Abstract
The physiological effects of caesium (Cs) on living cells are poorly understood. Here, we examined the physiological role of Cs+ on the activity of the potassium transporters in E. coli. In the absence of potassium (K+), Kup-mediated Cs+ uptake partially supported cell growth, however, at a much lower rate than with sufficient K+. In K+-limited medium (0.1 mM), the presence of Cs+ (up to 25 mM) in the medium enhanced growth as much as control medium containing 1 mM K+. This effect depended on the maintenance of basal levels of intracellular K+ by other K+ uptake transporters. Higher amounts of K+ (1 mM) in the medium eliminated the positive effect of Cs+ on growth, and revealed the inhibitory effect of high Cs+ on the growth of wild-type E. coli. Cells lacking Kdp, TrkG and TrkH but expressing Kup grew less well when Cs+ was increased in the medium. A kdp mutant contained an increased ratio of Cs+/K+ in the presence of high Cs+ in the medium and consequently was strongly inhibited in growth. Taken together, under excess Cs+ conditions Kup-mediated Cs+ influx sustains cell growth, which is supported by intracellular K+ supplied by Kdp.
Similar content being viewed by others
Introduction
Caesium (Cs) is an alkali metal in group 1 of the periodic table that is not an essential element for most living cells. Cs has gained special world-wide attentions due to the release of considerably large amounts of radioactive caesium (134Cs and 137Cs) after the power plant accidents in Chernobyl in 1986 and Fukushima in 2011. This has led to studies of the environmental impacts of Cs+ contamination1, 2, decontamination attempts3 and bioremediation efforts including the search for microorganisms highly tolerant to Cs+ 4, 5. The physiological effect on living organisms is poorly understood6, but toxicity of Cs+ to cells has been reported in microorganisms, animal cells and plant cells5, 7, 8. Application of Cs+ increased the doubling time of green algae and cyanobacteria9, 10. Due to the similar chemical properties of Cs+ and K+, it has been proposed that Cs+ enters the cell via K+ transport systems6, 11. However, Cs+ is frequently used as a K+ channel blocker, and most K+ transport systems do not facilitate the uptake of Cs+ instead of K+ 12, 13.
K+ is the most abundant cation in prokaryotic and eukaryotic cells. K+ plays an important role in the maintenance of intracellular osmolality, formation of membrane potential and regulation of enzyme activity14. K+ uptake transport systems can be divided into four classes; K+ channels, Trk/Ktr/HKT, Kdp and Kup/HAK/KT15. With respect to the conserved selectivity filter motif and their membrane topology, Trk and Kdp belong to the same family of K+ transporters. The structure of Kup has not been conclusively determined16. In Escherichia coli, only Kup has been reported to serve as a possible Cs+ uptake route but not any of the other major K+ uptake systems: Kdp and Trk (TrkG and TrkH)11. Kup is a low affinity K+ transport system that also transports Rb+ and Cs+ 11. Kup participates in adaptation to high osmolality stress17. Kup was originally isolated in a trkD mutant study as a K+ uptake system belonging to a different class than TrkG and TrkH18. Kup (predicted molecular mass: 69 kDa) consists of an N-terminal transmembrane region (approximately 440 amino acids) and a relatively long (approximately 180 amino acids) C-terminal hydrophilic domain16, 19. Several critical residues for Kup/HAK/KT transport function have been identified16. On the other hand, the response of E. coli to Cs+ remains to be elucidated. In plants, several groups reported Cs+ uptake actvity by members of the Kup/HAK/KT family. Barley HvHAK1 and Arabidopsis AtHAK5 have high affinity for K+ 20. AtHAK5, a high affinity type K+ transport system, contributes to Cs+ influx into roots under K+-depleted conditions20, 21. The expression of AtHAK5 is induced 9-fold due to K+-starvation in Arabidopsis thaliana 8. Here, we report that at high concentrations Cs+ inhibited growth but that Cs+ uptake mediated by Kup could partially substitute for K+ inside E. coli cells under conditions where the K+ concentration in the medium was limiting. This positive effect was dependent on K+ uptake transporters. An assay with mutant strains revealed that Kdp increased the internal K+ content in the cells. Both Kup and Kdp contributed to increased tolerance to both excess Cs+ and lack of K+ in the medium.
