Abstract
Soil microbes play important roles in plant growth and health. Little is known about the differences of soil microbes between healthy and bacterial wilt infected soils with Ralstonia solanacearum. By Illumina-MiSeq sequencing of 16S rRNA and 18S rRNA gene amplicons, we found the soil microbial composition and diversity were distinct between healthy and bacterial wilt infected soils. Soil microbial community varied at different plant growth stages due to changes of root exudates composition and soil pH. Healthy soils exhibited higher microbial diversity than the bacterial wilt infected soils. More abundant beneficial microbes including Bacillus, Agromyces, Micromonospora, Pseudonocardia, Acremonium, Lysobacter, Mesorhizobium, Microvirga, Bradyrhizobium, Acremonium and Chaetomium were found in the healthy soils rather than the bacterial wilt infected soils. Compared to bacterial wilt infected soils, the activities of catalase, invertase and urease, as well as soil pH, available phosphorous and potassium content, were all significantly increased in the healthy soils. In a conclusion, the higher abundance of beneficial microbes are positively related the higher soil quality, including better plant growth, lower disease incidence, and higher nutrient contents, soil enzyme activities and soil pH.
Similar content being viewed by others
Introduction
Bacterial wilt caused by Ralstonia solanacearum is a soil-borne disease to infect tomato, hot pepper, egg plants, potato, tobacco, banana, etc., and causes serious economic losses worldwide1. Recently, bacterial wilt is more and more epidemic in China. Many efforts have been engaged in control of this disease2,3,4, but unfortunately it is still popular worldwide including China.
Various soil bio-chemical factors not only influence pathogen growth and survive in soils but also affect the nutrient availability for plant productivity. Among them, soil microorganisms are very important factors both for plants and pathogens, which play critical roles in regulating soil fertility, cycling of nutrients, promoting plant health and protecting plants from diseases such as bacterial wilt5,6,7. On the other hand, other soil bio-chemical factors such as soil pH also influence soil microbial community structure8. Healthy soils with balanced soil microbial community are beneficial for promoting plants growth and prevention of plant diseases4, 9. However, it is still unclear for the soil microbial community structure in the unhealthy soils, especially in the bacterial wilt infected soils.
The objective of this study is to evaluate the characteristics and differences between bacterial wilt infected soils and healthy soils, including soil biological and chemical properties, and soil microbial community. We hypothesized that: (1) soil properties are correlated with plant health, soil microbial community, and bacterial wilt; (2) soil microbial community is shifted in the bacterial wilt infected soils; (3) soil microbial community varies in the healthy and bacterial wilt infected soils at different plant growth stages. For this purpose, we examined the difference of microbial community of tobacco (used as a model plant in this study) planting soils via Illumina-MiSeq sequencing between unhealthy soils (bacterial wilt infected soils) and healthy soils, to elucidate the correlation of soil microbial community and soil health in the field.
Results
Soil biological and chemical properties
Soil biological and chemical properties were different between healthy and bacterial wilt infected soils. The activities of urease, invertase and catalase in healthy soils were significantly (p < 0.01) higher than the bacterial wilt infected soils from 30 to 90 d post-transplantation (Table 1), while the acid phosphatase activity was similar between healthy and bacterial wilt infected soils except for 30 d post-transplantation. At 30 d, the acid phosphatase activity was significantly (p < 0.05) lower in the healthy soils than the bacterial wilt infected soils (Fig. S1).
The healthy soils contained less available N (AN) but more (p < 0.01) available P (AP) and K (AK) than the bacterial wilt infected soils from 30 d to 90 d post-transplantation (Table 2). The AK and AP content had the similar variable trends both in healthy and bacterial wilt infected soils, which reached the highest level at 30 d and 60 d, respectively, then both decreased thereafter until the end. The soil pH in healthy soils was significantly (p < 0.05) higher than the bacterial wilt infected soils from 30 d to 90 d post-transplantation (Table 2). However, there was no significant difference of soil organic matter (SOM) content between healthy and bacterial wilt infected soils from 30 d to 90 d (Fig. S1).
Plant growth and disease incidence
Tobaccos grew better in the healthy soils than the bacterial wilt infected soils. The height and stem circumference of tobaccos were significantly (p < 0.01) higher in the healthy soils than the bacterial wilt infected soils (Fig. S2). In the infected soils, severe bacterial wilt occurred, with significantly higher disease incidence than the healthy soils from 30 d to 90 d after transplantation. The disease index of bacterial wilt infected soils was 16.54 and 93.82 at 60 and 90 d post-transplantation, respectively, while it was 0 at all time points for the healthy soils (Table 3).
