Abstract
Pulmonary vessels have numerous variation and aberrant branching patterns. Mediastinal lingular artery (MLA), the most common aberrant branch, might contribute to greater blood flow to lingular division. Hence, we investigated a correlation between lingular division volume and MLA using three-dimensional CT volumetry. We included 199 consecutive patients who underwent surveillance chest CT to detect possible malignancies in April 2015. We measured lingular division volume and cross-sectional area of lingular arteries using three-dimensional CT volumetry. MLA was identified in 58 cases (29.1%). The MLA group had significantly greater lingular division volume (median ± quartile deviation: 378.3 ± 75.5 mL vs. 330.0 ± 87.5 mL; p = 0.021) and percentage lingular division to left lung volume (19.0 ± 2.62% vs. 16.6 ± 2.39%; p < 0.001) than the non-MLA group. Total cross-sectional area of lingular arteries of the MLA group was significantly larger than that of the non-MLA group (46.1 ± 9.46 vs. 40.2 ± 5.76 mm2; p = 0.003). The total cross-sectional area of the lingular arteries strongly correlated to the percentage of lingular division to left lung volume (r = 0.689, p < 0.001). This is the first report demonstrating a positive correlation between branching pattern of pulmonary artery and lung volume.
Similar content being viewed by others
Introduction
Complex lung architecture is often compared to a tree, which has intricate cellular structure generated by extremely elaborate process. Pulmonary vessels thus have numerous variation and aberrant branching patterns1,2,3. Particularly, the prevalence of the variant and aberrant branching is higher in left lung and it is well known that the most common variation is a mediastinal lingular artery (MLA)2,3,4. In recent years, three-dimensional computed tomography (3D-CT) combined with computer-aided diagnosis (CAD) has become widely used that can provide us accurate identification of peripheral branches of pulmonary vessels including intersegmental veins for simulation of surgery including segmentectomy5. In addition, this allows us to easily identify intersegmental plane as well as to calculate lung segment volume with automated algorithm. MLA may have greater diameter than interlobar lingular artery (ILA) as MLA is usually the first branch of the left main pulmonary artery. Thus, we hypothesized that MLA can largely affect lingular division volume via greater blood flow. In this study, we aimed to investigate relationship between lingular division volume and MLA using 3D-CT with CAD.
Results
Among 204 patients who underwent surveillance chest CT, three patients with of medically treated chronic obstructive pulmonary disease (COPD), one patient with interstitial lung disease (ILD) and one patient with history of pulmonary resection were excluded. Finally, a total 199 patients with medical record and HRCT data were analyzed (Fig. 1). No nodular lesions obstructing bronchi of greater than subsegment were detected on the CT. No patients were excluded due to infiltrative attenuation or pleural effusion. As shown in Table 1, our cohort included 113 men (56.8%) and 86 women (43.2%) with a median age of 64.0 years (range: 25–84 years). Ninety patients (45.2%) were former/current smokers. MLA was identified in 58 patients (29.1%; Fig. 2a) who were classified as “MLA group”. Among them 20 patients had one MLA and 38 patients had one MLA and one ILA, whereas there were no cases with two MLAs. In “non-MLA group”, 90 had one ILA, 51 had two ILAs (Fig. 2b). Number of lingular arteries identified was 1 in 110 cases (55.3%) and 2 in 89 cases (44.7%). A median diameter of lingular artery was 7.5 mm (range: 2.3–14.5 mm).
A patient selection chart shows that 204 patients who underwent surveillance chest CT to rule out possible intrathoracic malignancies in April 2015 at Aichi Cancer Center Hospital were included. Among them, 3 patients with history of COPD, 1 patient with history of ILD, and 1 patient with history of prior lung resection were excluded. The remaining 199 patients were selected as our study cohort.
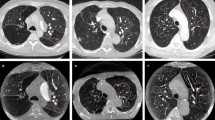
(a) A case of the “mediastinal lingular artery (MLA) group”. Three-dimensional computed tomography shows the MLA which branched as the first branch of the left main pulmonary artery (arrow). (b) A case of the “non-MLA group”mediastinal lingular artery”. Three-dimensional computed tomography shows theinterlobar lingular artery (ILA) which branched from interlobar portion of the left pulmonary artery (arrow).