Results
Cs+ can substitute for K+ in supporting E. coli growth under K+-depleted conditions
We tested whether Cs+, which is a similar alkali metal element, can act as a substitute for K+ during E. coli growth. In parallel, we tested the effect of Rb+ on E. coli growth since the elements can substitute potassium22. Growth tests of E. coli wild type (WT) and a knockout mutant of kup (Δkup) were carried out in minimal medium supplemented with K+, Cs+ or Rb+ (Fig. 1a). In the case of medium supplemented with Cs+ a background level of 4.6 μM K+ as determined by flame photometry was present. Overall, at the same concentrations, Cs+ did not support the same amount of growth of either WT or Δkup as K+. However, increasing the amount of Cs+ in the medium from 0.1 mM to 25 mM increased growth for both strains (Fig. 1a). The growth increase was higher for the WT (3.8-fold) than for the Δkup strain (2.6-fold). On the other hand, Rb+ could take over K+ under the conditions (Fig. 1a). These results indicate that Kup contributed to Cs+ uptake to sustain growth under depleted-K+ conditions.
Growth tests of E. coli WT and Δkup in minimal medium. E. coli WT (black bars) and Δkup (white bars) grown in minimal medium containing 0.1 mM K+ were collected by centrifugation, washed with K+-free buffer, then inoculated into minimal medium supplemented with K+, Cs+ or Rb+ (a), or with 0.1 mM K+ and various concentrations of Cs+ (b). Cell growth was determined after 24 h-incubation at 30 °C. *Represents significance by Student’s t-test comparing WT to Δkup at the same concentration (P < 0.05). (c) Difference in growth (OD600) between WT and Δkup calculated from the data in Fig. 1b.
To further evaluate utilization of Cs+ in E. coli under K+-limited condition, we examined the growth of WT and Δkup in minimal medium supplemented with low K+ (0.1 mM) and various concentrations of Cs+. The result showed that addition of Cs+ up to 25 mM Cs+ increased the growth of both strains (Fig. 1b). Higher concentrations of Cs+ (100–250 mM) impaired the growth. In order to determine the contribution of Kup to the observed growth, we subtracted the OD600 of the Δkup strain from that of the WT at each Cs+ concentration (Fig. 1c). This illustrated that E. coli utilized Kup for Cs+ uptake at concentrations of 0.5–25 mM effectively under K+-limited (0.1 mM) conditions and optimally at 5 mM. Under low K+ conditions, Kup therefore contributed to sustaining cell growth against excess external Cs+.
Kup contributes to uptake of Cs+ into E. coli at low external K+ concentrations
To further elucidate the role of Kup in taking up Cs+ as a substitute for K+ in E. coli, we measured the intracellular amount of K+ and Cs+ during the growth of E. coli. In medium with 0.1 mM K+ (Fig. 2a), the growth of both WT and Δkup increased at the same time as the Cs+ content in the cells in correlation with the Cs+ concentration in the medium. In contrast, the intracellular K+ was reduced as the amount of Cs+ increased. Nevertheless, the K+ content remained above 152 nmol/mg-proteins. The other K+ uptake transporters, TrkG, TrkH and Kdp, might contribute to K+ uptake in K+-containing medium. At high K+ (1 mM) (Fig. 2b), the positive effect of Cs+ on the cell growth of WT and Δkup disappeared and at higher concentrations of Cs+ growth of both E. coli strains was moderately inhibited. Interestingly, the growth of the WT was higher than that of Δkup in medium without Cs+ or with 0.1 mM Cs+ (Fig. 2b). This suggested that Kup functioned as a K+ uptake system. The reduced growth of Δkup in medium with 0 to 25 mM Cs+ was correlated with a decrease in intracellular K+ (Fig. 2b). This result also illustrated the contribution of other K+ uptake transporters to K+-loading into the cells. In contrast, the difference in Cs+ accumulation between both strains was relatively small, indicating that high K+ in the medium prevented Kup-mediated Cs+ uptake (Fig. 2b). These results showed that Kup-driven Cs+ uptake contributed to cell growth only under K+-limited conditions but had an adverse effect on growth under K+-sufficient conditions.
Determination of growth and K+ or Cs+ accumulation of E. coli WT and Δkup. E. coli WT and Δkup were grown in minimal medium supplemented with 0.1 mM K+ (a) or 1 mM K+ (b) and various Cs+ concentrations for 24 h at 30 °C. At that time, OD600 as well as intracellular K+ and Cs+ content were determined. The error bars represent mean ± S.D. (n = 4). *Represents significance by Student’s t-test comparing WT to Δkup at the same concentration (P < 0.05).
