Abstract
This study evaluates the relationship between obstructive sleep apnea (OSA) and asthma. Literature search was carried out in several electronic databases and random effects meta-analyses were performed to obtain pooled estimates of the prevalence of OSA, OSA risk and sleep disordered breathing (SDB) in asthma patients and pooled odds ratios of the prevalence between asthma and non-asthma patients. In adult asthma patients, the prevalence [95% confidence interval] of OSA, OSA risk, and SDB was 49.50 [36.39, 62.60] %, 27.50 [19.31, 35.69] %, and 19.65 [14.84, 24.46] % respectively. The odds of having OSA, OS risk and SDB by the asthma patients were 2.64 [1.76, 3.52], 3.73 [2.90, 4.57] and 1.73 [1.11, 2.36] times higher (p < 0.00001 for all) in asthma than in non-asthma patients, respectively. Adult asthma patients with OSA had significantly higher BMI in comparison with asthma patients without OSA. This study reveals that the prevalence of OSA in asthma patients is considerably higher; even higher than OSA risk and SDB. Sleep studies should be performed in asthma patients with symptoms suggestive of OSA/OSA risk/SDB.
Similar content being viewed by others
Introduction
Asthma and obstructive sleep apnea (OSA) may coexist1 to result in an overlap syndrome2 where a bidirectional relationship may deleteriously affect each other3. At least 5% of the general population suffer from asthma4, 5; Center for Disease Control and Prevention estimates the prevalence of asthma at 7.7% (6.3% in males and 9% in females)6. On the other hand, OSA is an under-diagnosed condition7. In a general population survey, of the 451 individuals who were invited to participate in sleep study, only 3.6% had OSA diagnosis but 24% had mild, 12.5% moderate, and 2.9% had severe OSA8. In a larger population sample of 2121 individuals, 84% men and 61% women had mild, and 50% men and 23% women were found to have moderate OSA9. More recently, Senaratna et al.10 after reviewing 24 relevant studies have estimated the prevalence of OSA ranging between 9% to 38%. These authors also found this disease more common in men11.
Asthma is a common respiratory disorder with complex interactions between airflow obstruction, hyper-responsiveness, reversible expiratory flow limitation and inflammation11, whereas OSA is characterized by snoring and interruptions in breathing during sleep with symptoms such as brief paroxysmal nocturnal dyspnea, choking during sleep, and nocturia along with daytime sleep, depression and memory loss12, 13.
Previously, many authors have described the relationship between asthma and OSA with regards to the prevalence and risk. Asthma has been found to be an independent risk factor for the development of habitual snoring in a prospective cohort study14. Sleep disordered breathing has also been observed in many studies with asthma patients15,16,17,18,19. A bidirectional relationship between asthma and OSA is also evident from a study in which not only the OSA patients were reported to exhibit many asthma symptoms but also a high prevalence of asthma was reported in OSA patients20.
Despite the recognition of high prevalence of OSA in asthma patients, highly variable prevalence estimates are reported in the individual studies. Because this area is not systematically reviewed, no summary estimates of the association between asthma and OSA are available for clinical and/or public health implications. Keeping in view this scenario, the present study was designed to carry out a systematic review of the relevant studies and to perform a meta-analysis of the indices that could display the relationship between asthma and OSA, OSA risk and sleep disordered breathing (SDB).
Materials and Methods
This meta-analysis is being reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement21.
Inclusion and exclusion criteria
Inclusion criterion was: clinical or epidemiological studies which examined the relationship between asthma and sleep disorders and reported the prevalence of OSA or OSA risk or SDB in asthma patients. Studies were excluded from the meta-analysis if reported only the sleep quality measures other than OSA, OSA risk or SDB, or provided qualitative information only.
Literature search
Electronic scientific databases (EMBASE, Google Scholar, Ovid SP, PubMed/Medline, and Web of Science) were searched for the relevant research articles. The MeSH and keywords used in different logical combinations and phrases were: Asthma, wheezing, nocturnal asthma, obstructive sleep apnea (OSA), apnea, hypoapnea, Epworth sleeping scale (ESS), Apnea index (AI), hypopnea, apnea-hypopnea index (AHI), sleep disordered breathing (SDB), oxygen desaturation, forced expiratory volume (FEV), sleep efficiency, arousal index, body mass index (BMI), clinical trial, cohort, and epidemiological survey. The search encompassed original research papers published by July 2016 in online journals in English language.