As shown in Table 2, there were no significant differences in clinical data including age, sex, smoking index, CT-CTR, height, body weight, body mass index, Goddard classification, and percent low-attenuation area (%LAA) in each lung between the two groups. Lingular division volume in the MLA group was significantly larger than that in the non-MLA group (378.3 ± 75.5 mL vs. 330.0 ± 87.5 mL; p = 0.021). The MLA group showed significantly greater lingular division/total lung volume compared with the non-MLA group (8.81 ± 1.26% vs. 7.72 ± 1.03%; p < 0.001). When compared with the non- MLA group, the MLA group showed significantly greater lingular division/left lung volume (19.0 ± 2.62% vs. 16.6 ± 2.39%; p < 0.001) as well as lingular division/left upper lobe volume (36.0 ± 4.00% vs. 31.0 ± 4.00%; p < 0.001).
Next, we investigated the correlation between the lingular division/left lung volume and continuous variables such as age, smoking index, CT-CTR, body mass index. The lingular division/left lung volume was not correlated with either age (r = 0.019, p = 0.729; Fig. S1a), body mass index (r = 0.107, p = 0.188; Fig. S1b), smoking index (r = 0.126, p = 0.118; Fig. S1c), or CT-CTR (r = 0.012, p = 0.884; Fig. S1d).
We then conducted case-control matching analysis to further minimize influence of possible confounders. As shown in Table 3, there were no significant differences in patient characteristics except for lingular division volume. The lingular division volume in the MLA group was larger than in the non-MLA group (378.3 ± 75.5 mL vs. 323.6 ± 84.5 mL; p = 0.028). The lingular division/total lung volume in the MLA group was also significantly greater than that in the non-MLA group and (8.81 ± 1.26% vs. 7.72 ± 1.03%; p < 0.001). Figure 3a showed percentage of either left upper division, left lingular division, or left lower lobe to left lung volume. There were no statistical differences in left lower lobe/left lung volume between the MLA and non-MLA groups (45.0 ± 4.67% vs. 45.9 ± 3.43%; p = 0.925). The MLA group showed significantly higher lingular division/left lung volume compared to the non-MLA group (19.0 ± 2.62% vs. 16.6 ± 2.22%; p < 0.001; Fig. 3b), while left upper division/left lung volume in the MLA group is significantly smaller than that in the non-MLA group (34.5 ± 4.26% vs. 38.0 ± 3.93%; p = 0.003). When compared with the non-MLA group, the MLA group had significantly higher lingular division/left upper lobe lung volume (36.0 ± 4.00% vs. 30.0 ± 4.50%; p < 0.001).
(a) The proportion of left upper division (LUD), left lingular division (LLD), and left lower lobe (LLL) to let lung volume in the original cohort. The MLA group showed significantly greater lingular division/left lung volume as well as left upper division/left lung volume compared with the non-MLA group (p < 0.001 and p = 0.003, respectively). Left lower lobe/left lung volume was not statistically different between the two groups (p = 0.925). (b) After matching, lingular division/left lung volume in the MLA group is significantly greater than that of the non-MLA group (p < 0.001). (c) Total cross-sectional area of lingular arteries in the MLA group is significantly greater than that in the non-MLA group (p = 0.003) in the matched cohort.
We further investigated total cross-sectional area of lingular arteries on 3D-CT in the matched cohort. The total cross-sectional area of lingular arteries in the MLA group was significantly greater than that in the non-MLA group (46.1 ± 9.46 vs. 40.2 ± 5.76 mm2; p = 0.003; Fig. 3c). In addition, we assessed correlation between lingular arteries and lingular division/left lung volume. As shown in Fig. 4, there was a strong positive correlation between the total cross-sectional area of lingular arteries and the lingular division volume/left lung volume (r = 0.689, p < 0.001).
Discussion
We demonstrated that the lingular division volume was highly correlated with the branching patterns of lingular arteries as well as the cross-sectional area of lingular arteries using the novel 3D-volumetry analyzing system. In addition, lingular division/left lung volume showed strong correlation to the total cross-sectional area of lingular arteries. Lingular division/left lung volume is considered to be a better index than lingular division/total lung volume because of less variability, while they both showed significant differences between the presence and absence of MLA. To our knowledge, this is the first report investigating a correlation between the branching patterns of pulmonary arteries and lung segment volume.
