Abstract
Chemicals in the aquatic environment can be harmful to organisms and ecosystems. Knowledge on effect concentrations as well as on mechanisms and modes of interaction with biological molecules and signaling pathways is necessary to perform chemical risk assessment and identify toxic compounds. To this end, we developed criteria and a pipeline for harvesting and summarizing effect concentrations from the US ECOTOX database for the three aquatic species groups algae, crustaceans, and fish and researched the modes of action of more than 3,300 environmentally relevant chemicals in literature and databases. We provide a curated dataset ready to be used for risk assessment based on monitoring data and the first comprehensive collection and categorization of modes of action of environmental chemicals. Authorities, regulators, and scientists can use this data for the grouping of chemicals, the establishment of meaningful assessment groups, and the development of in vitro and in silico approaches for chemical testing and assessment.
Similar content being viewed by others
Background & Summary
The intended and unintended release of micropollutants to the environment, the exposure of humans and ecosystems to those chemicals and the related potential of causing serious problems for the ecosystem and human health at various scales, has been defined as one of nine planetary boundaries1. More than 350,000 chemicals and mixtures thereof are in commerce2, and many of them are known or expected to end up in aquatic environments. Targeted screening analysis of surface waters and wastewater treatment plant effluents revealed the occurrence of up to 2000 chemicals3,4,5 in the aquatic environment. Some persistent substances, including the so-called “forever” perfluorinated compounds”, even end up far remote from production and use6, e.g., in high mountain7 or arctic8 areas.
Data on environmental occurrence in terms of measured environmental concentrations as well as on biological effects in terms of effect concentrations are sparse, scattered and still seldom interoperable. Nevertheless, some databases provide such information; for example, the NORMAN network collects chemical concentrations measured in European monitoring campaigns9, and the ECOTOXicology Knowledgebase (ECOTOX) developed by the US Environmental Protection Agency (US EPA) provides toxicity data for environmental species10. Such data is required for hazard and environmental risk assessment.
In the ideal case, experimental toxicity data is available for all chemicals and species present in the (aquatic) environment. In reality, scarcity of the relevant data for many chemicals often limits the risk prediction power. To fill gaps in the (eco)toxicity data, quantitative structure-activity relationship (QSAR) models are utilized. Lacking experimental data, these models estimate a chemical’s bioactivity or toxicity based on its structure11. While classical QSAR models are typically based on linear regression models to predict chronic or acute toxicity of chemicals from the calculated physico-chemical properties12,13, many of the modern QSARs apply machine-learning approaches to estimate effect concentrations based on molecular descriptors14,15,16. Furthermore, structure-based classification schemes exist to categorize chemicals based on their mode of toxic action (MoA). However, available tools to predict such MoA, for instance, the Verhaar scheme17, the EPA ASTER QSAR application18, and the EPA MOAtox database19, are limited in their ability to provide consistent predicted MoAs for a wide range of chemicals20.
Due to the current limitations in data availability and in the predictability of potential long-term effects with short-term acute toxicity testing, as well as the time lag and costs associated with chronic toxicity testing using animals, risk assessment is about to change towards evidence-based and integrated assessments considering mechanistic knowledge and evidence on a chemical’s mechanism of toxic action derived from in vitro bioassays or read across approaches21,22,23. The adverse outcome pathway (AOP) concept, introduced by Ankley et al.24 and implemented by the OECD25 helps to organize such knowledge from any kind of chemical-biological interaction studies and to inform such evidence-based approaches. Established AOPs provide mechanistic information, e.g., from a chemical binding to a biological receptor molecule exerting physiological events to the development of an adverse outcome. With this, AOPs contain information on a chemical’s MoA and offer cross-species considerations as well as read across options26. Using mechanistic information for grouping of chemicals according to their, e.g., similar biological MoA, has been proposed for the performance of risk assessment by EFSA27 and ECHA28. Thereby, EFSA defines a biologically plausible sequence of events in an organism leading to an observed effect as MoA. It refers to the major steps leading to an adverse health effect following interaction of the chemical with biological targets, but does not imply full understanding of the mechanism of action at the molecular level29.
