrophic cardiomyopathy (HCM) has a low risk for sudden cardiac death (SCD). The ESC clinical risk prediction model estimates the risk of SCD using clinical and echocardiographical parameters without taking into account cardiac magnetic resonance (CMR) parameters. Therefore, we compared the CMR characteristics of 149 patients with low, intermediate and high ESC risk scores. In these patients left and right ventricular ejection fraction and volumes were comparable. Patients with a high ESC risk score revealed a significantly higher extent of late gadolinium enhancement (LGE) compared to patients with intermediate or a low risk scores. During follow-up of 4 years an extent of LGE ≥20% identified patients at a higher risk for major adverse cardiac arrhythmic events in the low and intermediate ESC risk group whereas an extent of LGE <20% was associated with a low risk of major adverse cardiac arrhythmic events despite a high ESC risk score ≥6%. Hence, we hypothesize that the extent of fibrosis might be an additional risk marker.
Similar content being viewed by others
Introduction
Hypertrophic cardiomyopathy (HCM) is a complex genetic heart disease1,2,3,4. Although the overall risk for sudden cardiac death (SCD) is relatively low, some patients with HCM die suddenly from fatal arrhythmic events1, 5, 6. Therefore, the identification of patients at risk for life-threatening arrhythmias is important. Based on risk stratification the implantation of implantable cardioverter defibrillator (ICD) in patients with HCM and a high risk has shown to reduce mortality7,8,9,10. However, in contrast to patients with ischemic heart disease treated with an ICD for primary or secondary prevention, patients with HCM are generally younger and predominantly present with a preserved left ventricular function. Furthermore, the prevalence of atrial fibrillation is higher in patients with HCM compared to other patient subgroups11. The younger age of the physically active HCM patients is accompanied by a higher rate of ICD lead failures12 and might result in further morbidity and mortality. The higher prevalence of atrial fibrillation represents an additional risk for inappropriate shocks in these patients13. Therefore, risk stratification in patients with HCM is crucial to avoid over- or undertreatment. The recently published ESC clinical risk prediction model14 uses clinical and echocardiographic determined parameters such as age, family history of SCD, maximal left ventricular wall thickness, left atrial diameter, left ventricular outflow tract gradient, previous unexplained syncope and the occurrence of non-sustained ventricular tachycardia to estimate the probability of sudden cardiac death (SCD) at 5 years. However, this risk prediction model is just based on a multicenter, retrospective longitudinal cohort study15 since prospective randomized studies are missing. Furthermore, the ESC risk prediction model does not take into account cardiac magnetic resonance (CMR) parameters. Therefore, the aim of our study was to compare patients with low, intermediate and high ESC risk scores of SCD according to CMR characteristics in order to identify additional imaging risk factors that might help to refine risk stratification.
Methods
Study population
A total of 149 patients from our HCM registry that underwent CMR examination between January 2008 and December 2015 at our department were included in the study. All patients gave informed consent and the study was approved by the local ethics commission (Medizinische Ethikkommmision II, Medizinische Fakultät Mannheim) and was performed in adherence to the Declaration of Helsinki. All patients with HCM were diagnosed based on conventional criteria; left ventricular hypertrophy ≥15 mm on two-dimensional echocardiography in the absence of another disease that could account for the hypertrophy16. Non-obstructive HCM (HNCM) was defined as a pressure gradient ≤30 mmHg at rest and after provocation. Patients with a pressure gradient >30 mmHg at rest or after provocation were classified as obstructive HCM (HOCM). Non-sustained ventricular tachycardia (nsVT) was defined as three or more ventricular extra systoles at a rate of ≥120 BPM, lasting less than 30 seconds14.