In this study, the 3D-CT analysis was used to measure the lingular division volume and cross-sectional area of lingular arteries. This semi-automated measurement provided us highly reproducible anatomical data with high interobserver consistency as shown in Fig. S2. Since postoperative liver function is highly predicable by remnant liver volume, liver 3D-volumetry (so-called “virtual hepatectomy”) has been documented6,7,8,9. On the other hand, it has been used mainly to identify segmental bronchi and vessels such as intersegmental veins for preoperative evaluation of lung segmentectomy5, 10, as variations and aberrant branches of pulmonary vessels greatly affect technical difficulty and operative risk. In addition, “number of segment” method that calculates postoperative pulmonary function based on number of resected and remaining segments has been a gold standard to predict postoperative pulmonary function with or without adjustment by perfusion scintigraphy11, 12. Although one study reported usefulness of 3D-volumetry to evaluate lung volume for lung transplantation13, there is no previous literature investigating correlation between lung segment volume and segment artery branching patterns using 3D-CT volumetry to date. Thus, we should focus on the population with relatively healthy lung to validate our method. On the other hand, it is clinically significant to analyze patients with COPD and/or ILD because previous literature demonstrated structural changes of pulmonary arteries in these patients14,15,16,17,18. As shown in Fig. S3, our correlation analysis showed no significant correlation between lingular division/left lung volume and either Goddard classification score or %LAA in both the non-MLA and MLA groups. This suggests that influence of unrecognized COPD represented by Goddard score or %LAA can be ignored in this cohort. If decreased pulmonary artery flow is associated with decreased normal lung volume, it might be relevant to progression mechanisms of COPD and/or ILD. Lingular division may tend to be smaller in COPD patients who had been excluded from our study cohort, whereas influence of lingular artery type seems to be smaller than the current study cohort. Regardless of branching patterns of lingular arteries, the lingular artery dimension was likely to be associated with the lingular artery volume in the excluded 4 patients. Further study will clarify correlation between pulmonary artery flow and lung volume in patients with COPD and/or ILD. In addition, the current findings in normal population might also suggest a different role of pulmonary artery flow in normal lung development as below. The most important finding of our study is that the lingular division volume is significantly associated with the branching patterns and cross-sectional area of lingular arteries. MLA, the most common variation, was reportedly found in approximately 25% of healthy population1,2,3, which is consistent with that we identified 29.6% cases have one or two MLAs in our relatively healthy population. In previous reports, lung volume was affected by various factors including age19, sex20, smoking status19, CTR21, and body mass index22, 23, all of which were matched to further minimize possible effects on lung volume. Pulmonary emphysema is also characterized by the lung hyperinflation due to peripheral small airway obstruction24, 25. Thus, we excluded patients with history of COPD and additionally evaluate emphysema on HRCT as %LAA to focus on the analysis of normal lung. On the other hand, there is a small possibility that not only CTR but also biased heart position can affect left lung volume. Therefore, measuring lingular division/left lung volume can be a better way to evaluate lingular division volume.
