Abstract
Managing cardiovascular disease (CVD) risk factors is the key to prevent CVD. This study aimed to prevent CVD by introducing asymptomatic meibomian gland dysfunction (MGD), a condition associated with various CVD risk factors, as an early indicator for CVD in middle-aged population. Participants with and without asymptomatic MGD underwent standardized questionnaires, physical examinations, and laboratory investigations. One ophthalmologist completed the identification and grading of MGD by using slit-lamp biomicroscopy examination on the eyelid margins, meibomian gland orifices, and meibomian gland secretions. Standardized techniques were used to measure the CVD risk factor parameters. After adjusted for age and gender, CVD risk factors including elevated uric acid (P = 0.01), total cholesterol (Total-C, P < 0.001), low-density lipoprotein cholesterol (LDL-C, P < 0.001), fasting triglyceride (Fasting TG, P < 0.001), decreased high-density lipoprotein cholesterol (HDL-C, P = 0.04), and presence of hepatic steatosis (P = 0.008) were significantly associated with asymptomatic MGD. Stepwise logistic regression analysis revealed that LDL-C (OR: 1.03, 95% CI: 1.02–1.04) and Fasting TG (OR: 1.01; 95% CI: 1.00–1.01) levels were risk factors for having asymptomatic MGD (P < 0.001). Together, the results suggest that asymptomatic MGD may serve as an indicator for CVD.
Similar content being viewed by others
Introduction
Meibomian glands, or a row of sebaceous, holocrine glands that lie along the lid margin, are responsible for the lipid portion of the tear film1 through the secretion of meibum, which not only facilitates spread of the tear by retarding its evaporation, but also acts as a barrier against contamination2. Frequently encountered in clinical practice1, meibomian gland dysfunction (MGD) is characterized by outflow obstruction, increased meibum viscosity, duct dilatation, hyposecretion, and even acinar atrophy, which causes patient great distress. Several systemic factors such as aging3, androgen deficiency4, 5, anti-androgen use5, atopy6, menopause5, Parkinson’s disease7, rosacea8, Sjogren’s syndrome9, and Stevens-Johnson syndrome10 have been reported to be correlated with MGD. Given the lipid component of meibomian gland’s secretion, it is plausible to deduce that disorders regarding production or metabolism of lipid may also lead to MGD. Recently, numerous studies have found correlation between symptomatic MGD and dyslipidemia11,12,13,14. It remains unexplored whether this correlation still holds true in the population with asymptomatic MGD.
The importance of dyslipidemia lies in the fact that it is one of the major risk factors in cardiovascular disease (CVD)15. However, dyslipidemia is the only factor that has been discussed in conjunction with MGD, while other CVD risk factors such as obesity, diabetes mellitus, hypertension, metabolic syndrome, high-sensitivity C-reactive protein (hs-CRP), age, sex, uric acid level, and the presence of hepatic steatosis have not. Although it is well known that the above CVD risk factors16,17,18,19 as well as MGD11,12,13,14 are related to lipid and glucose metabolism, it remains unknown whether MGD is correlated with CVD risk factors.
One can never overemphasize the importance of early detection of CVD, which is one of the most common cause of death and also greatly impacts the lives of middle-aged population and their families. Having early prevention of CVD in mind, and that asymptomatic MGD is more common than symptomatic MGD20, current study aimed to identify the correlation between asymptomatic MGD and CVD risk factors in a middle-aged population using the screening profiles obtained during the participants’ voluntary physical examinations and laboratory investigations.
Results
The prevalence of asymptomatic MGD among middle-aged, community-based participants was 89/1329 (6.7%). The participant’s baseline characteristics and metabolic status with and without asymptomatic MGD are listed in Table 1. Most participants with asymptomatic MGD were men (86.5%). Participants with asymptomatic MGD had higher mean serum concentrations of Total-C (P < 0.001), LDL-C (P < 0.001), Fasting TG (P < 0.001), uric acid (P = 0.02), and higher probability of hepatic steatosis (P = 0.002) than those without. Conversely, participants with asymptomatic MGD had lower mean serum concentrations of HDL-C (P = 0.030) than those without. There were no significant differences in terms of WC, BMI, SBP, DBP, AC, PC, hs-CRP, age, sex, and the presence of metabolic syndrome between these two groups with or without asymptomatic MGD.