Soil bacterial diversity
Totally, 207426 effective sequence reads were obtained from all soil samples after filtering out low-quality reads and chimera sequences. The operational taxonomic units (OTUs) number, Chao 1, and Shannon index were used to evaluate and compare the diversity and richness of bacterial communities among different soil samples (Table 4). The number of OTUs in all soil samples ranged from 3538 to 6611, and the 16S rRNA gene diversity was higher in the healthy soils than the bacterial wilt infected soils. From 0 to 90 d post-transplantation, the number of OTUs and Shannon index were both higher in the healthy soils than the bacterial wilt infected soils, indicating the diversity of soil bacteria was higher in the healthy soils than the bacterial wilt infected soils. Analysis by Chao1, a higher richness of bacteria was also found in the healthy soils.
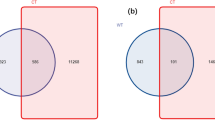
We further analyzed the temporal trends of OTUs number and Shannon index in the soils, and found they firstly decreased from 0 d to 60 d then increased at 90 d after transplantation both in healthy and bacterial wilt infected soils (Table 4). At 60 d, OTUs number decreased to 54.3% of the initial (0 d) in the bacterial wilt infected soil, possibly due to low soil pH at this time (Table 2). The overlapping bacterial OTUs between healthy and bacterial wilt infected soils were 11.6% (1145/9874) at 60 d post-transplantation (Fig. S3). More specific OTUs were found in the healthy soils (5052) than the bacterial wilt infected soils (3677). This result showed the soil bacterial diversity varied dramatically in the healthy soils when compared to the bacterial wilt infected soils.
Bacterial community composition
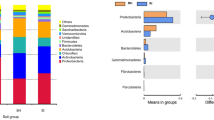
Bacteria were identified as 26 phyla in all soil samples. Proteobacteria was the most abundant phylum (27%), followed by Actinobacteria (14%), Acidobacteria (14%), Chloroflexi (8%) and Firmicutes (6%) (Fig. S4). Except for Acidobacteria, Chlamydiae, Chloroflexi and Spirochaetes, other phyla were more abundant in the healthy soils than the bacterial wilt infected soils (Fig. S4).
The abundance of bacterial phyla changed during different tobacco growth stages both in healthy soils and bacterial wilt infected soils (Fig. 1). For example, the abundance of Proteobacteria increased in the healthy soils at 30 d, while obviously decreased in the bacterial wilt infected soils at 30 d and 60 d. The abundance of Actinobacteria increased continuously from 0 d to 90 d in the healthy soils, while decreased at 30 d and then increased at 60 and 90 d in the bacterial wilt infected soils.
Different bacterial communities in the healthy and bacterial wilt infected soils
The bacterial difference between healthy and bacterial wilt infected soils was analyzed at the genus level. Abundances of 36 bacterial genera were significantly (p < 0.05) different between healthy and bacterial wilt infected soils (Fig. 2, Fig. S4), that was indicated by Heatmaps and hierarchical clustering analysis (Fig. 2). 19 bacterial genera were more abundant in the healthy soils, including Aeromicrobium, Agromyces, Bacillus, Blastococcus, Gemmatimonas, Micromonospora, Nocardioides, Pseudonocardia, Solirubrobacter, Bradyrhizobium, etc., while the rest 17 genera including Ralstonia, Aciditerrimonas, Actinospica, Byssovorax, Catenulispora, Conexibacter, Dongia, Acidobacteria Gp1-Gp3, Modestobacter, Mycobacterium, Pseudolabrys, Rhizomicrobium, Rudaea and Clostridium were more abundant in the bacterial wilt infected soils. Interestingly, the beneficial microorganisms (e.g. Bacillus, Bradyrhizobium, Nocardioides and Micromonospora) were more abundant in the healthy soils than the bacterial wilt infected soils.
Comparison of abundance of different soil bacterial genera and bacterial community between healthy and bacterial wilt infected soils. (a) and (b) Abundances of different soil bacterial genera were compared between healthy and bacterial wilt infected soils; (c) Relative abundance and hierarchical cluster analysis of bacterial genera; (d) PCoA analysis of soil bacterial community. H: healthy soils; D: bacterial wilt infected soils. Bars with asterisk (*) and double asterisks (**) indicate significant (p < 0.05) and very significant (p < 0.01) difference between healthy and bacterial wilt infected soils, respectively.