It is quite reasonable that centrally branched arteries have greater cross-sectional area which may contribute to higher blood flow to corresponding lung parenchyma. Regarding correlation between blood flow and organ volume, portal blood flow was associated with degree of liver regeneration after hepatectomy26 as well as after liver transplantation27. Our finding is more likely to be collateral evidence that blood flow can be a determinant of lung volume in normal lung development. However, there might be the following major concerns for this speculation: (1) whether cross-sectional area of lingular arteries does adequately reflect the blood flow and (2) whether the correlation between the cross-sectional area of lingular arteries and lingular volume might not be a cause-effect relationship. Physiologically, blood flow is directly proportional to the twice power of cross-sectional area of blood vessels if blood pressure is constant based on the Hagen-Poiseuille equation28. Individual differences of pulmonary artery pressure among healthy population are quite small29. Our study population did not include patients with chronic lung diseases which can cause secondary pulmonary hypertension. Because hypoxic pulmonary vasoconstriction is specifically seen when unilateral lung ventilation in general anesthesia30, it is unnecessary to consider it in the current setting. At this point, it is reasonable to consider that blood flow and total cross-sectional area of the lingular arteries can reflect blood flow in our population. On the other hand, we cannot directly measure blood flow of peripheral pulmonary arteries in a non-invasive way. Thus, the relationship between lingular division volume and blood flow should be speculative. Also, we do not have appropriate analytical methods and any data to answer the second question above. There are no studies investigating correlation between lingular volume and diameter of lingular arteries based on long-term changes of lung volume during lung development from birth to adolescent. Originally, relatively high prevalence of variation and aberrant branches of pulmonary vessels and bronchi are attributed to the complexity of the lung development. Normal lung development starts with formation of lung bud from ventral foregut during gestational fourth week31. After development of tracheal primordia and two main bronchi, the lung bud grows into adjacent splanchnic mesoderm where branching of vessels and bronchi are repeatedly induced during gestational period. Pulmonary vasculature is developed in parallel to bronchial tree. Even postnatally, increase of number of alveoli by septation of alveolar saccules continues to adolescence. During this period, vasculogenesis and remodeling of the capillary is essential for maturation of functioning alveoli. Lung parenchyma which consists largely of alveoli and alveolar duct is mainly generated in this “septation” process with regulation by angiogenic factors including vascular endothelial growth factor (VEGF)32. VEGF signaling are also essential for alveolarization which morphologically resembles “septation” in regeneration process after pneumonectomy in mice33,34,35. In addition, VEGF expression is significantly associated with better long-term compensatory restoration of pulmonary function after major lung resection36. Thus, some researchers consider that blood flow of pulmonary arteries can affect lung volume in some ways. Of note, we cannot clarify which of lingular volume or total cross-sectional area of lingular arteries is cause of the other, even if there is causal relationship. Nonetheless, our findings may shed a ray of light on the controversy whether pulmonary artery is an active determinant for normal lung development, which is of a great interest for researchers in this uncertain field.
The current findings using 3D volumetry are also useful for surgeon for better understanding of detailed lung anatomy. Lung segmentectomy has often been performed in patients with peripheral small-sized non-small cell lung cancer, even though the survival and functional benefit is still controversial. Relationship between vasculature and organ development will become more important in context toward regenerative medicine.
Limitations of our study include selection biases due to the retrospective nature. Although we identified one or more lingular arteries in all patients, possible influence of undetectable small arteries due to the limited spacial resolution of 3D-CT37 cannot be denied. In addition, our findings should be validated using the measurement by pulmonary perfusion scan and ventilation scan, which could provide regional quantitative data of blood flow and ventilation38,39,40. Thus, further study is required to confirm the correlation with these reliable modalities.
In conclusion, this is the first report demonstrating a significant influence of branching patterns and cross sectional area of pulmonary arteries on lung volume using novel 3D-CT volumetry, which shed a light on a role of vasculature in lung development.
Methods
Subjects and imaging data
This study was approved by Aichi Cancer Center Hospital Ethical Committee. Our study was performed in full accordance with the local IRB guidelines (No. 2015–1–247). Informed consent was obtained from all patients. Our study included 204 consecutive patients who underwent surveillance chest CT to rule out possible intrathoracic malignancies in April 2015 at Aichi Cancer Center Hospital. Patients who met the following criteria were excluded: 1) history of chronic pulmonary diseases, 2) recognizable pleural effusion or massive infiltrative attenuation on chest CT, 3) history of pulmonary resection and/or chest irradiation, 4) medically treated cardiac dysfunction, or 5) significant findings of emphysema, bronchiectasis, and/or bronchial wall thickening on high-resolution CT as previously described41.