Kup activity impaired growth of E. coli lacking TrkG, TrkH and Kdp under high external Cs+ concentrations
The impact of external Cs+ on cell growth depended not only on Kup but also on other K+ uptake systems (TrkG, TrkH and Kdp) as shown in Figs 1 and 2. To evaluate the contribution of these other K+ uptake transporters to adaptation to Cs+ stress, Kup was expressed in E. coli LB2003 which is lacking activity of four K+ transporters, Kup, Kdp, TrkG and TrkH23 (Fig. 3). The growth of both LB2003 harboring the empty vector (EV) and LB2003 expressing Kup increased with the increase in K+ concentration in the medium. The growth of LB2003 containing Kup decreased with higher concentrations of Cs+ in the medium, whereas the control strain containing EV was much less sensitive to Cs+ and even showed slightly increased growth at higher Cs+. Remarkably, high Cs+ concentrations, e.g. 20 mM Cs+ in the absence or presence of 1, 5, 10 or 20 mM K+ severely impaired growth of LB2003 expressing Kup compared to the control cells containing EV (Fig. 3e). Kup was therefore responsible for the hypersensitivity of the cells to excess Cs+. The growth of LB2003 expressing Kup was significantly lower compared with WT E. coli under the same conditions (e.g. 1 mM K+ and 0, 1, 10 mM Cs+, Figs 2b and 3b). The positive effect of Cs+ on WT cell growth (at 25 mM Cs+, Fig. 1a) largely disappeared in the K+ transporter mutant LB2003 (at 20 mM Cs+, Fig. 3a). This indicated that other K+ uptake systems (Trk and Kdp) besides Kup were important to sustain the growth of the wild-type cells during high Cs+ stress.
Comparison of growth of the K+ uptake system-deficient E. coli strain LB2003 transformed with Kup or the empty vector (EV) at various concentrations of K+ and Cs+. Cells grown in minimal medium containing 20 mM K+ were collected by centrifugation, washed with K+-free buffer and then inoculated into minimal medium supplemented with combinations of various concentrations (0–20 mM) of K+ and Cs+. Growth was determined after 24 h-incubation at 30 °C. (a) 0 mM K+, (b) 1 mM K+, (c) 5 mM K+, (d) 10 mM K+, (e) 20 mM K+. The error bars represent mean ± S.D (n = 3). LB2003 strain containing Kup (black bars) or EV (white bars).
Role of Kup in uptake of Cs+ into the cells and export of K+
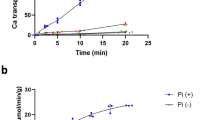
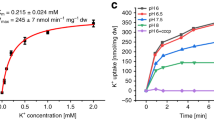
To dissect the effect of Cs+ on Kup-mediated K+ uptake, K+ content in LB2003 expressing Kup was measured in the buffer supplemented with different amounts of K+ and Cs+. The cells that were pretreated without or with 1 mM Cs+ showed a rapid increase in intracellular K+ content after K+ addition (Fig. 4a). In contrast, pretreatment with 10 or 100 mM Cs+ only led to a very slow increase of intracellular K+ content. The antagonistic effect of Cs+ influx on intracellular K+ content was more pronounced when Cs+ was added to LB2003 expressing Kup that had been pretreated with buffer containing 1 mM K+ (Fig. 4b). This effect was concentration-dependent, when low concentrations of Cs+ (0 or 1 mM) were added, the cellular K+ content continued to increase, whereas higher concentrations of Cs+ (10 or 100 mM) coincided with a loss of intracellular K+ (Fig. 4b). These results indicate that high Cs+ in the medium led to removal of K+ from the cells. This was confirmed by measurement of the intracellular concentrations of Cs+ of the cells treated with 1 mM K+ prior to addition of 1 mM or 100 mM Cs+ shown in Fig. 4b (Fig. 5). When 1 mM Cs+ was added to the cells the K+ content remained higher than that of Cs+, indicating that Kup transported K+ at a higher rate than Cs+ into the cells. Kup therefore recognized K+ as a preferential cation compared to Cs+ (Fig. 5a). Higher medium concentrations of Cs+ (10 mM) resulted in much higher Cs+ content compared with 1 mM Cs+ and a strong decrease in K+ content, suggesting that accumulated K+ was extruded from the cells (Fig. 5b).
High concentrations of Cs+ reduced K+ content in cells expressing Kup. Cs+ (0, 1, 10 or 100 mM) was added 20 min before (a) or 5 min after (b) K+ addition (1 mM final concentration). The error bars represent mean ± S.D. (n = 3). Filled and open symbols represent E. coli LB2003 transformed with Kup and EV, respectively.
