Abstract
Although a substantial body of previous functional magnetic resonance imaging (fMRI) studies have revealed different brain responses to external stimuli in chronic cigarette smokers compared with nonsmokers, only a few studies assessed brain spontaneous activity in the resting state in chronic smokers. The aim of this study was to investigate alterations of brain activity during the resting state in chronic smokers using fractional amplitude of low-frequency fluctuation (fALFF). In the present study, 55 smokers and 49 healthy nonsmokers were included. All the subjects underwent resting-state fMRI scans and the data were analyzed by the fALFF approach. The smokers showed significantly decreased fALFF in the left precuneus, right inferior temporal and occipital gyrus(ITG/IOG), while significantly increased fALFF in the right caudate. Subsequent correlation analysis revealed that the fALFF values of the left precuneus and right ITG/IOG were positively correlated with years of smoking across the smokers. This resting-state fMRI study suggests that the changed spontaneous neuronal activity, as reflected by the fALFF, in these regions may be implicated in the underlying the pathophysiology of smoking.
Similar content being viewed by others
Introduction
Cigarette smoking, one of the biggest threats to world health, is responsible for 6 million preventable deaths in 20111 and is estimated to cause 8.3 million deaths by the year 20302. Nicotine is primarily responsible for the highly addictive properties of cigarettes. Many functional magnetic resonance imaging (fMRI) studies have been performed to examine the effects of acute nicotine administration in smokers and non-smokers. A common finding from acute administration of nicotine/smoking is the globally reduced brain activity3. However, only a few studies4,5,6,7 reported alterations of regional spontaneous activity during resting state in chronic smokers. Resting-state functional magnetic resonance imaging (rs-fMRI) has recently been suggested as an important tool to explore the pathophysiological mechanisms underlying psychiatric and neurological diseases8.
The amplitude of low-frequency fluctuation (ALFF) of the blood-oxygenation level-dependent approach is effective and powerful for examining disease-related local brain activity in the resting state9. ALFF has been used in the studies of neuropsychiatric diseases, such as attention-deficit/hyperactivity disorder9, major depressive disorder10, Alzheimer’s disease11, and schizophrenia12. Although ALFF appears to be a promising method for detecting regional signal changes of spontaneous brain activity, certain cisternal areas have also shown significantly higher ALFF which is likely due to physiological noise. Thus, the fractional ALFF (fALFF) approach was developed to selectively suppress the noise in signals from nonspecific brain areas and thereby significantly improve the sensitivity and specificity of detecting spontaneous brain activity13. Using fALFF on 20 smokers and 19 nonsmokers, Chu et al.6 observed that fALFF was higher in the left middle occipital gyrus, left limbic lobe and left cerebellum posterior lobe but lower in the right middle frontal gyrus, right superior temporal gyrus, right extra nuclear, left postcentral gyrus and left cerebellum anterior lobe in smokers compared to nonsmokers. Recently, a fALFF study on 27 young adult smokers revealed that smokers had higher fALFF values in the right caudate7. The aim of the present study was to further investigate the fALFF of spontaneous brain dynamics in chronic smokers with a relatively large sample size.
Results
Demographic information
The results were from 55 smokers and 49 nonsmokers. Smokers and nonsmokers did not significantly differ in age (smokers mean = 39.4, SD = 6.9 years; nonsmokers mean = 37.3, SD = 8.0 years; t = 1.437, p = 0.154), gender (smokers: male/female = 55/0; nonsmokers: male/female = 49/0; p = 1.000) or handedness (smokers: right/left = 55/0; nonsmokers:49/0; p = 1.000), though there was a difference in years of education (smokers mean = 13.6, SD = 2.6 years; nonsmokers mean = 16.2, SD = 4.5 years; t = −3.566, p = 0.001). Therefore, years of education were included as a covariate in later analyses. Detailed demographic information and tobacco use parameters for smokers and non-smokers are summarized in Table 1.