Primary and secondary endpoints
Primary endpoints were: a) the prevalence of OSA (diagnosed with sleep studies only), OSA risk (a valid questionnaire-based evaluation) and SDB (one or more abnormal breathing and/or gas exchange patterns during sleep including habitual loud snoring at least 3–4 times/week, ≥3% desaturation/hour, upper airway resistance syndrome, and central sleep apnea) in asthma patients; and b) the pooled effect size of the odds ratios of the prevalence of OSA/OSA risk/SDB between asthma and non-asthma patients. Secondary endpoints were mean differences in BMI, % predicted FEV, and ESS score between asthma patients with OSA/OSA risk/SDB and without OSA/OSA risk/SDB.
Data extraction, synthesis and statistical analyses
Important information including outcome measures and outcomes, primary and secondary endpoints, participants’ demographic and clinical characteristics and other relevant information were obtained from the selected research articles of the respective studies and organized on datasheets. Data were extracted by two researchers independently who later cross checked the work of each other. Inter-rater reliability was good (kappa = 0.94).
Random effects meta-analyses were performed with STATA software (version 12; Stata Inc. Texas, USA) to achieve overall effect sizes of the prevalence of OSA, OSA risk, and SDB in asthma patients and to achieve a summary estimate of the odds ratio of the prevalence of OSA/OSA risk/SDB between asthma and non-asthma patients observed in the individual studies.
To assess the significance of differences in FEV (% predicted), BMI and ESS between asthma patients with and without OSA/OSA risk/SDB, meta-analyses of mean differences were carried out with RevMan software (version 5.3.5; Cochrane Collaboration) under random effects model. Between studies statistical heterogeneity was tested by tau2 and I2 indices.
Results
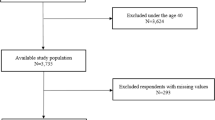
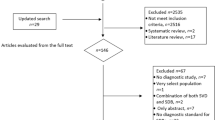
Data were acquired from 26 studies22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 (7675 patients) which fulfilled the eligibility criteria (Fig. 1). Important characteristics of the included studies are presented in Table S1. Average age of adult asthma patients was 48.9 ± 10 (range 42 ± 11 to 58.4 ± 15) years and that of asthmatic children 7.45 ± 3.2 (range 6.4 ± 4.4 to 9.1 ± 3.4) years. Proportion of males in this sample population was 41 ± 15% in adults and 62 ± 5.6% in children.
In adult asthma patients, the prevalence of OSA was 49.50 [36.39, 62.60] %, of OSA risk 27.50 [19.31, 35.69] %, and that of SDB was 19.65 [14.84, 24.46] % (Fig. 2). In Children, the prevalence of OSA and SDB in asthma patients was 63.04 [61.42, 64.67] % (one study data) and 22.34 [9.88, 34.79] % respectively.
The pooled analysis of odds ratios observed in the individual studies revealed that the odds of prevalence of the OSA, OSA risk and SDB was 2.64 [1.76, 3.52] (p < 0.00001), 3.73 [2.90, 4.57]; p < 0.00001 and 1.73 [1.11, 2.36]; p < 0.00001 (respectively) times higher in asthma patients than in non-asthma patients (Fig. 3).
Adult patients with asthma and OSA had significantly higher BMI than the asthma patients without OSA (mean difference: 2.15 [3.64, 0.67] kg/m2; p = 0.004; Fig. 4). In children, BMI z scores did not differ between asthma-OSA and non-asthma OSA patients. However, the number of included studies was less in this analysis (Fig. 4).
Although, there was no significant difference between the asthma patients with and without OSA in percent predicted FEV (mean difference: −2.28 [−6.79, 2.23] %; p = 0.32; Figure S1), the Epworth sleep scale score was significantly higher in the asthma patients with OSA (mean difference: 3.98 [1.30, 6.66]; p = 0.004; Figure S2) in comparison with non-OSA asthma patients.
Discussion
This meta-analysis has revealed that the prevalence of OSA, OSA risk and SDB in adult asthma patients is 50%, 27.5% and 20%, respectively, and the odds of having OSA, OSA risk and SDB is 2.64, 3.73, and 1.73 times higher (significantly) in asthma patients than in non-asthma patients. Asthma patients with OSA also had significantly higher BMI in comparison with non-asthma patients.