Loss of intracellular K+ due to Kup-driven Cs+ influx. The Cs+ content (gray symbols) was determined from the same E. coli LB2003 expressing Kup cells shown in Fig. 4b. Final concentration of Cs+ added in the medium was 1 mM (a) or 10 mM (b). The data representing K+ content (black symbols) is shown here for comparison, it is the same as that in Fig. 4b. The error bars represent mean ± S.D. (n = 3).
Requirement of Kdp to E. coli under high external Cs+ concentrations
The results above indicate that Kup conferred Cs+ tolerance to the cells by cooperating with other K+ uptake transporters in maintaining the intracellular K+ level. To further explore the importance of these other K+ uptake transporters in the presence of high Cs+, we performed growth tests of E. coli mutants carrying single deletions in the genes encoding TrkG, TrkH, Kdp or Kch in medium containing 0.1 mM K+ and varying concentrations of Cs+. We included Δkch in this experiment because E. coli also contains a K+ channel homolog, Kch, whose function is unknown24, 25. At 5 mM Cs+, the growth of Δkdp was similar to Δkup but lower than that of WT, ΔtrkG, ΔtrkH and Δkch (Fig. 6a). Higher concentrations of Cs+ (25 mM) severely impaired the growth of Δkdp. This suggested that under high Cs+ and low K+ conditions, Kdp contributed to the uptake of K+ into the cells while Kup took up Cs+. As expected, the content of K+ in Δkdp was lower than that in Δkup (Fig. 6b). Due to the lack of growth of Δkdp at 25 mM Cs+, the content of K+ and Cs+ was not measured under those conditions. Kdp is a high affinity K+ pump in E. coli 26. To further evaluate the connections between Kdp-mediated K+ uptake and Kup-mediated Cs+ uptake, the double knockout mutant ΔkdpΔkup was generated (Fig. 6c). The double knockout mutant grew less well than either single mutant under the same conditions (5 mM Cs+ and 0.1 mM K+). The content of K+ and Cs+ was comparable to that of Δkup. This implies that the reloading of K+ into ΔkdpΔkup might occur through Trk (TrkG and TrkH) and/or another unidentified K+ transport system. These results revealed that Kdp for K+ uptake contributes to sustain cell growth by Kup-mediated Cs+ uptake under excess Cs+ and limiting K+ conditions.
Requirement of K+ uptake transporters under high Cs+ conditions. (a) Growth assay of E. coli WT, Δkup, ΔkdpA, ΔtrkG, ΔtrkH and Δkch in minimal medium containing 0.1 mM K+ without or with 5 mM or 25 mM Cs+, which was performed in the same experimental condition to that of Fig. 1. (b) The content of K+ and Cs+ of WT, Δkup and ΔkdpA grown in minimal medium containing 0.1 mM K+ with 5 mM Cs+ measured from the data in Fig. 6a. N.D. Not detected. (c) The growth of WT and ΔkupΔkdpA and their content of K+ and Cs+.
Discussion
This study showed that Cs+ uptake by Kup promoted cell growth under K+-limiting conditions. The positive effect of Cs+ on E. coli growth was reduced when the amount of K+ in the medium was increased at the same time. Other K+ uptake systems, TrkG, TrkH and Kdp likely also contributed to cell tolerance to high Cs+. Specifically, Kdp helped to prevent depletion of intracellular K+ due excess Cs+ (Fig. 7). The activities of Kup and Kdp were coordinated to support cell growth during excess Cs+ conditions in E. coli.
Model explaining the coordinated activity of Kup with the K+ uptake transporters Kdp and Trk in response to excess Cs+ in E. coli. (a) Kup-mediated Cs+ uptake contributed to growth of E. coli WT in medium containing Cs+ as the only alkaline metal. Under sufficient K+ conditions, loss of Kup had no effect on growth. Contribution of high affinity Kdp uptake system may be small at high K+ and their expression may be suppressed at high K+ 31, 38. (b) As long as a low concentration of K+ was present, the WT was able to grow even with high amounts of Cs+. (c) K+ uptake by other K+ uptake transporters (Kdp, TrkG and TrkH) was important for the growth of E. coli in the presence of high Cs+. = , >>, > indicates equal, much higher and higher growth.