fALFF results
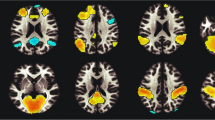
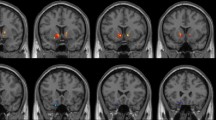
Using age, years of education as covariates, in comparison with nonsmokers, chronic smokers showed significantly decreased fALFF in the left precuneus, right inferior temporal (ITG) and occipital gyrus (IOG). In contrast, relative to nonsmokers, chronic smokers showed significantly increased fALFF in the right caudate (as shown in Fig. 1 and Table 2). No other difference in fALFF was observed between the two groups. Subsequent correlation analysis revealed that the fALFF values of the left precuneus (r = 0.287, p = 0.034; as shown in Fig. 2) and right ITG/IOG (r = 0.314, p = 0.019; as shown in Fig. 3) were positively correlated with smoking years across the smokers (as shown in Table 3). However, there were no correlations between the neuroimaging findings and the other cigarette smoking measures, including the cigarettes per day, FTND and age at start of smoking (as shown in Table 3).
Brain areas of fALFF difference between smokers and nonsmokers. Areas in blue colors are brain regions where fALFF was significantly decreased in smokers compared with nonsmokers. The areas with decreased fALFF were the left precuneus, right ITG and IOG. Areas in red colors show significantly increased fALFF in smokers compared with nonsmokers. The area showing increased fALFF was the right caudate.
Discussion
Many neuroimaging studies have sought to identify the effects of chronic cigarette smoking on structural and functional alterations in the brain. Structural MRI studies on smokers have found that chronic cigarette smoking is associated with gray matter deficits in the frontal, subcortical, parietal, occipital cortices, and the cerebellum14. Moreover, diffusion tensor imaging (DTI) studies have demonstrated altered fractional anisotropy in the prefrontal white matter, cingulum, corpus callosum in smokers14. Abnormal regional brain activity has been revealed in mesolimbic dopamine reward circuits, including the ventral tegmental area, nucleus accumbens, hippocampus, amygdala, cingulate, thalamus, striatum, and prefrontal cortex, as well as visuospatial attention circuits, including the dorsolateral and ventrolateral prefrontal cortex, parietal cortex, and extra-striate visual cortex in smokers15, 16. Furthermore, smokers also demonstrated changes in functional connectivity between the dorsal anterior cingulate cortex and ventral striatum17. Recently, several studies4,5,6,7 reported alterations of brain spontaneous activity during resting state in chronic smokers with a relatively small sample size. However, little is known about the chronic effects of cigarette smoking on the resting state spontaneous activity with a relatively large sample size, especially less was known about the association between the resting state abnormalities and cigarette smoking measures, which was very important to improve the understanding of the neural mechanism in smokers. In this study, compared with 49 healthy controls, 55 chronic smokers showed significantly decreased fALFF in the left precuneus, right ITG/IOG, which suggested that, during resting state, neural function was less active than nonsmokers in these brain regions. In contrast, smokers showed significantly increased fALFF in the right caudate, which suggested that, during resting state, neural function was more active than nonsmokers in this brain region. In addition, the fALFF values of left precuneus and right ITG/IOG were positively correlated with smoking years in smokers. Our findings provided insights into the effects of chronic cigarette use on spontaneous brain activity and the pathophysiological mechanisms of chronic cigarette smoking.