Correlations between Asymptomatic Meibomian Gland Dysfunction and Cardiovascular Disease Risk Factors
To assess the correlations between asymptomatic MGD and CVD risk factors, we divided continuous variables into three tertiles (Table 2). After adjusted for age and gender, participants having elevated uric acid (P = 0.01), Total-C (P < 0.001), LDL-C (P < 0.001), Fasting TG (P < 0.001), decreased HDL-C (P < 0.04), as well as having hepatic steatosis (P < 0.008) were significantly associated with asymptomatic MGD. No significant correlations were detected between asymptomatic MGD and parameters such as WC, BMI, SBP, DBP, AC, PC, and hs-CRP.
Risk Factors for Asymptomatic Meibomian Gland Dysfunction by using Multivariate Logistic Regression
The risk factors for asymptomatic MGD via multivariate logistic regression analysis were documented in Table 3. Higher LDL-C was a significant and independent risk factor for asymptomatic MGD (OR: 1.03; 95% CI: 1.02–1.04). Higher Fasting TG was also a significant but milder risk (OR: 1.01; 95% CI: 1.00–1.01) for asymptomatic MGD. Both results were consistent with the above findings.
Associations between the Severity of Asymptomatic Meibomian Gland Dysfunction and Risk of Having Dyslipidemia
To further evaluate the correlations between asymptomatic MGD and CVD risk factors, participants were categorized into three groups via findings in the slit lamp: no MGD, mild MGD, and moderate-to-severe MGD. We then calculated the odds ratio of dyslipidemia in terms of four indicators including Total-C, LDL-C, HDL-C and Fasting TG (Table 4). After adjusted for age and gender, analysis showed significant association between asymptomatic MGD and dyslipidemia except HDL-C, including Total-C ≧ 240 mg/dL, LDL-C ≧ 160 mg/dL, and Fasting TG ≧ 150 mg/dL. Also, the participants with severe asymptomatic MGD also had significantly higher risk of having Fasting TG ≧ 160 mg/dL than those who had asymptomatic MGD (OR: 2.70 versus 5.46).
Discussion
In order to establish the relationship between asymptomatic MGD and CVD, it is imperative to first identify the potential CVD risk factors for asymptomatic MGD. In this study, we found that dyslipidemia, uric acid, and hepatic steatosis were significantly associated with asymptomatic MGD. Furthermore, increased level of Total-C, LDL-C, Fasting TG, and decreased level of HDL-C were positively correlated with the severity of asymptomatic MGD.
The design of this study was built on the notion that meibomian gland, a sebaceous gland secreting the lipid layer of the tear film, is related to serum lipid. In fact, in 2008, Butovich et al. confirmed that the major component of human meibum is cholesteryl ester, an inactive form of cholesterol for the efficiency of transportation21. Consequently, this implies that the abnormality of serum lipid and glucose metabolism results in diseases such as MGD, CVD, as well as other risk factors of CVD. Therefore, it is crucial to discuss the association between MGD and dyslipidemia. Although some studies in the past have suggested that meibomian gland is capable of synthesizing lipid de novo22, 23, more recent studies, although differing in various degrees, have found correlations between meibomian lipids and serum lipid profile levels (Table 5). In 2010, Dao et al. studied the association between dyslipidemia and moderate to severe MGD and found a higher prevalence of elevated Total-C in patients with MGD than those without. In addition, since the incidence of decreased HDL-C levels was actually lower in patients with MGD, they concluded that it is mainly the elevated HDL-C levels that contributed to the increased Total-C13. In 2013, Bukhari analyzed the correlation between the severity of MGD and the severity of dyslipidemia and found no correlation between the two, but the severity of MGD is correlated with the increased prevalence of high Fasting TG and LDL-C levels12. Pinna et al., in 2013, investigated the correlation between MGD and hypercholesterolemia in young and middle-aged participants and their results, like Braich et al.’s study in 2015, showed that participants with MGD have higher mean levels of Total-C, LDL-C, Fasting TG, and HDL-C than those without. Nonetheless, the results differed in that Pinna et al. found MGD to be associated with higher serum concentrations of Total-C, LDL-C, and HDL-C as compared to Braich et al.’s higher serum concentrations of Total-C, LDL-C, and Fasting TG11, 14. Our results were