Several studies have found that other cations like Na+, Rb+ or Cs+ can substitute for intracellular K+ under K+-limited conditions. Rice accumulates Na+ to compensate for K+ starvation27, olive trees (Olea europaea L.) are able to replace K+ with Na+ 28, while pint bean subtitutes by Rb+ and Na+ for K+ limitation22, and turtle heart cells are capable of replacing K+ with Cs+ 29. However, an adverse effect of Cs+ accumulation has also been reported. In cyanobacterium Synechocystis PCC 6803, addition of 1 mM Cs+ increases the doubling time by 64%, compared to control medium9. Similarly, in the green algae Chlorella emersonii addition of 1 mM Cs+ into the medium increases the doubling time by 34%10. In E. coli and Bacillus subtilis, Cs+ toxicity is found to be dependent on external pH and the presence of other cations, especially K+ and Na+. Based on an agar diffusion assay, E. coli has a minimum inhibitory K+: Cs+ concentration ratio of 1: 630. Cs+ is therefore likely a partial substitute alkaline ion for K+, but as shown by this study, it does not fully compensate for K+.
What is the cause of Cs+ toxicity for the cells? Cs+ is known to compete with K+ binding sites on K+ channels and K+ dependent enzymes and thereby to inhibit their function. Hampton et al. emphasized that the toxicity of Cs+ is mainly caused by competition between K+ and Cs+ for K+ association sites on vital proteins, not by blockage of K+ uptake resulting in K+ starvation alone8. A further aspect of Cs+ cytotoxicity is that Cs+ interferes with protein expression8. The gene expression of K+-repleted cells differs from that of K+-depleted cells due to intracellular accumulation of Cs+. This implies that prolonged Cs+ accumulation in the cells alters the cellular response. To rescue the detrimental effect of Cs+ on cells under high Cs+ conditions, K+ uptake transporters likely function in replenishment of K+ when the K+ concentration in the medium is low30. Figure 3 shows that at 25 mM Cs+ in K+-limited medium (0.1 mM K+), the K+ inside of the cells was kept at a low level (152 nmol K+/mg of protein). Despite of this, the cells still grew well (Figs 2a and 7). The basal level of K+ in the cells likely alleviated the effect of the intracellular over-accumulation of Cs+. This explains well why K+ depletion in the presence of Cs+ severely hampered cell growth (Fig. 1a). Kup apparently facilitates the uptake of Cs+ as an electroneutral substitute for K+ as an osmo-protectants, and Cs+ does not act as a substitute for other physiological roles of K+ 10.
An alternative role of Kup under conditions of high external Cs+ may be the induction of the high affinity K+-uptake Kdp system, which takes up K+ into the cells at low K+. The K m value for K+ of Kdp is much lower than that of Trk, and both Kdp and Trk can distinguish K+ from Cs+ 11. In E. coli, Kdp is expressed upon K+ depletion26. The kdp operon is also induced in cells with low internal K+ due to intracellularly accumulated Cs+ 31. A relative increase of Cs+ accumulation induced by K+ loss was also reported in Chlorella10. K+ pumping by Kdp might be induced to supply K+ from low K+ in the medium because Kup-mediated Cs+ accumulation results in a lower intracellular K+/Cs+ ratio (Fig. 6). When Kup-mediated Cs+ uptake promoted E. coli growth in medium containing low amounts of K+, Kdp may enable the cells to maintain the proper intracellular balance of K+/Cs+ to protect themselves from Cs+ toxicity8.
The K m value of Kup for K+ and Cs+ was reported to be approximately 0.37 mM and 5 mM, respectively11, indicating that Kup preferred to transport K+ over Cs+. This property of Kup was also seen in our experiments (Figs 2 and 5a). The rate of K+ uptake by Kup was faster than that of Cs+ (Fig. 5a). Figure 2b also showed that lack of Kup resulted in a decrease of 8.8–25% in K+ accumulation in cells treated with varying concentrations of Cs+. Moreover, in the presence of 1 mM K+ in the medium there was no significant difference in Cs+ content in WT and Δkup (Fig. 2b), indicating that under sufficient K+ conditions Kup-mediated Cs+ uptake activity was low.
The remaining Cs+ influx activity in Δkup shown in Fig. 2b was not due to Kup function. Considering that Trk (TrkG and TrkH) and Kdp have only negligible Cs+ uptake activity11, E. coli likely possesses another, yet unidentified Cs+ uptake transport system in addition to Kup.
Another insight obtained in this study is that K+ efflux from K+-loaded cells was caused by Cs+ accumulation in the cells (Fig. 5). E. coli contains a glutathione-gated KefB/KefC system32, 33 and a K+/H+ antiporter34 in the inner membrane. It remains to be shown whether Kup provides a K+ efflux pathway across the inner membrane or whether other transporters mediate K+ efflux.
In summary, Kup took up Cs+ to support E. coli growth in K+-depleted medium. However, the growth rate of cells under Cs+ stress conditions depended on the activity of K+ uptake transporters. The temporary increase of intracellular Cs+ driven by the activity of Kup might in turn stimulate another K+-selective uptake system, Kdp to increase the cellular K+ content, which partially offset the effect of accumulated Cs+ in the cells. The relative broad selectivity of Kup, which is not found in Trk and Kdp, may contribute to increased plasticity in the cellular response to non-physiological levels of alkali metals in the environment.