This study observed decreased fALFF for chronic smokers in the left precuneus, which was positively correlated with smoking years in smokers. A recent meta-analysis of fMRI studies of smoking cue reactivity found a reliable cue reactivity effect in the precuneus16. The precuneus is a part of the superior parietal lobule, which is involved in planning and executing functions18. A meta-analysis demonstrated that parietal lobule was found to be related to the reward-circuit19, suggesting that the parietal cortex could be involved in addiction behavior. The precuneus is also an important component of the default mode network (DMN) identified in previous resting-state fMRI studies20. The DMN is one of the several brain networks that show spontaneous, synchronous low frequency fluctuations at rest. The DMN is thought to be associated with stimulus-independent thought that is detached from the external environment, and implicated in a series of internal self-referential and reflective activity including personal introspection, autobiographical memories, and thoughts about the future21. Many previous studies demonstrated reduced activity in the regions within the DMN after acute administration of nicotine or nicotinic cholinergic agonists in externally cued cognitively demanding tasks22,23,24,25. In addition, the effects of chronic cigarette smoking on DMN function have been investigated in many studies. A recent resting-state fMRI (rs-fMRI) study by Wu et al. revealed significantly decreased spontaneous neural activity in the DMN, such as in the bilateral posterior cingulate cortex/precuneus, bilateral ventral and dorsal medial prefrontal cortex (MPFC), and bilateral angular gyrus4. However, another rs-fMRI study by Yu et al. showed that heavy chronic smokers exhibited increased spontaneous neural activity in the posterior cingulate cortex relative to healthy controls5. One possible causes of this difference that should be noted is the different smoking states of data acquisition. Acute nicotine exposure has been associated with decreased DMN activity in the precuneus, posterior cingulate, and medial orbitofrontal cortex26. Wu et al.4 acquired data on heavy smokers who were allowed to smoke at liberty before scanning, but Yu et al.5 did not specify the smoking state of smokers. In addition, using brain network analysis, Lin et al.27 found that heavy smokers had decreased nodal efficiency in brain regions within the DMN, and these participants could smoke at liberty before imaging. In the present study (55 smokers vs 49 nonsmokers), we acquired data on smokers at a state similar to that in Wu et al.’s study, and decreased spontaneous neural activity in the left precuneus was found in smokers relative to nonsmokers. Another possible cause of this difference might be the relatively small sample size in Yu et al.’s study5 (only 16 smokers and 16 non-smokers). By contrast, Wu et al.’s study4 included a relatively large sample size (31 heavy smokers and 33 nonsmokers). Taken together, these studies suggested that cigarette smoking affected spontaneous brain activity in the DMN, including the precuneus, which is associated with self-referential and emotional processing. Furthermore, smokers showed reduced fALFF in the right ITG/IOG, which was positively correlated with years smoking in smokers. The temporal lobe is the olfactory and auditory center, which are known to take part in resisting smoking craving, such as self-talk, which was one of several strategies employed to resist craving28. The occipital lobe was the visual center and was found to be related to attention processing and the visuospatial analysis of environment29, 30. Several functional brain imaging studies reported that occipital areas were activated following smoking or nicotine exposure during visual task performance31, 32. Activated occipital areas have been regarded as neural correlates of nicotine-induced attention increase32, 33.
Notably, subsequent correlation analysis revealed that the fALFF values of the left precuneus and right ITG/IOG were positively correlated with smoking years in smokers. As mentioned above, compared to nonsmokers, smokers showed decreased fALFF in the left precuneus and right ITG/IOG; therefore, we expected to observe more attenuation in the smokers with longer smoking history. However, individuals with shorter smoking history showed weaker fALFF in the left precuneus and right ITG/IOG, whereas those with longer smoking history showed stronger fALFF which was closer to the fALFF value of the nonsmokers. Similar resting-state functional connectivity (rsFC) study showed widespread rsFC attenuation in the reward circuit in smokers and the rsFC was positively correlated with dependence severity33. Moreover, microstructural studies found that the generally higher fractional anisotropy (FA) seen in smokers may reflect an increase in FA that follows the initiation of smoking, but prolonged smoking appears to reverse this effect with a progressive decline in FA with increasing pack-years of smoking34, 35. In our opinion, these features might represent neuroplastic changes that develop over time to support the development of neurophysiologic dependence.
In addition, smokers showed increased fALFF in the right caudate. A recent fALFF study also revealed higher fALFF values of the right caudate in young adult smokers. The caudate is a part of the dorsal striatum and a key region of the nigrostriatal dopamine (DA) circuits, which are critical for habit formation36 and play important roles in craving and reward processing in addiction37, 38. Position emission tomography (PET) studies detected that smoking induced dopamine release in the caudate in smokers, which was significantly correlated with craving ratings39,40,41. Experimental evidence also revealed that the caudate mediated nicotine seeking following smoking abstinence and craving provoked by smoking cues42, 43. In addition, a recent study reported that the caudate morphology was correlated with craving measures in smokers44. Taken together, these neuroimaging findings indicate the important role of the caudate in chronic smoking.