Principal co-ordinates analysis (PCoA) showed the bacterial community were different between healthy soils and bacterial wilt infected soils at different time points. PC1 and PC2 explained 83.55% of the total bacterial community (Fig. 2). Healthy soils (H 30 d, H 60 d, and H 90 d) and bacterial wilt infected soils (D 30 d, D 60 d and D 90 d) were respectively clustered together and separated from each other at PC1 axis. In the healthy soils, H 0 d was well separated from other three time points (H 30 d, H 60 d, and H 90 d) at PC2 axis. D 0 d was also separated from other three time points (D 30 d, D 60 d and D 90 d) in the bacterial wilt infected soils, indicating the soil bacterial community changed obviously after transplanting.
Shifts of soil fungal diversity and community structure in bacterial wilt infected soils
All soil samples consist of 14235 fungal OTUs and 292463 reads. Sequences from fungi matched 6 main known phyla, including Ascomycota (58.35%) followed by Basidiomycota (18.47%) (Fig. S5). The temporal trends of fungal phyla in healthy soils were obviously different from the bacterial wilt infected soils (Fig. 3). OTUs number of different fungal phyla firstly decreased then increased in the bacterial wilt infected soils after transplantation; however, it varied irregularly in the healthy soils. For example, the abundance of Ascomycota decreased at 30 d, increased at 60 and decreased again at 90 d in the healthy soils, while it decreased at 30 d then continuously increased from 60 to 90 d in the bacterial wilt infected soils.
Soil fungal diversity and community structure. Temporal trends of different fungal phyla in healthy soils (a) and bacterial wilt infected soils (b) during different tobacco growth stages; (c) Abundance of different fungal genera were compared between healthy and bacterial wilt infected soils. Bars with asterisk (*) and double asterisks (**) indicate significant (p < 0.05) and very significant (p < 0.01) difference between healthy and bacterial wilt infected soils, respectively; (d) PCoA analysis of soil fungal community. H: healthy soils; D: bacterial wilt infected soils.
The fungal OTUs number ranged from 414 to 999 in different soil samples, much less than the soil bacterial abundance (Table 5). The healthy soil samples had higher OTUs number and Shannon index during all stages of tobacco growth when compared to the bacterial wilt infected soils. The average Shannon index was 4.3 in the healthy soils, much higher than the bacterial wilt infected soils of 3.4. Analysis by Chao1, it was found the healthy soils also had a higher fungal richness than the bacterial wilt infected soils (Table 5). Thereby, the healthy soils have a higher fungal diversity and richness than the bacterial wilt infected soils.
The percentage of overlapping fungal OTUs between healthy and bacterial wilt infected soils was only 5.28% (241/4563) at 60 d post-transplantation (Fig. S3), indicating these two different soil types have a great number of specific fungi species (2908 for healthy soils, and 1414 for bacterial wilt infected soils). This result showed the soil fungal types varied dramatically in the healthy soils when compared to the bacterial wilt infected soils.
The abundances of 11 fungal genera were significantly (p < 0.05) different between healthy and bacterial wilt infected soils (Fig. 3, Fig. S5). In the healthy soils, the abundances of Acremonium, Chaetomium, Sodiomyces, Halosphaeria, Lophotrichus, Mrakia and Phellinus were significantly (p < 0.05) higher, while the abundances of Basipetospora, Trichosporon, Paraglomus and Entophlyctis were significantly (p < 0.05) lower than the bacterial wilt infected soils. Further analysis found some beneficial fungi (e.g. Chaetomium and Acremonium) were more abundant in the healthy soils than the bacterial wilt infected soils, which are favorable for improving soil quality and inhibiting soil pathogens10,11,12.
PCoA analysis showed the soil fungal communities of healthy soils were different from the bacterial wilt infected soils. PC1 and PC2 explained 67.45% of the fungal community. Figure 3 clearly showed the healthy soils (H 0 d, H 30 d, and H 60 d) were well separated from the bacterial wilt infected soils (D 30 d, D 60 d, and D 90 d) at PC1 axis. Healthy soils (H 0 d, H 30 d, and H 60 d) and bacterial wilt infected soils (D 30 d, D 60 d, and D 90 d) were clustered together, respectively. H 90 d and D 0 d were separated alone. The results indicated the soil fungal community structure of bacterial wilt infected soils was dramatically shifted when compared with the healthy soils. The plant growth stages also obviously impacted on the dynamic and diversity of soil fungal community.