Methods
Growth test condition
E. coli strain BW25113 (wild type, WT), single knockout mutants of kup (Δkup), kdp (Δkdp), trkG (ΔtrkG) and trkH (ΔtrkH) in the BW25113 background were provided by the National BioResource Project (NIG, Japan)35. The double mutant of ΔkupΔkdp was generated by PCR-based mutagenesis36. Briefly, the kup gene was deleted in the Δkdp strain using PCR products amplified with two primers, 5′-AAGCACACATTTCATATTTCAACGAAAGACTAGTCTATGATTCCGGGGA TCCGTCGAC-3′ and 5-GAAAGGAGGCGTCTGGCGTTAGATTTCGACCTGAGTACCTG TAGGCTGGAGCTGCTTC-3. The kanamycin cassette was removed from the resultant E. coli mutant36. All strains were grown in medium containing 0.5% yeast extract, 0.5% KCl, and 1% polypeptone. Pre-cultures were grown in liquid minimal medium (46 mM Na2HPO4, 23 mM NaH2PO4, 8 mM (NH4)2SO4, 0.4 mM MgSO4, 6 mM FeSO4, 10 μg/ml thiamine and 1% glucose) supplemented with 1 mM KCl16. The pre-cultures were incubated at 30 °C for 24 h. Cells were harvested by centrifugation (15,000 rpm for 1 minute), washed with K+-free buffer (46 mM Na2HPO4, 23 mM NaH2PO4, 8 mM (NH4)2SO4) then re-suspended with the same buffer. The cell suspension was inoculated (1%) into the same liquid minimal medium containing various concentrations of KCl, RbCl and CsCl. The cultures were incubated at 30 °C for 24 h. The growth of E. coli WT and Δkup was determined by measuring the optical density at 600 nm (OD600). The cation content of the cell pellets was determined using an atomic absorption spectrometer (iCE 3500 Thermo Fisher Scientific AA Spectrometer).
Measurement of K+ uptake in E. coli
E. coli strain LB2003 (F−, thi, lacZ, gal, rha, ΔkdpFABC5, trkD1, ΔtrkA), which lacks activity of the three K+ uptake systems, was transformed with pPAB404 empty vector (EV) or pPAB404-EcKup-6xHis (Kup) as described previously16. The cation uptake procedure was essentially conducted as described previously with some modifications37. Briefly, E. coli LB2003 containing EV, Kup or Kdp were cultured in minimal medium supplemented with 30 μg/ml ampicillin and 20 mM KCl at 30 °C for 24 h. Pre-cultures were inoculated into the minimal medium to which 0.1 mM isopropyl β-D-1-thiogalactopryanoside (IPTG) was added and then incubated at 30 °C. Overnight-grown cultures were harvested by centrifugation. The cells were re-suspended in 120 mM Tris-HCl (pH 8.0) and 1 mM EDTA, incubated 30 min at 37 °C, collected by centrifugation and washed three times with 50 mM Tris-HCl pH 7.5, then re-suspended with the same buffer. After shaking at room temperature for 20 min, the concentration of the cell suspensions was adjusted to an OD578 of 3.0 with the same buffer. For the experiments where Cs+ was added prior to K+ supplementation, 10 mM of glucose and various concentrations (0, 1, 10, or 100 mM) of CsCl were added to the cells 20 minutes before K+ addition (t = 0 min). At t = 20 min, KCl was added at a final concentration of 1 mM. For the experiments where K+ was added prior to Cs+ supplementation, 10 mM of glucose was added at t = 0 min, then 1 mM KCl was added at t = 5 min and various concentrations (0, 1, 10, or 100 mM) of CsCl were added into the cells at t = 10 min. For sampling, aliquots of 1 mL were taken at different times (t = 1, 3, 6, 10, 15, and 20 min), and centrifuged for 1 min through silicone oil (Sigma Aldrich). The supernatant was removed by using an aspirator, then the pellet was disrupted by addition of 5% trichloroacetic acid and heating at 100 °C for 5 min. The total protein content in the cell pellets was measured by using the BCA protein assay kit (Micro BCA Protein Assay, Thermo Scientific). The K+ content of the cell pellets was determined using an atomic absorption spectrometer (iCE 3500 Thermo Fisher Scientific AA Spectrometer).