mostly in agreement with the previous findings in that participants with asymptomatic MGD had higher mean levels of Total-C, LDL-C, and Fasting TG than those without. However, lower mean level of HDL-C was observed in our study for participants with asymptomatic MGD than those without. In addition, after adjusted for age and gender, elevated Total-C, LDL-C, Fasting TG, and decreased HDL-C were significantly associated with asymptomatic MGD. Through multivariate logistic regression analysis, higher LDL-C and Fasting TG were independent risk factors for asymptomatic MGD (Table 3). Concisely, the most surprising findings were the lower mean level of HDL-C in participants with asymptomatic MGD, as well as the association between decreased HDL-C and asymptomatic MGD, which contradicted with the previous studies. We think that dyslipidemia may be the cause of MGD, but whether it is symptomatic or asymptomatic lies with the higher and lower serum level of HDL-C, respectively. Still, further studies are needed to support the observation. Despite the above discrepancies between our study and the previous ones, the lipid profile in our participants with asymptomatic MGD, specifically the lower mean HDL-C level, seemed to coincide with the current belief of what is known as susceptible to CVD18. Importantly, this should be the first study to have identified a correlation between the severity of asymptomatic MGD and the risk of dyslipidemia. Namely, the severity of asymptomatic MGD is positively correlated with increased risks of Total-C, LDL-C, and Fasting TG (Table 4). Collectively, based on our association analysis between dyslipidemia and asymptomatic MGD, as well as the correlation analysis between the severity of asymptomatic MGD and the risk of dyslipidemia, we speculate that asymptomatic MGD may be associated with CVD.
An interesting finding in our study was the association between the serum uric acid level and asymptomatic MGD. We not only found a higher serum uric acid level in the asymptomatic MGD group in comparison to that of the control, after adjusted for age and gender, we also found positive association between uric acid and asymptomatic MGD. Previously, it was detected that the mean level of uric acid for participants with (6.0 mg/dL) and without MGD (6.5 mg/dL) are both lower than the normal mean of 6.7 mg/dL for Taiwanese population according to Gosling et al.’s review24. One way to interpret the observed lower-than-average uric level in both groups was that our participants were collected from one single medical center and happened to be lower than the entire Taiwanese population. A second possible explanation was that Gosling et al. only collected male data to avoid the confounding hormone effects on uric acid level in pre-menopausal women24, which resulted in such bias. Nonetheless, the relationship between uric acid and asymptomatic MGD needs to be elucidated. Several studies have shown that serum uric acid level is associated with dyslipidemia, obesity, insulin resistance, hypertension25, 26, and metabolic syndrome16. Zhu et al.’s experimental study on mice showed that serum uric acid increases oxidative stress and reactive oxygen species, which leads to insulin resistance and abnormal glucose metabolism27. Knowing that glucose and lipid are molecularly interchangeable, perhaps this explains the reason that the above risk factors as well as asymptomatic MGD, are associated with CVD.
Our discovery of the association between the presence of hepatic steatosis and asymptomatic MGD was also an intriguing finding. A systematic review and meta-analysis by Gong et al. disclosed that hyperuricemia is associated with increased risk for nonalcoholic hepatic steatosis28. Studies have also shown that nonalcoholic hepatic steatosis is highly associated with metabolic syndrome, diabetes29, dyslipidemia, and cardiovascular disease30, 31. The specific mechanisms of fatty liver formation have not been well delineated and are currently believed to be multifactorial. Nonetheless, Rinella et al.’s systematic review proposed that the alteration of lipid and glucose metabolism generated by visceral adipose tissues is a major cause of fat buildup, which consequently causes organ inflammations and injuries32. This explanation concurs with our observation and further justifies that asymptomatic MGD is also the result of alteration in lipid and glucose metabolism, and therefore is associated with CVD risk factors.