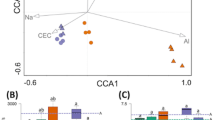
Relationships between microbial community structure and environmental variables
The relationships between microbial community structure and soil bio-chemical properties were analyzed with canonical correspondence analysis (CCA). Seven parameters including pH, invertase, catalase, soil organic matter (SOM), AK, AN and AP were selected for CCA based on the significant test, and the results showed 66.8% of community variation could be explained by these variables (Fig. 4). The healthy soils and bacterial wilt infected soils were grouped separately, and we found the first canonical axis (CCA1) was positively correlated with AN and soil organic matter, and the second canonical axis (CCA2) was positively correlated with pH, invertase and catalase, but negatively correlated with AP and AK. As important variables (represented by longer arrows), AP, AN, AK, catalase, invertase and pH play major roles in the shaping of soil bacterial community structure. The healthy soils (H 30 d, H 60 d and H 90 d) were positively correlated with AP and AK, while the bacterial wilt infected soils were positively correlated with AN but negatively correlated with pH, AP, AK, invertase and catalase.
Canonical correspondence analysis of the relationship between microbial community structure and soil properties. (a) soil bacterial community; (b) soil fungal community. The soil properties are indicated with arrows, including soil pH, invertase, catalase, soil organic matter (SOM), available potassium (AK), nitrogen (AN), and phosphorous (AP) content. H: healthy soils; D: Bacterial wilt infected soils. The percentage of variation is explained by each axis.
For fungi (Fig. 4), 52.8% of community variation can be explained by the variables. The healthy and bacterial wilt infected soils were grouped separately along CCA1. The first canonical axis was positively correlated with AN, and the second axis was positively correlated with AP, AK, SOM and catalase. As important variables, AN, pH, invertase and catalase played major roles in the shaping of soil fungal community structure. The healthy soils were positively correlated with AP and catalase, while the bacterial wilt infected soils (D 30 d, D 60 d and D 90 d) positively correlated with AN, and negatively correlated with pH, invertase and catalase.
Discussion
We hypothesized that soil properties are correlated with plant health, soil microbial community, and bacterial wilt. Here, we found soil bio-chemical properties were relevant to soil microbial community. Compared to bacterial wilt infected soils, the activities of soil oxidoreductase (catalase), and enzymes involved in C (invertase) and N (urease) cycling were significantly enhanced in the healthy soils, which are deduced to release more soil nutrients for plants and soil microorganisms. P additions can cause an increase of bacterial diversity, with increased Actinobacteria and Alphaproteobacteria, and decreased Acidobacteria in soils13, 14. Here, lower abundance of Acidobacteria, and higher abundance of Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes were found in the healthy soils with higher AP content. In addition, nitrogen is one of the important factors for plant growth. The optimal nitrogen application rate (168–210 kg/hm−2) can improve tobacco growth, while the excessive application of nitrogen fertilizers has negative effect on plant growth by decreasing soil pH15,16,17. We found the AN content was positively correlated with the microbial community of bacterial wilt infected soils, indicating the AN content may be an indicator for soil microbial community and plant health.
It was found that the bacterial wilt infected soils were acidic with a lower bacterial and fungal diversity than the healthy soils. As reported, soil pH strongly influences the composition of soil microbial community. The soils with near - neutral pH have a higher bacterial diversity than the acidic ones, and the abundances of many bacterial phyla are strongly correlated with soil pH18, 19. Soil acidity is linked to the decrease of available carbon for soil microbes20; thereby, the soil pH may act as an environmental filter (by stressing microbial cells) for selecting specific microbial groups and regulating soil microbial community composition21. Here, the healthy soils with high pH value had a higher abundance of Actinobacteria and Bacteroidetes than the bacterial wilt infected soils, consistently with the reports19. Conversely, the bacterial wilt infected soils had a higher abundance of Acidobacteria Gp1, Gp2, Gp3, and Aciditerrimonas (Fig. 2), which are potential indicators for acidic and diseased soils9, 21, 22. Notably, the low soil pH is positively correlated with R. solanacearum epidemic23, which can stress tobaccos making them more susceptible to infection of R. solanacearum.