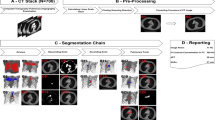
CT scan was performed using 64-detector-row CT scanner (Light Speed VCT; General Electric, CT, USA). Whole chest was scanned during a breath-hold at deep inspiration phase in supine position, and 1.25-mm thick high-resolution images were reconstructed using standard spatial-frequency reconstruction algorithm. Digital imaging and communications in medicine data was transferred to the commercially available workstation (Synapse Vincent; Fujifilm Medical Co., Tokyo, Japan). 3D images including pulmonary vessels, tracheo-bronchial trees, and lung parenchyma were reconstructed. Lingular division arteries (A4 and A5) and intersegmental veins (V3a and V3b), as well as lingular bronchi (B4 and B5) were identified using the images. Lingular division was isolated on the 3D-images based on the following process5: (1) calculation of the bronchial ventilation area and lingular artery perfusion area using an algorithm based on the direction and diameter of the bronchi and artery (Fig. 5a and b), and (2) visualization of intersegmental planes defined by intersegmental veins (V3a and V3b; Fig. 5c). Volumetry was automatically performed using the 3D images6. Measurement was performed twice by two experienced operator (HD and KS) and the best value was applied for analysis. Definition of MLA is a branch to lingular division as the first branch of the left main pulmonary artery (Fig. 2a). Normal lingular artery, branches from interlobar left pulmonary artery is called “ILA (interlobar artery)” (Fig. 2b). If cases had at least one MLA, they were categorized to MLA group. A diameter and cross-sectional area of each lingular artery were measured at the bifurcation (total 298 arteries). In case with two lingular arteries, cross-sectional area of the two arteries was totaled per subject.
(a and b) According to the 3D-CT images, lingular bronchi (B4 + 5) and segmental arteries (A4 + A5) are identified. Subsequently, the perfusion area is depicted using an algorithm based on the direction and diameter of the bronchi and artery. (c) Visualization of intersegmental plane defined by intersegmental veins (V3a and V3b).
For evaluation of emphysematous lung, %LAA, so-called “percent emphysema”, was defined as percentage of total voxels within the lung field that fell below −950 Hounsfield units42,43,44 based on 3D-CT analysis. Additionally, Goddard classification was also used to evaluate severity of pulmonary emphysema45. CT-based cardiothoracic ratio (CT-CTR) was measured as previously described46 because CT-CTR was highly concordant to CTR on chest X-ray.
Statistical analysis
Categorical variables were represented as counts with percentages. Continuous variables which did not show normal distribution were represented as a median with a quartile deviation (QD) or a range. Independent categorical data were compared by Pearson χ2 test and continuous data were compared by Mann-Whitney U-test. Correlation was tested using Spearman’s rank correlation. To further minimize the effect of measurable selection bias, a matching procedure was conducted. Variables which reportedly correlate to lung volume, including age19, sex20, smoking status19, CTR21, and body mass index22, 23 were matched 1 to 1, randomly choosing cases from the set of all possible matches. All tests were two-sided and p < 0.05 was considered to be significant. Statistical analysis was performed using SPSS (ver. 23; SPSS Inc., Chicago, IL).
References
Franser, R. G. & Pare, J. P. Diagnosis of Diseases of the chest. Vol. I: 2nd edition. 56 (PA: WB Saunders, 1977).
Yamashita, H. Variations in the pulmonary segments and the bronchiovascular trees. Roentgenologic anatomy of the lung. 70–107 (Igaku-Shoin, 1978).
Shiels, T. W., LoCiero, J. III & Ponn, R. B. Surgical anatomy of the lungs, General Thoracic Surgery. 5th ed. 63–75 (Lippincott Williams & Wilkins, 2000).
Subotich, D., Mandarich, D., Milisavljevich, M., Filipovich, B. & Nikolich, V. Variations of pulmonary vessels: some practical implications for lung resections. Clin Anat. 22, 698–705, doi:10.1002/ca.v22:6 (2009).
Saji, H. et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 17, 227–232, doi:10.1093/icvts/ivt120 (2013).
Ohshima, S. Volume analyzer SYNAPSE VINCENT for liver analysis. J. Hepatobiliary Pancreat Sci. 21, 235–238, doi:10.1002/jhbp.81 (2014).
Simpson, A. L. et al. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 219, 199–207, doi:10.1016/j.jamcollsurg.2014.02.027 (2014).
Sato, F. et al. A study of the right intersectional plane (right portal scissura) of the liver based on virtual left hepatic trisectionectomy. World J Surg. 38, 3181–3185, doi:10.1007/s00268-014-2718-5 (2014).
Oshiro, Y. et al. Novel 3-dimensional virtual hepatectomy simulation combined with real-time deformation. World J Gastroenterol. 21, 9982–9992, doi:10.3748/wjg.v21.i34.9982 (2015).