In spite of the above findings that support the association of asymptomatic MGD and CVD, we did not observe any associations among asymptomatic MGD and other CVD risk factors that are supposedly interrelated to dyslipidemia: these include the WC, BMI, SBP, DP, fasting glucose, PC glucose, and metabolic syndrome. Obesity is the consequence of fat accumulation, and is inevitably interrelated to dyslipidemia. Common assessments for obesity include BMI and WC. Lately, studies have found no differences in BMI between groups with and without asymptomatic MGD12, 14. In addition to BMI, we have included WC for obesity indicator to reinforce the evaluation. Unfortunately, no significant differences were observed among MGD, BMI and WC in groups with and without asymptomatic MGD. Obesity and hepatic steatosis are both the result of lipid accumulation, but only hepatic steatosis was found to be correlated with asymptomatic MGD. A possible explanation is that visceral adiposity specifically33, rather than fat accumulation in general34, is the major contributor to the complications of obesity. As for blood pressure, it is well known that most causes of hypertension cannot be determined. Hence, even though there are many factors that may contribute to hypertension, they all play a certain but limited role. Similar to our results, those published by Viso et al. also failed to demonstrate any significant difference in blood pressure between patients with and without asymptomatic MGD20. With regard to hyperglycemia, Viso et al. found that asymptomatic MGD, but not symptomatic MGD, is associated with diabetes, which is unexpected and difficult to explain. More recent studies reinforced the idea by looking at the serum glucose level but found no significant differences between groups with and without MGD12, 14. In our study, fasting glucose and PC glucose were, for the first time, added to the comparison, but no significant differences were found between groups with and without asymptomatic MGD. Finally, it makes sense to include metabolic syndrome into this study, for metabolic syndrome encompasses dyslipidemia, visceral obesity, hypertension, and hyperglycemia19 and is known to greatly increase the risk of CVD35. To our knowledge, this is the first study to illustrate the relation between metabolic syndrome and asymptomatic MGD. Unfortunately, no association were found in this study. Taken together, in order to better evaluate the association among the above CVD risk factors and asymptomatic MGD, visceral rather than general obesity should be focused; on the other hand, too many disrupting mechanisms are involved in hypertension and hyperglycemia, which lead to the lack of association between the presence of metabolic syndrome and asymptomatic MGD. Accordingly, further studies are needed to unravel the missing links.
We were also unable to identify any association among asymptomatic MGD, hs-CRP, age, and gender. This should be the first and only report on the relation of hs-CRP and asymptomatic MGD. It is well known that hs-CRP is a non-specific inflammatory biomarker. Other studies also demonstrated that CRP can be found in atherosclerotic plaques that are responsible for acute coronary syndromes36, implicating that the elevated hs-CRP observed may be mainly attributed to atherosclerotic involvements and may not necessarily have any relation with asymptomatic MGD. The lack of association in hs-CRP between groups with and without asymptomatic MGD, therefore, probably depended on the underlying atherosclerotic involvement. As for the lack of difference in age between groups with and without MGD, our study was preconditioned so that the participants enrolled were between the age of 30 and 60 in order to focus on the middle-aged population, which inevitability reduced the variation. The strength of this preconditioning was that the differences in gender between groups with and without asymptomatic MGD could be more evidently identified. In a recent study by Braich et al.11, the odds of having MGD is moderately increased in the population of elderly (age > 65) male. Our study, which focused on the middle-aged population, found no such difference in terms of sex, suggesting that the etiology of asymptomatic MGD involving gender differences is of late-onset, but the exact mechanism is still uncertain.
There were some limitations in our study. Firstly, the ophthalmic examinations were completed by a single ophthalmologist, which minimized the interobserver error but at the same time reduced the reproducibility. Secondly, all our participants were collected from a single medical center, resulting in selection bias. Thirdly, the majority of participants with (86.5%) and without asymptomatic MGD (79.9%) were comprised of male, which made the differences between gender difficult to distinguish. Lastly, our retrospective cross-sectional study failed to demonstrate the temporal sequence between asymptomatic MGD and the CVD risk factors.
In summary, in the middle-aged Taiwanese population, our study suggests that the association between asymptomatic MGD and CVD is dependent on elevated Total-C, LDL-C, Fasting TG, decreased HDL-C, increased uric acid, and the presence of hepatic steatosis. Since early diagnosis of CVD can increase the survival rate drastically, we recommend that MGD screening should be used for such purpose. Further larger prospective studies on the association between asymptomatic MGD and CVD are also warranted.