We also hypothesized that the soil microbial community is shifted in the bacterial wilt infected soils, which was proven in this study. The soil bacterial and fungal communities of healthy soils were very different from the bacterial wilt infected soils. We also found the healthy soils had higher microbial diversity, as reported, the soil microbial diversity is positively correlated with the plant resistance to pathogens24,25,26. Healthy soils had higher abundances of beneficial microbes (e.g. Bacillus, Agromyces, Micromonospora, Pseudonocardia, Acremonium, Lysobacter, Bradyrhizobium, Mesorhizobium, Microvirga, Acremonium, and Chaetomium), which can improve soil nutrients, promote plant growth and control soil-borne diseases27. For example, Bacillus is generally effective for suppressing bacterial wilt caused by R. solanacearum 28,29,30. Agromyces, Micromonospora and Pseudonocardia play important roles in degradation of xylan through production of xylanase31,32,33. Micromonospora, Acremonium and Lysobacter can produce antimicrobial compounds to protect plants from pathogens infection34,35,36. Bradyrhizobium is reported to suppress fungal pathogens and root-knot nematodes37. Mesorhizobium, Microvirga and Bradyrhizobium are beneficial for plants growth by nitrogen-fixation38. Both Acremonium and Chaetomium are potential for bio-control of plant disease via production of lytic enzymes and antimicrobial metabolites10,11,12. Thereby, we infer these beneficial bacteria and fungi are positively correlated with soil quality and plant health. Whereas, Ralstonia is more abundant in the bacterial wilt infected soils, which can cause serious bacterial wilt in the tobacco field.
Lastly, we hypothesized that the soil microbial community varies in healthy and bacterial wilt infected soils at different plant growth stages. Here, we found the soil microbial communities were distinct at different tobacco growth stages. The composition of root exudates differs at different plant growth stages, with strong impact on soil microbial communities39, 40. For example, phenol and phenolic acids have been identified in the tobacco root exudates41, 42, which may greatly impact on microbial biomass and diversity in soils43, 44. Here, the bacterial OTUs number increased at 90 d post-transplantation, this may be explained by the increased tobacco root exudates for promoting soil bacterial growth at this stage. It is noted that some bacterial (e.g. Proteobacteria, Actinobacteria) and fungal (e.g. Ascomycota, Basidiomycota) abundances obviously changed with different trends at different tobacco growth stages in healthy and bacterial wilt infected soils. Proteobacteria play key roles in the soil carbon, sulfur and nitrogen cycles, including purple nonsulfur bacteria (Rhodospirillum and Rhodopseudomonas)45, free-living aerobic nitrogen fixers (Azotobacter, Azomonas, Azospirillum and Beyerinckia)46, and nitrifying bacteria (Nitrobacter, Nitrococcus and Nitrospira)47. The abundance of Proteobacteria in healthy soils increased in 30 d because fertilizers are adequate at this stage, that may be beneficial for promoting the C, N, S cycles in soils. However, the abundance of Proteobacteria in healthy soils decreased at 60 and 90 d, because most of fertilizers were exhausted and the composition of root exudates also changed at these stages. On the other hand, the abundance of Proteobacteria greatly decreased in the bacterial wilt infected soils at 30 d possibly due to poor soil environments such as soil acidification in the tobacco field. Most of Actinobacteria can produce antibiotics inhibiting plant pathogens and controlling plant diseases48. The abundance of Actinobacteria increased continuously from 0 d to 90 d in the healthy soils, and always were higher than the bacterial wilt infected soils at all time points.
In addition, the abundance of Ascomycota was higher in the healthy soils than the bacterial wilt infected soils from 0 d to 60 d, which can enhance soil C cycle and plant nutrient uptake49, 50. The abundance of Basidiomycota decreased at 30 d and then increased at 60 d and 90 d in the healthy soils, while it decreased both at 30 and 60 d in the bacterial wilt infected soils. Both Ascomycetes and Basidiomycota are important decomposers in carbon cycle, which can secrete digestive enzymes to break down organic substances (e.g. cellulose, lignocellulose, lignin in plant litters) into smaller molecules49, 50. Thereby, the increase of Basidiomycota abundance is favorable for degradation of plant litters and promotion of C cycle in soils49, 50.
In conclusions, we found the soil microbial composition and diversity were distinct between healthy and bacterial wilt infected soils. The soil microbial community varied at different plant growth stages possibly due to the changed root exudates and soil pH. Healthy soils exhibited a higher microbial diversity and abundance of beneficial microbes such as Bacillus, Agromyces, Micromonospora, Pseudonocardia, Acremonium, Lysobacter, Mesorhizobium, Microvirga, Bradyrhizobium, Acremonium and Chaetomium, but a lower abundance of pathogens such as R. solanacearum. High abundances of beneficial microbes are positively related with the high soil quality, which is indicated by better plant growth, lower disease incidence, and higher soil pH, nutrient and enzyme activities.