References
Mizuno, T. & Kubo, H. Overview of active cesium contamination of freshwater fish in Fukushima and Eastern Japan. Sci. Rep. 3, 1742, doi:10.1038/srep01742 (2013).
Aliyu, A. S., Evangeliou, N., Mousseau, T. A., Wu, J. & Ramli, A. T. An overview of current knowledge concerning the health and environmental consequences of the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. Environ. Int. 85, 213–228, doi:10.1016/j.envint.2015.09.020 (2015).
Yanaga, M. & Oishi, A. Decontamination of radioactive cesium in soil. J. Radioanal. Nucl. Chem. 303, 1301–1304, doi:10.1007/s10967-014-3541-z (2015).
Tomioka, N., Uchiyama, H. & Yagi, O. Cesium Accumulation and Growth Characteristics of Rhodococcus erythropolis CS98 and Rhodococcus sp. Strain CS402. Appl. Environ. Microbiol. 60, 2227–2231 (1994).
Kato, S. et al. Enrichment and isolation of Flavobacterium strains with tolerance to high concentrations of cesium ion. Sci. Rep. 6, 20041, doi:10.1038/srep20041 (2016).
Avery, S. V. Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Ind. Microbiol. 14, 76–84, doi:10.1007/BF01569888 (1995).
John, E. An A-Z Guide to The Elements. Oxford Univ. Press 539, doi:978-0-19-960563-7 (2001).
Hampton, C. R. et al. Cesium Toxicity in Arabidopsis. Plant Physiol. 136, 3824–3837, doi:10.1104/pp.104.046672 (2004).
Avery, S. V., Codd, G. A. & Gadd, G. M. Caesium accumulation and interactions with other monovalent cations in the cyanobacterium Synechocystis PCC 6803. J. Gen. Microbiol. 137, 405–413, doi:10.1099/00221287-137-2-405 (1991).
Avery, S. V., Codd, G. & Gadd, M. Replacement of cellular potassium by caesium in Chlorella emersonii: differential sensitivity of photoautotrophic and chemoheterotrophic growth. J. Gen. Microbiol. 138, 69–76, doi:10.1099/00221287-138-1-69 (1992).
Bossemeyer, D., Schlosser, A. & Bakker, E. P. Specific cesium transport via the Escherichia coli Kup (TrkD) K+ uptake system. J. Bacteriol. 171, 2219–2221, doi:10.1128/jb.171.4.2219-2221.1989 (1989).
Uozumí, N., Gassmann, W., Cao, Y. & Schroeder, J. I. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. J. Biol. Chem. 270, 24276–24281, doi:10.1074/jbc.270.41.24276 (1995).
Kim, E. J., Kwak, J. M., Uozumi, N. & Schroeder, J. I. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10, 51–62, doi:10.1105/tpc.10.1.51 (1998).
Epstein, W. The Roles and Regulation of Potassium in Bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75, 293–320, doi:10.1016/S0079-6603(03)75008-9 (2003).
Uozumi, N. & Dreyer, I. Structure-function correlates in plant ion channels. Comprehensive Biophysics 6, (Elsevier Ltd., 2012).
Sato, Y. et al. Defining membrane spanning domains and crucial membrane-localized acidic amino acid residues for K+ transport of a Kup/HAK/KT-type Escherichia coli potassium transporter. J. Biochem. 155, 315–323, doi:10.1093/jb/mvu007 (2014).
Trchounian, A. & Kobayashi, H. Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett. 447, 144–148, doi:10.1016/S0014-5793(99)00288-4 (1999).
Dosch, D. C., Helmer, G. L., Sutton, S. H., Salvacion, F. F. & Epstein, W. Genetic analysis of potassium transport loci in Escherichia coli: Evidence for three constitutive systems mediating uptake of potassium. J. Bacteriol. 173, 687–696, doi:10.1128/jb.173.2.687-696.1991 (1991).
Schleyer, M. & Bakker, E. P. Nucleotide sequence and 3′-end deletion studies indicate that the K+- uptake protein kup from Escherichia coli is composed of a hydrophobic core linked to a large and partially essential hydrophilic C terminus. J. Bacteriol. 175, 6925–6931, doi:10.1128/jb.175.21.6925-6931.1993 (1993).
Rubio, F. & Rodríguez-Navarro, A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 109, 34–43, doi:10.1034/j.1399-3054.2000.100106.x (2000).
Qi, Z. et al. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J. Exp. Bot. 59, 595–607, doi:10.1093/jxb/erm330 (2008).