Methods
Participant Recruitment
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Linkou, Taiwan (Registration Number: 201600086B0) and adhered to the tenets of the Declaration of Helsinki.
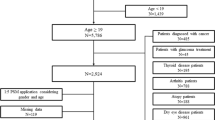
A retrospective single-center cross-sectional study of eyes with asymptomatic MGD from middle-aged participants was conducted. The clinical records of 1,979 participants registered for the Health Care Program at the Chang Gung Memorial Hospital during an interval of four years were reviewed. Participants with age between 30 and 60 years old were enrolled (n = 1,329). The exclusion criteria included those with thyroid dysfunction, alcoholism, dyslipidemia under lipid-lowering therapy, positive serology for hepatitis B or hepatitis C virus, and ophthalmic abnormalities such as cataract, glaucoma, uveitis, previous ocular surgery, or symptomatic MGD. A group of participants with asymptomatic MGD (n = 89) who met the criteria was eligible for this study. Likewise, another group of age-matched participants (n = 199) who met the identical criteria, except that MGD was absent in all eyes, was used for the control group.
History Taking and Physical Biometrics
Baseline characteristics, including history takings and physical biometrics, were carried out by the medical personnel in the Center of Health Promotion at Chang Gung Memorial Hospital. Demographic data as well as medical and surgical histories were recorded during history takings. Baseline characteristics such as systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference (WC), presence of metabolic syndrome, body weight, and height (calculated into BMI, kg/m2) were obtained. Asymptomatic MGD was determined through modified questionnaire37 by excluding the six symptoms: the sensation of dryness, grittiness, burning, redness, lash crusting, and eyelids getting stuck.
Slit-lamp Biomicroscopy
The ophthalmic examinations of all participants were completed by a single ophthalmologist (Chen HC) using SL-3E slit-lamp biomicroscopy (Topcon, Paramus, New Jersey). The eyelid margins, meibomian gland orifices, and meibomian gland secretions were carefully examined for signs of MGD and these were classified according to modified previous criteria38. MGD was graded on a scale of 0 (no obstruction) to 4 (complete obstruction). Meibum quality was graded on a scale of 0 (clear) to 4 (toothpaste-like). Participants graded 2 or lower on either of these scales in either eyes were diagnosed with mild MGD, while others moderate-to-severe.
Laboratory Investigation
Serum lipid profiles including total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and fasting triglyceride (Fasting TG) were obtained in all participants. Fasting glucose (AC), 2-hour postprandial glucose (PC), uric acid, and hs-CRP were also checked in sera from all participants. All biochemical data were measured by the Department of Clinical Laboratory at Chang Gung Memorial Hospital. The lipid profiles were determined by enzymatic colorimetric method (Total-C and Fasting TG) and accelerator selective detergent method (LDL-C and HDL-C) using 7600-210 Clinical Analyzers (Hitachi, Tokyo, Japan). Dyslipidemia was defined as Total-C ≧ 240 mg/dL, LDL-C ≧ 160 mg/dL, and HDL-C < 40 mg/dL18.
Abdominal Ultrasonography
Abdominal ultrasonography was performed and interpreted by the gastroenterologists using HDI-5000 SonoCT (Philips Co., Bothell, Washington) in all participants. Hepatic steatosis, also known as fatty liver disease, was diagnosed by characteristics of echoic patterns according to conventional criteria39. Hepatic steatosis was graded into mild and moderate-to-severe groups according to the ultrasonic findings.
Statistical Analysis
Unless otherwise specified, data were presented in the forms of means ± standard deviation or frequencies. A P value < 0.05 was considered as statistically significant. Odds ratio with 95% confidence interval was used to compare the possibility of asymptomatic MGD. All data were analyzed using SPSS software version 13.0 (SPSS Inc. Chicago, Illinois).
To compare the differences of basic characteristics and cardiovascular risk factors between participants with and without asymptomatic MGD, Student’s t test and χ2 test were used to compare the means of continuous variables and gender differences, respectively.