Both asthma and OSA have airway obstruction in the pathogenesis and have many diurnal and nocturnal symptoms in common48. Obstructive sleep apnea is identified as an independent risk factor for asthma exacerbation40, and OSA is reported to be more prevalent among patients with severe asthma than in moderate asthma which may be linked to the potential pathophysiologic interaction between OSA and asthma severity30. Moreover, a significant alleviation in asthma symptoms has been reported with the long-term use of continuous positive air pressure (CPAP) in patients with both asthma and OSA49, 50.
Obesity and related comorbidities, including SDB and gastro-esophageal reflux are found to be highly prevalent in asthma patients in many epidemiological studies31, 35, 51. A high body mass index may be a worsening factor in both conditions52. Although, obesity is a well-recognized risk factor for asthma in adult patients, but a causal relationship is still lacking53. There is also some evidence to suggest that hypothyroidism may also play a role in obesity mediated OSA severity54.
An important aspect that needs to be further studied is the association between AHI and asthma severity. In the included studies of present meta-analysis, some observations indicate that there exists a positive relationship between SDB and asthma severity e.g. Goldstein et al.27 mentioned that percentage of snoring patients was 33%, 38%, and 43% in mild, moderate, and severe asthma patients, respectively. Ross et al.35 also found that percentage of patients with snoring and desaturation was higher in severe asthma (55%) than in moderate asthma (20%) patients. Julien et al.30 found that sleeping efficiency and arousal index were significantly higher in severe than in moderate asthma patients. Zidan et al.47 found that percentage of OSA-asthma patients was 5.6% in well-controlled asthma patients, 61% in partially controlled and 33.3% in uncontrolled asthma patients.
Based on the above-mentioned findings, it can be presumed that the relationship between OSA and asthma have therapeutic implications. Effective treatment of OSA can favorably impact asthma control; CPAP is one such treatment49, 50. The CPAP therapy is also found to reduce gastroesophageal reflux disease and inflammation in OSA patients which may be beneficial for both asthma and OSA. However, CPAP has also been reported to increase bronchial hyper-responsiveness in non-asthma OSA patients55, 56 and cause sleep abnormalities in non-OSA asthma patients57 which makes it necessary to further investigate the effectiveness and consequences of this therapy.
Asthma-OSA overlap appears to be an area where patient-centered healthcare is more important because not only asthma is associated with significantly more comorbidities in comparison with other diseases58 but it is also evident that OSA has relationship with obesity and hypothyroidism54. Therefore, consideration of relevant therapies may have better impact e.g. bariatric surgery is found to be associated with improvement in symptoms of OSA and asthma37. Vitamin D deficiency has been reported to be associated with OSA severity59 and vitamin D treatment in asthma patients is found to reduce exacerbations60. Thus, it seems imperative that while managing asthma patients, physicians should examine the symptoms suggestive of OSA and consider differential diagnostics, especially in unstable, under-controlled and overweight patients that may also help in choosing a therapeutic strategy.
Conclusion
The prevalence of OSA in adult asthma patients is estimated at 50% in this meta-analysis and the odds of having OSA is 2.64 times higher in asthma patients than in the non-asthma patients. Moreover, the prevalence of OSA risk and SDB are estimated at 27.5% and 20% respectively. The odds of having OSA risk and SDB is 3.73 and 1.73 time higher (respectively) in asthma patients than in non-asthma patients. The prevalence of OSA was higher than the prevalence of SDB in asthma patients which indicates the existence of a bilateral relationship between OSA and asthma to result in a more severe phenotype. Asthma patients with OSA also had significantly higher BMI in comparison with non-OSA asthma patients. Therefore, a closer look at the symptoms suggestive of OSA is necessary in asthma patients especially in more severe forms and overweight individuals.
Statement of Ethics
This study does not involve ethical review by virtue of its design.
References
Larsson, L. G., Lindberg, A., Franklin, K. A. & Lundback, B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 95, 423–9 (2001).
Prasad, B., Nyenhuis, S. M. & Weaver, T. E. Obstructive sleep apnea and asthma: associations and treatment implications. Sleep Med Rev. 18, 165–71 (2014).
Min, Y. Z., Subbarao, P. & Narang, I. The Bidirectional Relationship Between Asthma and Obstructive Sleep Apnea: Which Came First? J Pediatr. doi:10.1016/j.jpeds.2016.05.058 (2016).
Simmons, J. C. Improving care and quality of life for all asthma patients. Qual Lett Healthc Lead. 13, 2–13 (2001).
Becker, A. B. & Chan-Yeung, M. Primary prevention of asthma. Curr Opin Pulm Med. 8, 16–24 (2002).