ESC risk score
ESC risk score was calculated according to the following formulas: Prognostic index = [0.15939858 × maximal wall thickness(mm)] − [0.00294271 × maximal wall thickness2 (mm2)] + [0.0259082 × left atrial diameter (mm)] + [0.00446131 × maximal(rest/Valsalva) left ventricular outflow tract gradient (mm Hg)] + [0.4583082 × family history SCD] + [0.82639195 × NSVT] + [0.71650361 × unexplained syncope] − [0.01799934 × age at clinical evaluation (years)] and Probability of SCD at 5 years = 1 − 0.998exp(Prognostic index) 14. A risk of SCD at 5 years <4% was considered as low, ≥4–<6% as intermediate and ≥6% as high risk14.
Image acquisition
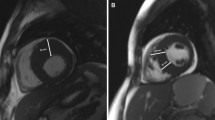
All studies were performed using a 1.5 Tesla and 3.0 Tesla whole body imaging system (Magnetom Avanto and Skyra, Siemens Medical Systems, Healthcare Sector, Erlangen, Germany) using a six-element (Avanto) phased-array body coil and a 18-element body matrix coil (Skyra). Cine images were acquired using a retrospective-gated balanced segmented steady state free precession (trueFISP) sequence in three long-axis views (2-, 3-, and 4-chamber view) and in multiple short-axis views, covering the entire left ventricle (LV) from base to apex. Late gadolinium enhancement (LGE) images were obtained 10–15 min after intravenous administration of 0.2 mmol·kg−1 Gadoteric acid (Dotarem, Guerbet, Roissy CdG Cedex, France, Germany), using either an inversion recovery turbo Fast Low Angle Shot sequence or a phase-sensitive inversion recovery true fast imaging with steady state precission sequence at the same position as the long and short-axis cine acquisitions in end diastole17. The inversion time was adjusted per patient to optimally null signal from normal myocardium typically between 250 and 300 ms.
CMR Image analysis
Left ventricular mass and volumes18 as well as right ventricular19 and left atrial volumes20 were determined using CMR as previously described. Right atrial volumes were evaluated on the 4-chamber view and/or a right ventricular 2-chamber view using the biplane area length method21. MAPSE measurements were assessed on 4-chamber view cine images. The distance between the basal septal mitral annulus (septal MAPSE) and a reference point outside the heart were measured in end-diastole and end-systole. Septal MAPSE was calculated by subtracting the end-systolic (ESL) from the end-diastolic length (EDL).
To determine TAPSE, the distance between the cutting edge of the tricuspid annulus with the RV free wall and a reference point outside the RV apex were measured in end-diastole (EDL) and end-systole (ESL). The point outside the RV apex was chosen in extension to the right ventricular apex and had to stay unchanged at end-diastole and end-systole. TAPSE was defined as the difference between EDL and ESL. LGE was assessed by a semi quantitative score as previously described22. One representative basal, midventricular and apical slice which revealed visually the greatest amount of LGE was chosen for analysis. Then, each segment was visually scored by an experienced observer for the total amount of LGE, irrespective of distribution throughout the width of the segment (segmental extent of LGE) using the following scoring scheme: 0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75% and 4 = 76–100%. By summing all the 17-segmental scores the total size of myocardial LGE was calculated for the entire left ventricle. The resulting summed score for the total left ventricle was thereafter expressed as a percentage of the maximum possible score.
Follow-up and definition of study endpoints
Follow-up data for non-cardiac and cardiac death was analysed retrospectively by review of the corresponding medical records in our electronic Hospital Information System or by telephone interview with the patient or the treating family doctor in all 149 patients.
Among the 149 HCM patients included in the study, 120 (80.5%) patients were regularly examined at our outpatient clinic and a complete arrhythmic follow-up including regular holter ECG monitoring and complete ICD analysis could be provided. 29 (19.5%) were followed-up by referring cardiologist in private practice. For these 29 patients a complete documentation of arrhythmic events could not be provided and these patients were excluded from the arrhythmic follow-up. Another 3 (2.0%) patients were also excluded from analysis due to myomectomy shortly after MRI. Therefore, only 117 (78.5%) patients were included in the final analysis for major adverse cardiac events including sudden cardiac death (SCD), electrical storm, adequate ICD shock, adequate antitachycardia pacing (ATP), sustained ventricular tachycardia on holter electrocardiogram (ECG) or ECG.