Valdez-Aguilar, L. A. & Reed, D. W. Influence of potassium substitution by rubidium and sodium on growth, ion accumulation, and ion partitioning in bean under high alkalinity. J. Plant Nutr. 31, 867–883, doi:10.1093/aobpla/plv105 (2008).
Stumpe, S. & Bakker, E. P. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 167, 126–136, doi:10.1007/s002030050425 (1997).
Munsey, T. S. et al. Functional properties of Kch, a prokaryotic homologue of eukaryotic potassium channels. Biochem. Biophys. Res. Commun. 297, 10–16, doi:10.1016/S0006-291X(02)02095-8 (2002).
Kuang, Q., Purhonen, P., Jegerschöld, C. & Hebert, H. The projection structure of Kch, a putative potassium channel in Escherichia coli, by electron crystallography. Biochim. Biophys. Acta - Biomembr. 1838, 237–243 (2014).
Haupt, M., Bramkamp, M., Coles, M., Kessler, H. & Altendorf, K. Prokaryotic Kdp-ATPase: Recent insights into the structure and function of KdpB. J. Mol. Microbiol. Biotechnol. 10, 120–131, doi:10.1159/000091559 (2005).
Horie, T. et al. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 26, 3003–3014, doi:10.1038/sj.emboj.7601732 (2007).
Erel, R., Ben-Gal, A., Dag, A., Schwartz, A. & Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 34, 1102–1117, doi:10.1093/treephys/tpu081 (2014).
Guerin, M. & Wallon, G. The reversible replacement of internal potassium by caesium in isolated turtle heart. J. Physiol 293, 525–537, doi:10.1113/jphysiol.1979.sp012905 (1979).
Perkins, J. & Gadd, G. M. The influence of pH and external K+ concentration on caesium toxicity and accumulation in Escherichia coli and Bacillus subtilis. J. Ind. Microbiol. 14, 218–225, doi:10.1007/BF01569931 (1995).
Jung, K., Krabusch, M. & Altendorf, K. Cs+ induces the kdp operon of Escherichia coli by lowering the intracellular K+ concentration. J. Bacteriol. 183, 3800–3803, doi:10.1128/JB.183.12.3800-3803.2001 (2001).
MacLean, M. J., Ness, L. S., Ferguson, G. P. & Booth, I. R. The role of glyoxalase I in the detoxification of methylglyoxal and in the activation of the KefB K+ efflux system in Escherichia coli. Mol. Microbiol. 27, 563–571, doi:10.1046/j.1365-2958.1998.00701.x (1998).
Miller, S., Ness, L. S., Wood, C. M., Fox, B. C. & Booth, I. R. Identification of an ancillary protein, YabF, required for activity of the KefC glutathione-gated potassium efflux system in Escherichia coli. J. Bacteriol. 182, 6536–6540, doi:10.1128/JB.182.22.6536-6540.2000 (2000).
Radchenko, M. V. et al. Potassium/proton antiport system of Escherichia coli. J. Biol. Chem. 281, 19822–19829, doi:10.1074/jbc.M600333200 (2006).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008, doi:10.1038/msb4100050 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–5, doi:10.1073/pnas.120163297 (2000).
Matsuda, N. et al. Na+-dependent K+ uptake Ktr system from the cyanobacterium Synechocystis sp. PCC 6803 and its role in the early phases of cell adaptation to hyperosmotic shock. J. Biol. Chem. 279, 54952–54962, doi:10.1074/jbc.M407268200 (2004).
Roe, A. J., McLaggan, D., O’Byrne, C. P. & Booth, I. R. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol. Microbiol. 35, 1235–1243, doi:10.1046/j.1365-2958.2000.01793.x (2000).
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (16H04906 and 16H06558 to NU, 15K18678 to SH) from the Ministry of Education, Culture, Sports, Science, and Technology and from the Japan Society for the Promotion of Science. We thank Kei Nanatani for technical suggestions regarding the measurement of K+ uptake activity. We thank Anke Reinders for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
E.T., N.H. and Y.-H.S. performed and analyzed the experiments. E.T., S.H. and N.U. analyzed the results. N.U. designed the study and E.T. and N.U. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanudjaja, E., Hoshi, N., Su, YH. et al. Kup-mediated Cs+ uptake and Kdp-driven K+ uptake coordinate to promote cell growth during excess Cs+ conditions in Escherichia coli . Sci Rep 7, 2122 (2017). https://doi.org/10.1038/s41598-017-02164-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02164-7
This article is cited by
-
Potassium transporter KUP9 participates in K+ distribution in roots and leaves under low K+ stress
Stress Biology (2022)
-
Evidence for potassium transport activity of Arabidopsis KEA1-KEA6
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.