Center for Disease Control and Prevention. Asthma. Most recent Data. https://www.cdc.gov/asthma/most_recent_data.htm. Last accessed on January 2 (2017).
Simpson, L. et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 17(3), 967–73 (2013).
Arnardottir, E. S., Bjornsdottir, E., Olafsdottir, K. A., Benediktsdottir, B. & Gislason, T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 47(1), 194–202 (2016).
Heinzer, R. et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 3(4), 310–8 (2015).
Senaratna C. V. et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. doi:10.1016/j.smrv.2016.07.002. (2016).
Alkhalil, M., Schulman, E. & Getsy, J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med. 5, 71–8 (2009).
Brzecka, A., Pawelec-Winiarz, M., Piesiak, P., Nowak, E. & Jankowska, R. Suppression of chronic nocturnal cough during continuous positive airway pressure (CPAP) treatment in a patient with asthma and obstructive sleep apnea syndrome. Pneumonol Alergol Pol 79, 121–6 (2011).
Qiao, Y. X. & Xiao, Y. Asthma and Obstructive Sleep Apnea. Chin Med J (Engl). 128, 2798–804 (2015).
Knuiman, M., James, A., Divitini, M. & Bartholomew, H. Longitudinal study of risk factors for habitual snoring in a general adult population: The Busselton Health Study. Chest 130, 1779–83 (2006).
Fagnano, M., Bayer, A. L., Isensee, C. A., Hernandez, T. & Halterman, J. S. Nocturnal asthma symptoms and poor sleep quality among urban school children with asthma. Acad Pediatr. 11, 493–9 (2011).
Janson, C. et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 9, 2132–8 (1996).
Karachaliou, F., Kostikas, K., Pastaka, C., Bagiatis, V. & Gourgoulianis, K. I. Prevalence of sleep-related symptoms in a primary care population - their relation to asthma and COPD. Prim Care Respir J. 16, 222–8 (2007).
Kozyrskyj, A. L., Kendall, G. E., Zubrick, S. R., Newnham, J. P. & Sly, P. D. Frequent nocturnal awakening in early life is associated with nonatopic asthma in children. Eur Respir J. 34, 1288–95 (2009).
Shen, T. C. et al. Risk of Obstructive Sleep Apnea in Adult Patients with Asthma: A Population-Based Cohort Study in Taiwan. PLoS One. 10, e0128461 (2015).
Alharbi, M. et al. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J. 18, 328–30 (2009).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7), e1000097, doi:10.1371/journal.pmed.1000097 (2009).
Auckley, D., Moallem, M., Shaman, Z. & Mustafa, M. Findings of a Berlin Questionnaire survey: Comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 9, 494–9 (2008).
Braido, F. et al. Sleep apnea risk in subjects with asthma with or without comorbid rhinitis. Respir Care. 59, 1851–6 (2014).
Ciftci, T. U., Ciftci, B., Guven, S. F., Kokturk, O. & Turktas, H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 99, 529–34 (2005).
Ekici, A. et al. Association of asthma-related symptoms with snoring and apnea and effect on health-related quality of life. Chest. 128, 3358–63 (2005).
Ferguson, S. et al. Factors associated with systemic hypertension in asthma. Lung. 192, 675–83 (2014).
Goldstein, N. A. et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 50, 1128–36 (2015).
Guven, S. F., Dursun, A. B., Ciftci, B., Erkekol, F. O. & Kurt, O. K. The prevalence of obstructive sleep apnea in patients with difficult-to-treat asthma. Asian Pac J Allergy Immunol. 32, 153–9 (2014).
Jamrozik, E., Knuiman, M. W., James, A., Divitini, M. & Musk, A. W. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. 14, 814–21 (2009).
Julien, J. Y. et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 124, 371–6 (2009).
Kheirandish-Gozal, L., Dayyat, E. A., Eid, N. S., Morton, R. L. & Gozal, D. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 46, 913–8 (2011).
Kim, M. Y. et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol. 110, 253–7 (2013).
Li, S. et al. Habitual snoring in school-aged children: environmental and biological predictors. Respir Res. 11, 144 (2010).
Madama, D., Silva, A. & Matos, M. J. Overlap syndrome–Asthma and obstructive sleep apnea. Rev Port Pneumol. 22, 6–10 (2016).
Ross, K. Sleep-disordered breathing and childhood asthma: clinical implications. Curr Opin Pulm Med. 19, 79–83 (2013).