The definition of cardiac event required the documentation of significant arrhythmia. ICD therapies were classified appropriate when they occurred in response to ventricular tachycardia or ventricular fibrillation. Electrical storm (ES) was defined as ≥3 separate ventricular tachycardia events ≤24 h. In case of out-of-hospital death not followed by autopsy, sudden unexpected death was classified as cardiac death.
Statistical Analysis
All data are presented as a mean ± standard deviation. Continuous parameters were compared using a 2-tailed student’s t-test. The Mann-Whitney U test was applied for nonparametric data. Box plot analysis was used. All results were considered statistically significant when p < 0.05. Analyses were performed with Statistical Package for Social Sciences (SPSS for windows 14.0, Chicago, IL, USA).
Receiver operating characteristic (ROC) curves were used in the whole group of patients to find the optimal cut-off values for extent LGE% and probability of SCD according to the ESC risk score (with maximizing the sum of sensitivity and specificity) to predict major adverse cardiac arrhythmic events. Sensitivity was calculated in all patients with a low and intermediate ESC risk score (ESC risk score <6%) to evaluate how good an extent LGE ≥20% is at detecting a major adverse cardiac arrhythmic event. Sensitivity was calculated as number of true positives/(number of true positives + number of false negatives). In patients with a high ESC risk score (ESC risk score ≥6%) the negative predictive value of a LGE <20% was calculated to estimate the probability that despite a high ESC risk score the HCM patients will not suffer a major adverse cardiac arrhythmic event. The negative predictive value is defined as number of true negatives/(number of true negatives + number of false negatives). In patients with a low to intermediate ESC risk score (ESC risk score <6%) survival estimates and cumulative event rates were compared by the Kaplan–Meier method by using the time-to-first event for each endpoint. The time to event curves were estimated with the Kaplan–Meier method and compared with the log-rank test.
Results
In our study cohort of 149 patients with HCM 121 (81%) belonged to the low, 18 (12%) to the intermediate and 10 (7%) to the high risk group. The probability of SCD at 5 years according to the ESC risk score14 was 1.8 ± 0.8% in the low risk, 4.6 ± 0.7% in the intermediate and 12.2 ± 8.1% in the high risk group. Table 1 presents the clinical characteristics as well as the distribution of the traditional risk factors according to the ESC risk groups. Compared to patients with a low and intermediate risk, those in the high risk group revealed the highest percentage of patients with hypertrophic obstructive cardiomyopathy (HOCM). Looking at the distribution of the traditional risk factors in the ESC risk groups we observed a significant lower percentage of syncope, SWT >30mm and family history of SCD in the low risk group compared to those patients with an intermediate or high ESC risk. Whereas the prevalence of non-sustained ventricular tachycardias showed a significant increase from the low to the intermediate and from the intermediate to the high risk group.
Comparing CMR characteristics (Table 2), left and right ventricular ejection fraction as well as left and right ventricular volumes were comparable among the different risk groups. Patients with an intermediate and high ESC risk score had a significantly higher SWT (23 ± 6mm and 24 ± 5mm, respectively) compared to the low risk group (18 ± 4, Table 2). Whereas the left atrial end-diastolic volume index (LA-EDVI) was significantly higher in the high risk group (74 ± 45 ml/m²) compared to the intermediate (48 ± 17 ml/m²) and the low risk group (49 ± 30 ml/m²) (Table 2). However, patients with a high ESC risk score revealed a significantly higher extent of fibrosis defined as extent of late gadolinium enhancement (extent LGE %) 33 ± 19% (Fig. 1A, Fig. 2, dark grey bar) compared to patients with an intermediate risk score and an extent LGE % of 15 ± 16 (p = 0.02 Fig. 1B, Fig. 2 light grey bar) and patients with a low risk score and an extent LGE % of 10 ± 11% (p < 0.0001, Fig. 1C, Fig. 2 crosshatched bar).