Materials and Methods
Soil sampling
The study site was located in Enshi county (29.97°N, 109.38°W), Hubei province, central China. The fields are tobacco planting soils with subtropical humid climate (an annual rainfall of 1400–1500 mm and annual average temperature of 16 °C). Tobacco has been cultivated continuously for more than 15 years. Tobacco in certain fields exhibited severe bacterial wilt infected by R. solanacearum, whereas tobacco in other fields grew well without bacterial wilt. The soil samples were collected separately from three healthy fields in which the tobacco grew well without bacterial wilt (healthy soils), and three bacterial wilt infected fields where the tobaccos exhibited severe bacterial wilt. Bulk soil samples were obtained from the healthy and bacterial wilt infected soils at four time points (0 d - transplanting stage, 30 d - rosette stage, 60 d - fast growing stage, and 90 d - maturation stage post-transplantation) (Fig. S6). For each field (667 m2), a composite sample was collected from a mixture of 20 random soil cores (5 cm diameter, 15 cm depth). Each soil sample was partitioned into two subsamples, one for DNA extraction and another for analysis of bio-chemical properties after air-dry.
Analysis of soil bio-chemical properties
Urease, catalase, acid phosphatase and invertase were respectively measured according to Guan51. After being suspended with water (soil: water = 1: 2.5, w/v), the soil pH was measured using a pH meter (Mettler-Toledo FE20; Switzerland). Soil available N, P, K, and organic matter (SOM) were respectively measured by the methods described by Bao52. All tests were performed in triplicates.
Tobacco growth and disease incidence of bacterial wilt
Tobacco stem circumference and height, as well as disease incidence and disease index of bacterial wilt, were recorded at 90 d post-transplantation, respectively. Disease incidence was calculated by the percentage of diseased tobaccos in each field. Disease index was evaluated using a disease score method: 0 = no symptom, 1 = below one half of tobacco leaves wilted, 3 = one half to two-thirds of tobacco leaves wilted, 5 = above two-thirds of tobacco leaves wilted, 7 = all leaves wilted, and 9 = stems collapsed or tobaccos died53. Disease index was calculated using the formula:
r is the disease severity, N is the number of infected tobaccos with a rating of r, n is the total number of tobaccos tested, and R is the value of the highest disease severity in each field.
Soil DNA extraction
0.4 g soils were used to extract soil microbial genomic DNA using soil DNA extraction kits (FastDNA Spin Kit, MP Biomedicals, USA) according to the manufacturer’s instructions. DNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA).
Microbial rRNA gene amplification and Illumina sequencing
The extracted soil genomic DNA was used as template to amplify 16S rRNA and 18S rRNA genes, respectively. The V3 - V4 regions of 16S rRNA gene were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′)54, and the V5 - V7 regions of 18S rRNA gene were amplified using primers 0817F (5′-TTAGCATGGAATAATRRAATAGGA-3′) and 1196R (5′-TCTGGACCTGGTGAGTTTCC-3′)55. Amplicons were sequenced on Illumina-MiSeq platform (Illumina Inc., USA) at SHBIO Technology (Shanghai, China). The sequence quality was statistically analyzed by CASAVA1.8. The raw sequence data was preliminarily filtrated using the FASTX Toolkit 0.0.13 software package, removing the low mass base at the tail of the sequence (Q value less than 20), and finally removing the sequences with lengths less than 35 bp. The length of the valid reads was approximately 250 bp.
Statistical analysis
The operational taxonomic units (OTUs) at 97% similarity were used to perform rarefaction analysis and calculate the richness and diversity index. Sample normalization was conducted by rarefaction. Chao and Shannon indices were calculated as measures of microbial richness and diversity. The taxonomic differences between groups were compared by least-significant-difference (LSD) test with Holm-Bonferroni adjustment. P < 0.05 was considered with statistical significance. Principal co-ordinates analysis (PCoA) was performed based on OTU data to calculate the difference of microbial communities between healthy and bacterial wilt infected soils. We also performed hierarchical clustering analysis by Multi-experiment Viewer 4.9.0 based on microorganisms with significant difference between health and bacterial wilt infected soils. Heatmaps were generated using custom R scripts. Canonical correspondence analysis (CCA) using vegan package in R version 3.2.5 is performed to analyze the relationships between microbial community structure and environmental variables. Seven environmental factors including pH, catalase, invertase, AP, AN, SOM and AK were chosen to perform CCA analysis.
References
Liu, L. et al. Bioorganic fertilizer enhances soil suppressive capacity against bacterial wilt of tomato. PLoS One 10, e0121304 (2015).