There are some limitations that should be mentioned. Education levels were not well matched in the two groups. However, education level was set as a covariate of no interest in the group analysis. In addition, in this study, we didn’t evaluate sex effects on outcome measures and the findings may be specific to male smokers, since only the male subjects were recruited in this study as most smokers are male in China.
In conclusion, in the present study smokers, compared to matched nonsmokers, showed significantly decreased fALFF in the left precuneus, right ITG and IOG, while increased fALFF in the right caudate. Furthermore, the fALFF values of the left precuneus and right ITG/IOG were positively correlated with smoking years across the smokers. Our findings provided new evidence on neuroimaging measures and smoking behaviors, which may improve our understanding of the neurobiological underpinning of chronic cigarette smoking. Longitudinal studies will be needed to further investigate the casual relationship between the neuroplasticity and addiction.
Materials and Methods
Participants
Fifty-five nicotine-dependent male smokers and 49 age-matched healthy male nonsmokers were recruited via advertisements. The inclusion criteria for smokers were as follows: (1) smoking ≥10 cigarettes per day for ≥2 years, (2) meeting DSM-IV criteria for nicotine dependence, (3) having an afternoon breath carbon monoxide (CO) level >10 ppm, (4) no current smokeless tobacco use, (5) being right-handed, (6) being 22–55 year-old. Additionally, demographic and smoking data were obtained from all participants by a questionnaire prior to scanning. Nicotine dependence levels of smokers were assessed with the FTND45. The FTND includes 6 items and produces a score from 0 to 10, with higher scores indicating more severe nicotine dependence. The nonsmokers were identified as those smoking no more than 20 cigarettes in their lifetime with expired CO ≤ 3 ppm. Participants with histories of major illnesses, other substance abuse (besides nicotine), psychotropic medication use, neurological and psychiatric diseases, and systemic diseases (i.e., diabetes or hypertension) were excluded. Additional exclusion criteria for all participants included MRI contraindications such as claustrophobia and metal implants. All the procedures were reviewed and approved by the Institutional Review Boards of the Second Affiliated Hospital of Zhejiang University School of Medicine, and all the procedures were carried out in accordance with the approved guidelines. All subjects provided signed informed consents prior to study participation.
Image acquisition
All the data were acquired using a 3.0 T GE Signa MR scanner equipped with a birdcage coil. Foam padding and earplugs were used to limit head movement and reduce scanner noise for the subject. During the data acquisition, the subjects were instructed to keep their eyes closed, but not to fall asleep, and to relax their minds and move as little as possible. The functional images were collected axially using an echo-planar imaging (EPI) sequence. The imaging parameters were as follows: repetition time = 2000 ms; echo time = 30 ms; slices = 30; thickness = 4 mm; gap = 1 mm; field of view (FOV) = 240 × 240 mm2; resolution = 64 × 64; and flip angle = 80°. The scan lasted for 370 s. Three-dimensional axial Fast Spoiled Gradient Recalled (3D-FSGPR) images were collected using the following parameters: TR/TE = 5056 ms/1.116 ms; Flip angle = 15°; FOV = 24 × 21.6 cm; matrix = 256 × 256; slices = 136; thickness = 1.2 mm; and space = 0 mm. After the scan, the subjects were asked whether they were stayed awake or not during the whole procedure. In the current study, smokers were allowed to smoke as usual prior to scanning to avoid withdrawal symptoms during scanning.
Fractional amplitude of low-frequency fluctuation (fALFF) analysis
The EPI data were preprocessed with the Dam Processing Assistant for Resting State fMRI (DPARSF) (http://www.restfmri.net/forum/DPARSF) that works with SPM8 (http://www.fil.ion.ucl.ac.uk/spm) on the Matlab 7.5 platform. The first ten volumes of the scanning sessions were removed to allow for the magnetization to reach a steady state and participants’ adaptation to the scanning environment. For each subject, the images were slice-timing corrected and realigned. None of the subjects’ head motion exceeded 1 mm of movement or 1 rotation in any direction. After realignment, all of the data were normalized to Montreal Neurological Institute space, resampled with 3 mm × 3 mm × 3 mm resolution and smoothed with a Gaussian kernel of 6 mm full width.