The box plots illustrate the extent LGE % in patients with a low ESC risk score (white crosshatched box plot), patients with intermediate ESC risk score (light gray box plot) and low ESC risk score (dark gray box plot). Extent LGE % is given as mean ± standard deviation below the box plots, the line in the box plots indicates the median value of the data. The p-values for the comparison of groups are also indicated. The figure also illustrates that patients with a high ESC risk score (defined as risk for SCD at 5 years ≥6%) reveal a significantly higher amount of LGE than those HCM patients with an intermediate (risk for SCD at 5 years ≥4–<6%) or low ESC risk score (risk for SCD at 5 years <4%). A risk of SCD at 5 years <4% was considered as low, ≥4–<6% as intermediate and as high risk. Abbreviations: HCM: hypertrophic cardiomyopathy, LGE: late gadolinium enhancement, SCD: sudden cardiac death.
During the median follow-up period of 4 years in the whole study group of 149 HCM patients 4 (2.7%) patients died due to non-cardiac cause: lung cancer (n = 1), breast cancer (n = 1), malignant melanoma (n = 1) and a haemorrhagic shock after a peripheral vascular intervention (n = 1). A cardiac death occurred in 12 (8.1%) patients due endocarditis (n = 1), SCD (n = 9) and HTX (n = 2). In the final analysis for major adverse cardiac arrhythmic events, 117 patients with a complete arrhythmic documentation were included. During the median follow-up period the likelihood to suffer from a major adverse arrhythmic events was 14.5%. Among the 17 HCM patients who suffered a major adverse arrhythmic event, SCD occurred in 9 patients, 2 patient suffered ES, 1 experienced an adequate ICD shock. Sustained VT on holter ECG was found in another 4 patients and 1 patient presented with torsade de point tachycardia at the emergency room.
ROC curves of extent LGE % showed an AUC of 0.86 (95%CI 0.75–0.96; p < 0.0001 (Fig. 3) and the optimal cut-off values for extent LGE% to predict major adverse cardiac arrhythmic events based on maximizing the sum of sensitivity and specificity was determined for an extent LGE of 20% (sensitivity: 88%; specificity: 82%). The ROC curve for the probability of SCD according to the ESC risk score revealed an AUC of 0.73 (95%CI 0.59–0.87; p = 0.002 (Fig. 3). The cut-off value for the probability of SCD according to the ESC risk score was 2.6% (sensitivity 71%, specificity 70%). Table 3 illustrates the distribution of patients with major adverse arrhythmic event and event-free patients according to ESC risk score classification in low, intermediate and high risk patients with HCM. Among the different risk groups, a further subdivision was created depending on the presence of an extent LGE ≥20% or <20%. This data shows that in the group of patients with a low or intermediate ESC risk score (<6%) the sensitivity of an extent LGE ≥20% to predict a major adverse arrhythmic event is 84.6%. The ability of extent LGE ≥20% for further risk stratification in the low and intermediate ESC risk group is further underlined by the significant higher event-rate in the group of HCM patients with an extent LGE ≥20% compared to HCM patients with an extent LGE % below the cut-off in Kaplan–Meier survival analysis (Fig. 4) (Log-rank, p < 0.0001). In the group of patients with an ESC risk <6% and an extent LGE ≥20% (n = 26), 11 major adverse cardiac arrhythmic events occurred during the follow-up period resulting in an annual event rate of 10.5% in these patients (Table 3). In patients with a high ESC risk score ≥6% none of the patients with an extent LGE <20% suffered from a major adverse arrhythmic event (Table 3). Therefore, an extent LGE <20% exhibits a negative predictive value of 100% in the ESC high risk group.
ROC Curves of extent LGE% and ESC risk score to predict major adverse cardiac arrhythmic events in patients with HCM. AUC for extent LGE% was 0.86 (95%CI 0.75–0.96; p < 0.0001) and for ESC risk score was 0.73 (95%CI 0.59–0.87; p = 0.002). ROC indicates receiver operating characteristic; AUC area under curves; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; CI, confidence interval.