Gamliel, A., Austerweil, M. & Kritzman, G. Non-chemical approach to soilborne pest management-organic amendments. Crop Prot 19, 847–853 (2000).
Liu, Y. et al. Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol Fert Soils 49, 447–464 (2012).
Janvier, C. et al. Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol Biochem 39, 1–23 (2007).
Faoro, H. et al. Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic Forest. App Environ Microbiol 76, 4744–4749 (2010).
Lauber, C. L., Strickland, M. S., Bradford, M. A. & Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40, 2407–2415 (2008).
Rousk, J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4, 1340–1351 (2010).
Zhang, T. et al. Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund region, Svalbard (high arctic). Front Microbio l 7, 227 (2016).
Benizri, E. et al. Replant diseases: bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol Biochem 37, 1738–1746 (2005).
Anisha, C. & Radhakrishnan, E. K. Gliotoxin-producing endophytic Acremonium sp. from Zingiber officinale found antagonistic to soft rot pathogen Pythium myriotylum. Appl Biochem Biotechnol 175, 3458–3467 (2015).
Soytong, K., Kanokmedhakul, S., Kukongviriyapa, V. & Isobe, M. Application of Chaetomium species (Ketomium®) as a new broad spectrum biological fungicide for plant disease control: a review article. Fungal Divers 7, 1–15 (2001).
Yao, Y. R. et al. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J Microb Biot 31, 549–556 (2015).
Leff, J. W. et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci USA 112, 10967–10972 (2015).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Lu, Y. X., Li, C. J. & Zhang, F. S. Transpiration, potassium uptake and flow in tobacco as affected by nitrogen forms and nutrient levels. Ann Bot 95, 991–998 (2005).
Wang, G. Y., Li, C. J. & Zhang, F. S. Effects of different nitrogen forms and combination with foliar spraying with 6-enzylaminopurine on growth, transpiration, and water and potassium uptake and flow in tobacco. Plant Soil 256, 169–178 (2003).
Li, J. et al. Optimal nitrogen application rate for flue-cured tobacco in tobacco-rice rotation region of Liuyang. Chinese Agricultural Science Bulletin 32, 58–63 (2016).
Lauber, C. L., Hamady, M., Knight, R. & Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75, 5111–5120 (2009).
Fierer, N. et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci USA 109, 21390–21395 (2012).
Högberg, M. N., Högberg, P. & Myrold, D. D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150, 590–599 (2007).
Dimitriu, P. A. & Grayston, S. J. Relationship between soil properties and patterns of bacterial b-diversity across reclaimed and natural boreal forest soils. Microb Ecol 59, 563–573 (2010).
Jones, R. T. et al. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3, 442–453 (2009).
Michel, V. V. & Mew, T. Effect of a soil amendment on the survival of Ralstonia solanacearum in different soils. Phytopathology 88, 300–305 (1998).
Luan, F. G. et al. Analysis of microbial diversity and niche in rhizosphere soil of healthy and diseased cotton at the flowering stage in southern Xinjiang. Genet Mol Res 14, 1602–1611 (2015).
Bailey, K. L. & Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res 72, 169–180 (2003).
Bulluck, L. R. & Ristaino, J. B. Effect of synthetic and organic soil fertility amendments on southern blight, soil microbial communities, and yield of processing tomatoes. Phytopathology 92, 181–189 (2002).
Raaijmakers, J. M. et al. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321, 341–361 (2009).
Chen, D. et al. Isolation of Bacillus amyloliquefaciens S20 and its application in control of eggplant bacterial wilt. J Environ Manage 137, 120–127 (2014).
Hu, H. Q., Li, X. S. & He, H. Characterization of an antimicrobial material from a newly isolated Bacillus amyloliquefaciens from mangrove for biocontrol of capsicum bacterial wilt. Biol Control 54, 359–365 (2010).
Tan, S. et al. Two Bacillus amyloliquefaciens strains isolated using the competitive tomato root enrichment method and their effects on suppressing Ralstonia solanacearum and promoting tomato plant growth. Crop Prot 43, 134–140 (2013).
Rivas, R. et al. Agromyces ulmi sp. nov, a xylanolytic bacterium isolated from Ulmus nigra in Span. Int J Syst Evol Microbiol 54, 1987–1990 (2004).
Ann, M. H. & Maria, V. Micromonospora: An important microbe for biomedicine and potentially for biocontrol and biofuels. Soil Biol Biochem 42, 536–542 (2010).