Shaarawy, H. & Affarab, N. Assessment of the prevalence of obstructive sleep apnea in patients with stable uncontrolled asthma, impact of continuous positive airway pressure treatment. Egyptian J Chest Dis Tuberculosis. 62, 183–7 (2013).
Simard, B. et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 14, 1381–8 (2004).
Sulit, L. G., Storfer-Isser, A., Rosen, C. L., Kirchner, H. L. & Redline, S. Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med. 171, 659–64 (2005).
Taillé, C. et al. Obstructive Sleep Apnoea Modulates Airway Inflammation and Remodelling in Severe Asthma. PLoS One. 11, e0150042 (2016).
Ten Brinke, A. et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 26, 812–8 (2005).
Teodorescu, M. et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 138, 543–50 (2010).
Teodorescu, M. et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma. 49, 620–8 (2012).
Teodorescu, M. et al. Asthma Control and Its Relationship with Obstructive Sleep Apnea (OSA) in Older Adults. Sleep Disord. 2013, 251567 (2013).
Teodorescu, M. et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract. 3, 566–75 (2015).
Teodorescu, M. et al. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 313, 156–64 (2015).
Yigla, M., Tov, N., Solomonov, A., Rubin, A. H. & Harlev, D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 40, 865–71 (2003).
Zidan, M., Daabis, R. & Gharraf, H. Overlap of obstructive sleep apnea and bronchial asthma: Effect on asthma control. Egyptian J Chest Dis Tuberculosis. 64, 425–30 (2015).
Schechter, M. S. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 109, e69 (2002).
Brzecka, A., Pawelec-Winiarz, M., Piesiak, P., Nowak, E. & Jankowska, R. Suppression of chronic nocturnal cough during continuous positive airway pressure (CPAP) treatment in a patient with asthma and obstructive sleep apnea syndrome. Pneumonol Alergol Pol. 79, 121–6 (2011).
Kauppi, P., Bachour, P., Maasilta, P. & Bachour, A. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep Breath. doi:10.1007/s11325-016-1340-1 (2016).
Kaiser, P. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 47, 311–4 (2012).
Jubber, A. S. Respiratory complications of obesity. Int J Clin Pract. 58, 573–580 (2004).
Beuther, D. A., Weiss, S. T. & Sutherland, E. R. Obesity and asthma. Am J Respir Crit Care Med. 174, 112–9 (2006).
Zhang, M. et al. Role of hypothyroidism in obstructive sleep apnea: a meta-analysis. Curr Med Res Opin. 32, 1059–64 (2016).
Devouassoux, G. et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol. 119, 597e603 (2007).
Korczynski, P. et al. Continuous positive airway pressure treatment increases bronchial reactivity in obstructive sleep apnea patients. Respiration 78, 404e10 (2009).
Martin, R. J. & Pak, J. Nasal CPAP in nonapneic nocturnal asthma. Chest. 100, 1024e7 (1991).
Su, X. et al. Prevalence of Comorbidities in Asthma and Non-asthma Patients: A Meta-analysis. Medicine (Baltimore). 95, e3459, doi:10.1097/MD.0000000000003459 (2016).
Mete, T. et al. Obstructive sleep apnea syndrome and its association with vitamin D deficiency. J Endocrinol Invest. 36(9), 681–5 (2013).
Martineau, A. R. et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 9, CD011511 (2016).
Acknowledgements
This study was supported by Liaoning Province Science and Technology Project (2014021022).
Author information
Authors and Affiliations
Contributions
D.L.K. conceived and designed the study. Z.Q. and H.S. carried out the literature search and extracted data. H.Y.J. and Z.F.W. conducted the meta-analyses/analyses. W.W. majorly wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kong, DL., Qin, Z., Shen, H. et al. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Sci Rep 7, 4088 (2017). https://doi.org/10.1038/s41598-017-04446-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04446-6
This article is cited by
-
The prevalence of obstructive sleep apnea in Japanese asthma patients
Allergy, Asthma & Clinical Immunology (2024)
-
Chronic intermittent hypoxia increases airway hyperresponsiveness during house dust mites exposures in rats
Respiratory Research (2023)
-
Low arousal threshold: a common pathophysiological trait in patients with obstructive sleep apnea syndrome and asthma
Sleep and Breathing (2023)
-
Do nocturnal asthma attacks influence sleep parameters and inflammatory markers? A cross-sectional population-based study
Sleep and Breathing (2023)
-
Predictive factors for obstructive sleep apnea in adults with severe asthma receiving biologics: a single-center cross-sectional study
Sleep and Breathing (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.