Kaplan-Meier event-free survival curves in patients with HCM according to extent of LGE%. According to this analysis, extent LGE ≥20% was an indicator of worse outcomes during follow-up compared with patients with extent LGE <20% (Log-rank, p < 0.0001). HCM indicates hypertrophic cardiomyopathy; LGE, late gadolinium enhancement.
Discussion
Our study showed that HCM patients with a high risk of SCD according to the ESC clinical risk prediction model revealed the highest extent of LGE % compared to patients with HCM and lower ESC risk scores. The extent LGE % showed a better predictive value to estimate major adverse cardiac arrhythmic events than ESC risk score. Furthermore, in patients with a low and intermediate ESC risk score <6% a high extent LGE % identified a subgroup of patients with an increased risk for major adverse cardiac arrhythmic events. On the other hand, in patients with high ESC risk score ≥6% an extent LGE <20% was associated with a low risk of major adverse cardiac arrhythmic events. Therefore, we concluded that these results support the hypothesis that the extent of LGE might play a role as an additional risk marker that could help to further improve risk stratification in these patients.
Most patients with HCM have a normal life expectancy15, 23. However, despite all efforts some patients with HCM still suffer from SCD5. Up to now, the risk for SCD was estimated clinically by evaluating parameters that reflect the severity of the underlying myocardial disease. In the last years, considerable improvements of risk stratification in these patients have been made. The new ESC risk prediction model14 is a validated risk prediction model for SCD that does not intend to render the physician’s judgement superfluous but helps to stratify patients to three risk groups with close interactions and smooth transitions treating the SCD risk as a continuum15.
Nevertheless, not all patients at risk are identified by conventional risk markers24,25,26. Therefore, intensive research activities have been undertaken to find new risk markers27, 28 aiming at identifying HCM patients who profit most from the implantation of a lifesaving ICD. Contrast enhanced CMR has emerged as an interesting additional imaging tool which is able to determine accurately the LV wall thickness due to high resolution imaging29 also in regions in which hypertrophy might be underestimated echocardiographically30. Moreover, CMR offers the possibility to detect areas of LGE. However, the precise pathophysiological basis for the occurrence of LGE in HCM is still ambiguous. Currently, LGE is mostly considered to represent areas of replacement fibrosis. This hypothesis is supported by two case reports31, 32 correlating areas of histological fibrosis to LGE in explanted hearts of patients with end-stage HCM. In the current literature, LGE is detected in 33–86% of patients with HCM33,34,35,36. The myocardial fibrosis is pathophysiologically assumed to be associated with re-entrant ventricular arrhythmia and myocardial dysfunction37. On holter monitoring, an association between LGE and NSVT could be shown38, 39. Besides, Fluechter et al.40 revealed that the extent of LGE % was higher in HCM patients with inducible ventricular tachyarrhythmias during programmed ventricular stimulation. Several small studies35, 38, 39, 41, 42 could show a correlation between the presence of LGE and adverse outcome in HCM, but there was only a trend towards an increased risk of SCD in the pooled data analysis28. The problem about the published data is the limited reliability due to selection and referral bias, differences in scanning protocols and LGE quantification14. Therefore, prospective multicenter longitudinal studies are needed to prove the role of the extent of LGE as an independent risk in patients with HCM. Due to the individually variable disease course in patients with HCM additional risk factors must therefore be established to decide which patients profit most form a more aggressive medical and device therapy.
The presence and extent of LGE seems to be a promising additional risk marker that has the potential to further improve the risk stratification in these patients beyond the known and well-established clinical risk markers.
Conclusion
Patients with HCM who have a high ESC risk score reveal the highest extent of LGE compared to patients with HCM and an intermediate or low ESC risk score. During the follow-up an extent LGE ≥20% identified patients at a higher risk for major adverse cardiac arrhythmic events in the low and intermediate ESC risk group; whereas an extent LGE <20% was associated with a lower risk of major adverse cardiac arrhythmic events despite a high ESC risk score ≥6%. Therefore, the results of our study support the role of extent of LGE % as an additional risk marker that could help to further improve risk stratification in patients with HCM.