To assess the correlations between asymptomatic MGD and potential CVD risk factors, continuous variables were divided into tertiles, and standardized relative risks of asymptomatic MGD were then calculated by comparing the rates of asymptomatic MGD in each group relative to that of the lowest group. Trends of the rate of asymptomatic MGD among these three groups were assessed by χ2 test. Stepwise multiple logistic regression analysis was performed to identify the independent risk factors associated with asymptomatic MGD.
To further evaluate the associations between intensity of asymptomatic MGD and dyslipidemia, age- and gender-adjusted relative risks were calculated for the presence of mild or moderate-to-severe MGD related to the absence of MGD.
Data Availability
The datasets for the analysis of the current study are readily available from the corresponding author on reasonable request.
References
Bron, A. J. & Tiffany, J. M. The contribution of meibomian disease to dry eye. Ocul Surf 2, 149–165 (2004).
Driver, P. J. & Lemp, M. A. Meibomian gland dysfunction. Surv Ophthalmol 40, 343–367 (1996).
Borchman, D., Foulks, G. N. & Yappert, M. C. & Milliner, S. E. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Invest Ophthalmol Vis Sci 53, 475–482, doi:10.1167/iovs.11-8341 (2012).
Krenzer, K. L. et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab 85, 4874–4882, doi:10.1210/jcem.85.12.7072 (2000).
Sullivan, D. A. et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci 966, 211–222 (2002).
Bron, A. J., Benjamin, L. & Snibson, G. R. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond) 5(Pt 4), 395–411, doi:10.1038/eye.1991.65 (1991).
Tamer, C., Melek, I. M., Duman, T. & Oksuz, H. Tear film tests in Parkinson’s disease patients. Ophthalmology 112, 1795, doi:10.1016/j.ophtha.2005.04.025 (2005).
Buechner, S. A. Rosacea: an update. Dermatology 210, 100–108, doi:10.1159/000082564 (2005).
Shimazaki, J., Goto, E., Ono, M., Shimmura, S. & Tsubota, K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology 105, 1485–1488, doi:10.1016/S0161-6420(98)98033-2 (1998).
Sotozono, C. et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 114, 1294–1302, doi:10.1016/j.ophtha.2006.10.029 (2007).
Braich, P. S., Howard, M. K. & Singh, J. S. Dyslipidemia and its association with meibomian gland dysfunction. Int Ophthalmol 36, 469–476, doi:10.1007/s10792-015-0149-4 (2016).
Bukhari, A. A. Associations between the grade of meibomian gland dysfunction and dyslipidemia. Ophthal Plast Reconstr Surg 29, 101–103, doi:10.1097/IOP.0b013e31827a007d (2013).
Dao, A. H. et al. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am J Ophthalmol 150, 371–375 e371, doi:10.1016/j.ajo.2010.04.016 (2010).
Pinna, A., Blasetti, F., Zinellu, A., Carru, C. & Solinas, G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology 120, 2385–2389, doi:10.1016/j.ophtha.2013.05.002 (2013).
Patel, S. A., Winkel, M., Ali, M. K., Narayan, K. M. & Mehta, N. K. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med 163, 245–253, doi:10.7326/M14-1753 (2015).
Choi, H. et al. Serum uric acid concentration and metabolic syndrome among elderly Koreans: The Korean Urban Rural Elderly (KURE) study. Arch Gerontol Geriatr 64, 51–58, doi:10.1016/j.archger.2016.01.005 (2016).
Cook, D. G. et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 149, 139–150 (2000).
National Cholesterol Education Program Expert Panel on Detection, E. & Treatment of High Blood Cholesterol in, A.. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Alberti, K. G., Zimmet, P., Shaw, J. & Group, I. D. F. E. T. F. C. The metabolic syndrome–a new worldwide definition. Lancet 366, 1059–1062, doi:10.1016/S0140-6736(05)67402-8 (2005).
Viso, E., Rodriguez-Ares, M. T., Abelenda, D., Oubina, B. & Gude, F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 53, 2601–2606, doi:10.1167/iovs.11-9228 (2012).
Butovich, I. A., Millar, T. J. & Ham, B. M. Understanding and analyzing meibomian lipids–a review. Curr Eye Res 33, 405–420, doi:10.1080/02713680802018419 (2008).