In 2016, Busch et al.3 researched mechanisms of action of and knowledge on MoAs for more than 400 environmentally relevant chemicals and showed that diverse pesticides, pharmaceuticals, and industrial chemicals can have the same or similar MoAs, for example, acting on the nervous or endocrine system of vertebrates. This is of relevance, especially for non-target organisms that are unintended co-exposed to mixtures of such chemicals in the aquatic environment. Chemical regulation and authorization, however, does, in most cases, not consider co-exposures and is separated into regulatory silos according to the intended chemical use domain. Recent efforts discuss the implementation of a mixture assessment factor30 or the development of cumulative assessment groups of chemicals27 to cope with this issue. MoAs or mechanistic information are, however, not considered yet within these recent environmental mixture risk assessment considerations.
Here, we aimed to extend the previous list of 426 environmentally relevant chemicals to a comprehensive list of 3,387 compounds and provide information on their MoAs as well as on their toxicity to support environmental risk assessment. Therefore, we undertook the effort to research, merge, curate, and provide data on biological MoAs, as well as curated effect concentrations for each compound for three biological quality elements (BQE) of the European Water Framework Directive, namely algae, crustaceans, and fish (https://www.eea.europa.eu/themes/water/european-waters/water-quality-and-water-assessment/water-assessments/quality-elements-of-water-bodies). The data should be provided in a FAIR format to enable standardization, comparability, reproducibility, and acceleration for ecotoxicological hazard and risk assessment31.
Based on information from databases, lists of regulatory directives, monitoring projects, and respective chemical suspect lists, we compiled a curated list of 3,387 compounds of freshwater environmental occurrences e.g.32,33,34,35,36. We researched all chemicals on this list for their use and product group, their biological MoAs, and compiled ecotoxicity data ready to be used in environmental risk assessment based on data from the US EPA ECOTOXicology Knowledgebase10 (ECOTOXDB) and QSAR predictions. Out of all 3,387 compounds, 2,890 were identified as parent substances, 374 as transformation products (TP), and 96 as both, parent and TP. Only in a few cases (27 chemicals) of mainly industrial chemicals (e.g., 3-dodecylbenzene-1-sulfonic acid.), such a classification could only be assumed, and was, therefore, not assigned (Tables A, B, and C, available on Zenodo)37. Further details on the curated use group classifications, the MoA categories, and the toxicity data for all compounds are explained and summarized below.
Methods
Use groups, mode of action and accompanying information
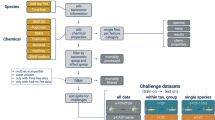
Overall strategy
For each compound in the dataset, a systematic search in different data sources and databases was performed. All information was obtained from the following databases listed in Table 1.
We did not use specific search terms within those databases but collected all information on MoA and use that was available for the respective compound. For compounds with uncertain or limited information available, results of a literature research were included to fill as many remaining gaps as possible. There, we searched the web of Science and PubMed databases and used the compound name in combination with “toxicity” or “mode of action”. Results of these searches were not systematically stored, but relevant information transferred to our collection. Data were retrieved between January 2021 and April 2023. All collected data were sorted and categorized at higher levels: based on the results, each compound was assigned as a parent or transformation product; assigned to a specific use group; and if possible, to a mode of action and target or non-target taxa, as pointed out in detail below. Overall, we followed a step-wise approach where all information for a respective compound was collected first, and sorted and annotated according to our systematic and standardized broad categories of use groups and MoAs in a second step. Finally, we curated all further information and developed standardized terms for detailed use information as well as for specific MoA information, although this was challenging and some chemicals remained with individual descriptions (Tables A and C, available on Zenodo)37.