Perspectives
In patients with HCM the identification of patients at risk for SCD is crucial. Drugs have not been proven to be effective to prevent SCD and ICD implantation is the only treatment strategy that has been shown to extend life. Therefore, risk stratification to identify the small proportion of patients who might benefit from prophylactic ICD implantation is a crucial part of the patient management in HCM. In contrast to patients with coronary artery disease, patients with HCM who undergo ICD implantation are young and might face serious ICD related complications during their lifetime. CMR using LGE has emerged as an important noninvasive imaging modality to visualize myocardial fibrosis that is supposed to be the underlying structural cause of the ventricular tachyarrhythmias. In our study, we could show that there is a significant increase in the amount of LGE with increasing clinical risk underlining the importance of visualizing and quantification of potential substrate. Furthermore, our study revealed a significantly higher risk for major cardiac arrhythmic events in patients with HCM and an extent LGE ≥20% belonging to the low and intermediate ESC risk score group. In these patients, a primary preventive ICD implantation would have been deferred based on the ESC risk prediction score but the additional determination of the extent LGE% identified a subset of patients at increased risk for major cardiac arrhythmic events. On the other hand, despite a high ESC risk score ≥6%, an extent LGE <20% seemed to identify a low risk patient group. None of these patients experienced a major cardiac arrhythmic event during follow-up of 4 years. Therefore, the absence of extent LGE might corroborate the decision to decide against ICD implantation in selected high risk patients.
Further standardized prospective multicenter and multivendor studies are needed to clearly depict the value of LGE CMR and establish the extent of LGE as a risk factor in future risk stratification models.
References
Maron, B. J. Hypertrophic cardiomyopathy: a systematic review. Jama 287, 1308–1320 (2002).
Su, M. et al. Rare variants in genes encoding MuRF1 and MuRF2 are modifiers of hypertrophic cardiomyopathy. Int J Mol Sci 15, 9302–9313 (2014).
Wigle, E. D., Rakowski, H., Kimball, B. P. & Williams, W. G. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92, 1680–1692 (1995).
Maron, B. J. et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 42, 1687–1713 (2003).
Elliott, P. M. et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 36, 2212–2218 (2000).
Maron, B. J. Sudden death in young athletes. N Engl J Med 349, 1064–1075 (2003).
Weinstock, J., Bader, Y. H., Maron, M. S., Rowin, E. J. & Link, M. S. Subcutaneous Implantable Cardioverter Defibrillator in Patients With Hypertrophic Cardiomyopathy: An Initial Experience. J Am Heart Assoc 5, e002488 (2016).
Maron, B. J. et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 342, 365–373 (2000).
Gersh, B. J. et al. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58, e212–260 (2011).
Maron, B. J. et al. Prevention of sudden cardiac death with implantable cardioverter-defibrillators in children and adolescents with hypertrophic cardiomyopathy. J Am Coll Cardiol 61, 1527–1535 (2013).
Olivotto, I. et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 104, 2517–2524 (2001).
Morrison, T. B. et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol 21, 671–677 (2010).
Lin, G. et al. Device complications and inappropriate implantable cardioverter defibrillator shocks in patients with hypertrophic cardiomyopathy. Heart 95, 709–714 (2009).
Elliott, P. M. et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35, 2733–2779 (2014).
O’Mahony, C. et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 35, 2010–2020 (2014).
Maron, B. J. et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113, 1807–1816 (2006).
Simonetti, O. P. et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218, 215–223 (2001).
Papavassiliu, T. et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 236, 57–64 (2005).
Alfakih, K. et al. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 17, 323–329 (2003).
Sievers, B. et al. Assessment of left atrial volumes in sinus rhythm and atrial fibrillation using the biplane area-length method and cardiovascular magnetic resonance imaging with TrueFISP. J Cardiovasc Magn Reson 6, 855–863 (2004).