Iwano, S., Usami, N., Yokoi, K. & Naganawa, S. Segmentectomy simulation using a virtual three-dimensional safety margin. Ann Thorac Surg. 93, e37–39, doi:10.1016/j.athoracsur.2011.09.050 (2015).
Nakahara, K. et al. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg. 39, 260–265, doi:10.1016/S0003-4975(10)62591-X (1985).
Kim, H. K. et al. Vibration response imaging in prediction of pulmonary function after pulmonary resection. Ann Thorac Surg. 94, 1680–1686, doi:10.1016/j.athoracsur.2012.07.019 (2012).
Konheim, J. A. et al. Predictive equations for lung columes from computed tomography for size matching in pulmonary transplantation. J Thorac Cardiovasc Surg. 151, 1163–1169, doi:10.1016/j.jtcvs.2015.10.051 (2016).
Nakahara, Y. et al. Exercise hypoxaemia as a predictor of pulmonary hypertension in COPD patients without severe resting hypoxaemia. Respirology. 22, 120–125, doi:10.1111/resp.12863 (2017).
Lindenmaier, T. J. et al. Pulmonary Artery Abnormalities in Ex-smokers with and without Airflow Obstruction. COPD. 13, 224–234, doi:10.3109/15412555.2015.1074666 (2016).
Sheikh, K., Coxson, H. O. & Parraga, G. This is what COPD looks like. Respirology. 21, 224–236, doi:10.1111/resp.12611 (2016).
Stone, I. S. et al. Lung Deflation and Cardiovascular Structure and Function in Chronic Obstructive Pulmonary Disease. A Randomized Controlled Trial. Am J Respir Crit Care Med. 193, 717–726, doi:10.1164/rccm.201508-1647OC (2016).
Schulman, L. L., Lennon, P. F., Wood, J. A. & Enson, Y. Pulmonary vascular resistance in emphysema. Chest. 105, 798–805, doi:10.1378/chest.105.3.798 (1994).
Griffith, K. A. et al. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 163, 61–68, doi:10.1164/ajrccm.163.1.9906089 (2001).
Schwartz, J., Katz, S. A., Fegley, R. W. & Tockman, M. S. Sex and race differences in the development of lung function. Am Rev Respir Dis. 138, 1415–1421, doi:10.1164/ajrccm/138.6.1415 (1988).
Tomita, H. et al. Changes in Cross-Sectional Area and Transverse Diameter of the Heart on Inspiratory and Expiratory Chest CT: Correlation with Changes in Lung Size and Influence on Cardiothoracic Ratio Measurement. PLoS One. 10, e0131902, doi:10.1371/journal.pone.0131902 (2015).
Brazzale, D. J., Pretto, J. J. & Schachter, L. M. Optimizing respiratory function assessments to elucidate the impact of obesity on respiratory health. Respirology. 20, 715–721, doi:10.1111/resp.2015.20.issue-5 (2015).
Cibella, F. et al. An elevated body mass index increases lung volume but reduces airflow in Italian schoolchildren. PLoS One. 10, e0127154, doi:10.1371/journal.pone.0127154 (2015).
Schuhmann, M. et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med. 191, 767–774, doi:10.1164/rccm.201407-1205OC (2015).
Herth, F. J. et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med. 4, 185–193, doi:10.1016/S2213-2600(16)00045-X (2016).
Nobuoka, T. et al. Portal blood flow regulates volume recovery of the rat liver after partial hepatectomy: molecular evaluation. Eur Surg Res. 38, 522–532, doi:10.1159/000096292 (2006).
Jiang, S. M. et al. Role of splanchnic hemodynamics in liver regeneration after living donor liver transplantation. Liver Transpl. 15, 1043–1049, doi:10.1002/lt.v15:9 (2009).
Goldsmith, H. L., Cokelet, G. R. & Gaehtgens, P. Robin Fåhraeus: evolution of his concepts in cardiovascular physiology. Am J Physiol. 257, H1005–1015 (1989).
Kovacs, G., Berghold, A., Scheidl, S. & Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 34, 888–894, doi:10.1183/09031936.00145608 (2009).