After the above preprocessing, fALFF images were computed in a similar way to the REST software (http://restingfmri.sourceforge.net) as described previously17, 18. First, the time series of each voxel was transformed with a Fast Fourier Transform (FFT) to obtain the power spectrum. Then, the square root was calculated at each frequency of the power spectrum, and the averaged square root was acquired as ALFF across 0.01–0.08 Hz at each voxel. Finally, fALFF was calculated using the ratio of power spectrum of low-frequency (0.01 Hz to 0.08 Hz) to that of the entire frequency range.
The statistical analysis of fALFF was performed using Statistical Parametric Mapping 8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm). To investigate the fALFF differences between smokers and nonsmokers, a two-sample t test was performed on the individually normalized fALFF maps in a voxel by voxel manner with age and years of education as covariates of no interest. Multiple comparisons were corrected at a threshold of alpha <0.05 determined by the newest version of AFNI 3dClustSim Program. The following parameters were used: single voxel p = 0.005, cluster size = 27 voxels (729 mm3), FWHM = 6 mm, cluster connection radius r = 5 mm. Subsequently, we performed a post-hoc correlation analysis in order to assess whether smoking measures were related to the fALFF properties or not. A Pearson’s correlation analysis was performed to assess the relationship between neuroimaging findings (the fALFF values in regions showing differences between smokers and nonsmokers) and cigarette smoking measures (i.e., smoking years, cigarettes per day, FTND, age at start of smoking).
References
Asma, S. et al. CDC Grand Rounds: global tobacco control. MMWR Morb Mortal Wkly Rep 63, 277–80 (2014).
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3, e442 (2006).
Brody, A. L. Functional brain imaging of tobacco use and dependence. J Psychiatr Res 40, 404–18 (2006).
Wu, G., Yang, S., Zhu, L. & Lin, F. Altered spontaneous brain activity in heavy smokers revealed by regional homogeneity. Psychopharmacology (Berl) 232, 2481–9 (2015).
Yu, R. et al. Regional homogeneity changes in heavy male smokers: a resting-state functional magnetic resonance imaging study. Addict Biol 18, 729–31 (2013).
Chu, S. et al. Spontaneous brain activity in chronic smokers revealed by fractional amplitude of low frequency fluctuation analysis: a resting state functional magnetic resonance imaging study. Chin Med J (Engl) 127, 1504–9 (2014).
Feng, D. et al. Intra-regional and inter-regional abnormalities and cognitive control deficits in young adult smokers. Brain Imaging Behav 10, 506–16 (2016).
Barkhof, F., Haller, S. & Rombouts, S. A. Resting-state functional MR imaging: a new window to the brain. Radiology 272, 29–49 (2014).
Zang, Y. F. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29, 83–91 (2007).
Liu, F. et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 146, 401–6 (2013).
He, Y. et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage 35, 488–500 (2007).
Guo, W. et al. Dissociation of functional and anatomical brain abnormalities in unaffected siblings of schizophrenia patients. Clin Neurophysiol 126, 927–32 (2015).
Zou, Q. H. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172, 137–41 (2008).
Wang, C., Xu, X., Qian, W., Shen, Z. & Zhang, M. Altered human brain anatomy in chronic smokers: a review of magnetic resonance imaging studies. Neurol Sci 36, 497–504 (2015).
Franklin, T. R. et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology 32, 2301–9 (2007).
Engelmann, J. M. et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage 60, 252–62 (2012).
Hong, L. E. et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66, 431–41 (2009).
Johnson-Frey, S. H. The neural bases of complex tool use in humans. Trends Cogn Sci 8, 71–8 (2004).
Liu, X., Hairston, J., Schrier, M. & Fan, J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35, 1219–36 (2011).