Sievers, B., Addo, M., Breuckmann, F., Barkhausen, J. & Erbel, R. Reference right atrial function determined by steady-state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 9, 807–814, doi:10.1080/10976640701545552 (2007).
Doesch, C. et al. Visual estimation of the extent of myocardial hyperenhancement on late gadolinium-enhanced CMR in patients with hypertrophic cardiomyopathy. Magn Reson Imaging 28, 812–819 (2010).
Maron, B. J., Casey, S. A., Hauser, R. G. & Aeppli, D. M. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol 42, 882–888 (2003).
Maron, B. J. et al. Sudden cardiac arrest in hypertrophic cardiomyopathy in the absence of conventional criteria for high risk status. Am J Cardiol 101, 544–547 (2008).
Bongioanni, S. et al. Extensive myocardial fibrosis in a patient with hypertrophic cardiomyopathy and ventricular tachycardia without traditional high-risk features. Circ Cardiovasc Imaging 2, 349–350 (2009).
Maron, B. J. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 121, 445–456 (2010).
Maron, M. S. et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 118, 1541–1549 (2008).
Green, J. J., Berger, J. S., Kramer, C. M. & Salerno, M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 5, 370–377 (2012).
Moon, J. C. & McKenna, W. J. The emerging role of cardiovascular magnetic resonance in refining the diagnosis of hypertrophic cardiomyopathy. Nat Clin Pract Cardiovasc Med 6, 166–167 (2009).
Rickers, C. et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 112, 855–861 (2005).
Moon, J. C. et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 43, 2260–2264 (2004).
Papavassiliu, T., Schnabel, P., Schroder, M. & Borggrefe, M. CMR scarring in a patient with hypertrophic cardiomyopathy correlates well with histological findings of fibrosis. Eur Heart J 26, 2395 (2005).
Olivotto, I. et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 52, 559–566 (2008).
Spirito, P. et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342, 1778–1785 (2000).
Bruder, O. et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 56, 875–887 (2010).
Kwon, D. H. et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 54, 242–249 (2009).
Noureldin, R. A. et al. The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14, 17 (2012).
Adabag, A. S. et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 51, 1369–1374 (2008).
O’Hanlon, R. et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 56, 867–874 (2010).
Fluechter, S. et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 12, 30 (2010).
Prinz, C. et al. Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol 29, 358–363 (2013).
Rubinshtein, R. et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 3, 51–58 (2010).
Acknowledgements
This study was supported by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
Author information
Authors and Affiliations
Contributions
C.D., E.T. and T.P. wrote the main manuscript text; C.D. and T.P. prepared figures and tables; C.D., T.P., J.B., A.S. and S.O.S. analyzed the cardiac magnetic resonance images; E.T., I.A., B.R., J.K., M.B. and I.E.B. collected patient’s data and calculated E.S.C. risk score; C.D., T.P. and E.T. performed statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doesch, C., Tülümen, E., Akin, I. et al. Incremental benefit of late gadolinium cardiac magnetic resonance imaging for risk stratification in patients with hypertrophic cardiomyopathy. Sci Rep 7, 6336 (2017). https://doi.org/10.1038/s41598-017-06533-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06533-0
This article is cited by
-
Gendiagnostik bei kardiovaskulären Erkrankungen
Die Kardiologie (2023)
-
Prognostic significance of cardiac magnetic resonance-based markers in patients with hypertrophic cardiomyopathy
The International Journal of Cardiovascular Imaging (2021)
-
Risk stratification in hypertrophic cardiomyopathy
Herz (2020)
-
Multimodality imaging predictors of sudden cardiac death
Heart Failure Reviews (2020)
-
Myocardial tissue characterization by gadolinium-enhanced cardiac magnetic resonance imaging for risk stratification of adverse events in hypertrophic cardiomyopathy
The International Journal of Cardiovascular Imaging (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.