Carlsson, A. J., Bindslev, L., Santesson, J., Gottlieb, I. & Hedenstierna, G. Hypoxic pulmonary vasoconstriction in the human lung: the effect of prolonged unilateral hypoxic challenge during anaesthesia. Acta Anaesthesiol Scand. 29, 346–351, doi:10.1111/aas.1985.29.issue-3 (1985).
Cardoso, W. V. Molecular regulation of lung development. Annu Rev Physiol. 63, 471–494, doi:10.1146/annurev.physiol.63.1.471 (2001).
Pugh, C. W. & Ratcliffe, P. J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 9, 677–684, doi:10.1038/nm0603-677 (2003).
Takahashi, Y. et al. Thyroid transcription factor-1 influences the early phase of compensatory lung growth in adult mice. Am J Respir Crit Care Med. 181, 1397–1406, doi:10.1164/rccm.200908-1265OC (2010).
Ding, B. S. et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 147, 539–553, doi:10.1016/j.cell.2011.10.003 (2011).
Ding, B. S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 505, 97–102, doi:10.1038/nature12681 (2014).
Takahashi, Y. et al. Predictors of long-term compensatory response of pulmonary function following major lung resection for non-small cell lung cancer. Respirology. 22, 364–371, doi:10.1111/resp.12904 (2017).
Sugimoto, M. et al. Carbon dioxide-enhanced virtual MDCT cholangiopancreatography. J Hepatobiliary Pancreat Sci. 17, 601–610, doi:10.1007/s00534-009-0201-8 (2010).
Kurimoto, A. et al. Parenchyma-preserving hepatectomy based on portal ramification and perfusion of the right anterior section: preserving the ventral or dorsal area. J Hepatobiliary Pancreat Sci. 23, 158–166, doi:10.1002/jhbp.v23.3 (2016).
Perisinakis, K., Seimenis, I., Tzedakis, A. & Damilakis, J. Perfusion scintigraphy versus 256-slice CT angiography in pregnant patients suspected of pulmonary embolism: comparison of radiation risks. J Nucl Med. 55, 1273–1280, doi:10.2967/jnumed.114.137968 (2014).
Ohno, Y. et al. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. AJR Am J Roentgenol. 182, 73–78, doi:10.2214/ajr.182.1.1820073 (2004).
Takahashi, Y. et al. Qualitative Analysis of Preoperative High-Resolution Computed Tomography: Risk Factors for Pulmonary Complications After Major Lung Resection. Ann Thorac Surg. 101, 1068–1074, doi:10.1016/j.athoracsur.2015.09.009 (2016).
Gevenois, P. A., de Maertelaer, V., De Vuyst, P., Zanen, J. & Yernault, J. C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 152, 653–657, doi:10.1164/ajrccm.152.2.7633722 (1995).
Wang, Z. et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 23, 975–984, doi:10.1007/s00330-012-2683-z (2013).
Cho, M. H. et al. NETT Genetics, ECLIPSE, and COPD Gene Investigators. A Genome-Wide Association Study of Emphysema and Airway Quantitative Imaging Phenotypes. Am J Respir Crit Care Med. 192, 559–569, doi:10.1164/rccm.201501-0148OC (2015).
Goddard, P. R., Nicholson, E. M., Laszlo, G. & Watt, I. Computed tomography in pulmonary emphysema. Clin Radiol. 33, 379–387, doi:10.1016/S0009-9260(82)80301-2 (1982).
Gollub, M. J. et al. Shall we report cardiomegaly at routine computed tomography of the chest? J Comput Assist Tomogr. 36, 67–71, doi:10.1097/RCT.0b013e318241e585 (2012).
Acknowledgements
We thank to Dr. Kei Suzuki, Boston University School of Medicine, for statistical support and editing this manuscript.
Author information
Authors and Affiliations
Contributions
H.D. and Y.T. wrote the main manuscript and prepared all figures. All authors reviewed and approved this manuscript. D.H., Y.T., T.H., M.K., and Y.S. designed this study and are responsible for interpretation of the data. H.D. and Y.T. conducted most of the analysis with the help of K.S., Y.S. takes responsibility for the integrity of the data and accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dejima, H., Takahashi, Y., Hato, T. et al. Mediastinal pulmonary artery is associated with greater artery diameter and lingular division volume. Sci Rep 7, 1273 (2017). https://doi.org/10.1038/s41598-017-01384-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01384-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.