Duerden, J. M. & Tiffany, J. M. Lipid synthesis in vitro by rabbit Meibomian gland tissue and its inhibition by tetracycline. Biochim Biophys Acta 1042, 13–18 (1990).
van Haeringen, N. J. & Glasius, E. Cholesterol in human tear fluid. Exp Eye Res 20, 271–274 (1975).
Gosling, A. L., Matisoo-Smith, E. & Merriman, T. R. Hyperuricaemia in the Pacific: why the elevated serum urate levels? Rheumatol Int 34, 743–757, doi:10.1007/s00296-013-2922-x (2014).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N Engl J Med 359, 1811–1821, doi:10.1056/NEJMra0800885 (2008).
Gagliardi, A. C., Miname, M. H. & Santos, R. D. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 202, 11–17, doi:10.1016/j.atherosclerosis.2008.05.022 (2009).
Zhu, Y. et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun 447, 707–714, doi:10.1016/j.bbrc.2014.04.080 (2014).
Gong, S., Song, J., Wang, L., Zhang, S. & Wang, Y. Hyperuricemia and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 28, 132–138, doi:10.1097/MEG.0000000000000507 (2016).
Mofrad, P. et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37, 1286–1292, doi:10.1053/jhep.2003.50229 (2003).
Arulanandan, A. et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 13, 1513–1520 e1511, doi:10.1016/j.cgh.2015.01.027 (2015).
Chang, Y. et al. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol 108, 1861–1868, doi:10.1038/ajg.2013.349 (2013).
Rinella, M. E. Nonalcoholic fatty liver disease: a systematic review. JAMA 313, 2263–2273, doi:10.1001/jama.2015.5370 (2015).
Di Chiara, T., Argano, C., Corrao, S., Scaglione, R. & Licata, G. Hypoadiponectinemia: A Link between Visceral Obesity and Metabolic Syndrome. J Nutr Metab 2012, 175245, doi:10.1155/2012/175245 (2012).
Hall, J. E. et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285, 17271–17276, doi:10.1074/jbc.R110.113175 (2010).
Reynolds, K. & He, J. Epidemiology of the metabolic syndrome. Am J Med Sci 330, 273–279 (2005).
Inoue, T. et al. Local release of C-reactive protein from vulnerable plaque or coronary arterial wall injured by stenting. J Am Coll Cardiol 46, 239–245, doi:10.1016/j.jacc.2005.04.029 (2005).
Schein, O. D., Munoz, B., Tielsch, J. M., Bandeen-Roche, K. & West, S. Prevalence of dry eye among the elderly. Am J Ophthalmol 124, 723–728 (1997).
Foulks, G. N. & Bron, A. J. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf 1, 107–126 (2003).
Angulo, P. Nonalcoholic fatty liver disease. N Engl J Med 346, 1221–1231, doi:10.1056/NEJMra011775 (2002).
Acknowledgements
‘’This study was supported by the Chang Gung Memorial Hospital (CMRPG3F1471~2 and CMRPG3G0031~3) and the Ministry of Science and Technology (MOST 104-2314-B-182A-007-) to Chen HC. The funding organizations had no role in the design or conduct of this research; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.C.C. designed the research. A.C. and H.T.C. contributed equally significantly to this study. A.C., H.T.C., and H.C.C. contributed to the data acquisition and research execution. A.C., H.T.C., H.C.C., Y.T.C., and Y.H.H. analyzed and interpreted the acquired data. A.C., H.C.C., C.C.S., C.H.H., D.H.K.M., W.C.W., and C.C.L. prepared and reviewed the manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, A., Chen, HT., Chen, HC. et al. Asymptomatic Meibomian Gland Dysfunction and Cardiovascular Disease Risk Factors in a Middle-Aged Population in Taiwan - A Cross-sectional Analysis. Sci Rep 7, 4935 (2017). https://doi.org/10.1038/s41598-017-05368-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05368-z
This article is cited by
-
Meibomian Gland Dysfunction Clinical Practice Guidelines
Japanese Journal of Ophthalmology (2023)
-
Risk factors for the occurrence of visual-threatening posterior capsule opacification
Journal of Translational Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.