Zimmermann, W. Xylanolytic enzyme activities produced by mesophilic and thermophilic actinomycetes grown on graminaceous xylan and lignocellulose. FEMS Microbiol Lett 55, 181–185 (1988).
Donald, T. W., Shoshannah, R., Stephen, T. D. & James, B. G. A protective endophyte of maize: Acremonium zeae antibiotics inhibitory to Aspergillus flavus and Fusarium verticillioides. Mycol Res 109, 610–618 (2005).
Ismet, A. et al. Production and chemical characterization of antifungal metabolites from Micromonospora sp. M39 isolated from mangrove rhizosphere soil. World J Microb Biot 20, 523–528 (2004).
Ji, G. H. et al. Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Bio Control 45, 288–296 (2008).
Siddiqui, I. & Shaukat, S. Mixtures of plant disease suppressive bacteria enhance biological control of multiple tomato pathogens. Biol Fertil Soils 36, 260–268 (2002).
Ardley, J. K. et al. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int J Syst Evol Microbiol 62, 2579–2588 (2012).
Okubo, A., Matsusaka, M. & Sugiyama, S. Impacts of root symbiotic associations on interspecific variation in sugar exudation rates and rhizosphere microbial communities: a comparison among four plant families. Plant Soil 64, 1–12 (2016).
Yang, C. H. & Crowley, D. E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol 66, 345–351 (2000).
Gao, X. et al. Identification of chemical compositions of root exudates from flue-cured tobacco and their influence to seed germination. Chin Tob Sci 33, 87–91 (2012).
Hu, Y. S. et al. Identification of allelochemicals in cucumber root exudates and its allelopathy to radicle and Fusarium oxysporum. Ecol Environ 16, 954–957 (2007).
Tan, S. et al. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol 64, 15–22 (2013).
Zhou, X. & Wu, F. Effects of amendments of ferulic acid on soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.). Eur J Soil Biol 50, 191–197 (2012).
Adessi, A. & De Philippis, R. Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: a review. Int J Hydrogen Energy 39, 3127–3141 (2014).
Nosheen, A. et al. Protein quantity and quality of safflower seed improved by NP fertilizer and Rhizobacteria (Azospirillum and Azotobacter spp.). Front Plant Sci 7, 104 (2016).
Saijai, S. et al. Analysis of microbial community and nitrogen transition with enriched nitrifying soil microbes for organic hydroponics. Biosci Biotechnol Biochem 27, 1–8 (2016).
Barka, E. A. et al. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80, 1–43 (2015).
Unterseher, M., Peršoh, D. & Schnittler, M. Leaf-inhabiting endophytic fungi of European Beech (Fagus sylvatica L.) co-occur in leaf litter but are rare on decaying wood of the same host. Fungal Diversity 60, 43–54 (2013).
Purahong, W. et al. Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol Ecol. 25, 4059–4074 (2016).
Guan, S. M. Soil enzyme and its research method (Agriculture Press, 1986).
Bao, S. D. Soil and agricultural chemistry analysis (eds Jiang, R. F. et al.) (China Agriculture Press, 2013).
Yi, Y. J. et al. Isolation, identification and field control efficacy of an endophytic strain against tobacco bacterial wilt (Ralstonia solanacarum). Ying Yong Sheng Tai Xue Bao. 18, 554–558 (2007).
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41 (2013).
James, B. & Jack, H. R. PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66, 4356–4360 (2000).
Acknowledgements
We are very thankful for Tan J, Xiang BK and Peng WX for their helps in soil sampling and field experiments.
Author information
Authors and Affiliations
Contributions
R.W., H.C.Z., L.G.S. and S.C. performed the experiments. H.C.Z., R.W. and X.Y.Z. analyzed the data. G.F.Q. and X.Y.Z. designed the study and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, R., Zhang, H., Sun, L. et al. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7, 343 (2017). https://doi.org/10.1038/s41598-017-00472-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00472-6
This article is cited by
-
Impact of planting Phallus rubrovolvatus on physicochemical and microbial properties and functional groups of soil
Annals of Microbiology (2023)
-
Tobacco microbial screening and application in improving the quality of tobacco in different physical states
Bioresources and Bioprocessing (2023)
-
Impact of Parthenium hysterophorus L. invasion on soil fungal communities in the Yellow River Delta
Annals of Microbiology (2023)
-
Rhizobia modulate the peanut rhizobacterial community and soil metabolites depending on nitrogen availability
Biology and Fertility of Soils (2023)
-
Plant and soil-associated microbiome dynamics determine the fate of bacterial wilt pathogen Ralstonia solanacearum
Planta (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.