Lerman, C. et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71, 523–30 (2014).
Zhang, D. & Raichle, M. E. Disease and the brain’s dark energy. Nat Rev Neurol 6, 15–28 (2010).
Sutherland, M. T. et al. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry 78, 711–20 (2015).
Bentley, P., Husain, M. & Dolan, R. J. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron 41, 969–82 (2004).
Hahn, B. et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27, 3477–89 (2007).
Thiel, C. M. & Fink, G. R. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience 152, 381–90 (2008).
Tanabe, J. et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl) 216, 287–95 (2011).
Lin, F., Wu, G., Zhu, L. & Lei, H. Altered brain functional networks in heavy smokers. Addict Biol 20, 809–19 (2015).
Hartwell, K. J. et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol 16, 654–66 (2011).
Corbetta, M. et al. A common network of functional areas for attention and eye movements. Neuron 21, 761–73 (1998).
Vanni, S., Tanskanen, T., Seppa, M., Uutela, K. & Hari, R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci USA 98, 2776–80 (2001).
Ghatan, P. H. et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 136, 179–89 (1998).
Lawrence, N. S., Ross, T. J. & Stein, E. A. Cognitive mechanisms of nicotine on visual attention. Neuron 36, 539–48 (2002).
Shen, Z. et al. Severity of dependence modulates smokers’ functional connectivity in the reward circuit: a preliminary study. Psychopharmacology (Berl) 233, 2129–37 (2016).
Hudkins, M., O’Neill, J., Tobias, M. C., Bartzokis, G. & London, E. D. Cigarette smoking and white matter microstructure. Psychopharmacology (Berl) 221, 285–95 (2012).
Jacobsen, L. K. et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci 27, 13491–8 (2007).
Volkow, N. D., Fowler, J. S., Wang, G. J. & Swanson, J. M. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9, 557–69 (2004).
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C. & Fiez, J. A. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84, 3072–7 (2000).
Volkow, N. D. et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26, 6583–8 (2006).
Barrett, S. P., Boileau, I., Okker, J., Pihl, R. O. & Dagher, A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 54, 65–71 (2004).
Brody, A. L. et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatry 161, 1211–8 (2004).
Brody, A. L. et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 63, 808–16 (2006).
McClernon, F. J., Kozink, R. V., Lutz, A. M. & Rose, J. E. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 204, 25–35 (2009).
Sweitzer, M. M. et al. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol Psychiatry 76, 681–8 (2014).
Janes, A. C., Park, M. T., Farmer, S. & Chakravarty, M. M. Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology 40, 406–11 (2015).
Fagerstrom, K. O. & Schneider, N. G. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12, 159–82 (1989).
Acknowledgements
This work was supported by the Natural Science Foundation of China Surface Project (81171310) and Medical and Health Scientific Research Fund Project of Zhejiang Province (2017KY080). Y.Y. was supported by the Intramural Research Program of the National Institute on Drug Abuse, the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
C.W., Y.Y. and M.Z. were responsible for the study concept and design. C.W., Z.S., W.Q. and H.Y. contributed to the acquisition of imaging data. P.H., L.X., X.G. and Q.G. assisted with data analysis and interpretation of findings. C.W. drafted the manuscript. Y.Y. and M.Z. provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, C., Shen, Z., Huang, P. et al. Altered spontaneous brain activity in chronic smokers revealed by fractional ramplitude of low-frequency fluctuation analysis: a preliminary study. Sci Rep 7, 328 (2017). https://doi.org/10.1038/s41598-017-00463-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00463-7
This article is cited by
-
Reciprocal modulation between cigarette smoking and internet gaming disorder on participation coefficient within functional brain networks
Brain Imaging and Behavior (2022)
-
Altered patterns of fractional amplitude of low-frequency fluctuation and regional homogeneity in abstinent methamphetamine-dependent users
Scientific Reports (2021)
-
Increased interregional functional connectivity of anterior insula is associated with improved smoking cessation outcome
Brain Imaging and Behavior (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.