Use group classification
Based on the collected information, all compounds were assigned either as parent compound or as transformation product (TP) in column “parent_or_TP” (Tables A and C, available on Zenodo)37. Respective parent compound(s) are listed for all TPs in column “TP_of”. In the context of this dataset, TPs comprise both, metabolites and environmental degradation products. Some chemicals are both, they occur as TP of another compound, but are also parent compounds in another context. These chemicals were labeled as ‘parent + TP’. In our statistical analyses, we counted them as parent substances. In the next step, the compounds were grouped according to their use into eight categories: (1) Industrial Chemical, (2) Pesticide, (3) Biocide, (4) Pharmaceutical, (5) Drug of abuse, (6) Natural, (7) Food additive, and (8) Metal (column”use_group”). The groups “drug of abuse” and “biocide” are listed as separate use groups in the dataset but were included into the use group categories of “Pharmaceutical/Drug of abuse” and “Pesticide/Biocide”, respectively, in the analyses and figures. As “drugs of abuse” we defined psychoactive drugs that are not used as pharmaceuticals such as, e.g., Nicotine, or 3,4-Methylenedioxyamphetamine (MDA). Multiple use groups can be assigned to a compound depending on its range of applications.
The category of pharmaceuticals and drugs is the most common in the dataset with 1,162 compounds and additional 139 TPs, followed by pesticides and biocides with 696 compounds and 204 TPs, industrial chemicals with 726 and 19 TPs, naturally occurring compounds with 93 and 4 TPs, metals with 19 compounds and food additives with 11 compounds (Fig. 1). 279 compounds (8 TPs) were assigned to more than one use group and are summarized as multiple use compounds in the graphs. These include food additives which predominantly have an additional application, e.g., in the industrial sector as fragrance. Moreover, many naturally occurring compounds such as alkaloids or hormones are frequently applied as pesticides, pharmaceuticals or in industrial applications.
Detailed information on the application and usage of all compounds in each use group category were collected and added to the table in additional columns (“use_group_details” and “additional_use_info”). Pesticides were further categorized systematically into insecticides (I), acaricides (A), herbicides (H), fungicides (F), and other specific use groups, e.g., molluscicides and nematicides. Detailed application areas of pharmaceuticals were included and annotated according to their area of action, ranging from antibiotics over contraceptives to psychotropic or radioactive agents, just to mention a few examples. This listing contains more than 100 terms and was curated based on the retrieved information, but does not necessarily consider medical term standards. Moreover, specific applications of industrial chemicals are listed and respective terms were taken from the collected information or curated by us. If chemicals belong to different use groups, this is indicated in the “use_group” column where all broad application domains are listed, separated with “,”. In the “use_group_details” applications in different sectors, e.g., as industrial chemical and insecticide, are listed with “ + ” while multiple uses within a sector are separated with “/”. This listing is far from being comprehensive but covers the most common use domains for the investigated chemicals, whereas “most common” was a subjective decision by the curator based on the retrieved information. All abbreviations used in the mentioned columns of Table C are explained next to the column header explanations in Table A (available on Zenodo)37. The “additional_use_info” column contains additional non-systematic and non-standardized information on the use of the chemical.
Most industrial chemicals are used for multiple applications, e.g., as colorants, fragrances, plasticizers, additives, flame retardants, solvents, reagents, or intermediates in the manufacture of other products. Furthermore, 105 compounds within the industrial chemicals group have an application as personal care (PC) products such as stabilizer, surfactant or UV filter in sunscreen products e.g., benzophenone-3. Compounds that are found in diesel exhaust, cigarette smoke or which are generally released in combustion processes of natural compounds are summarized as combustion products (59 compounds) within the group of industrial chemicals. Contrary to the multiple applications of industrial chemicals, pesticides are more specific in their use. Within the group of pesticides, 126 (40 TPs) compounds are assigned as pure insecticides, and 99 compounds (10 TPs) as both, insecticide and acaricide. The dataset includes 251 (83 TPs) herbicides, 124 (41 TPs) fungicides, and 73 compounds (26 TPs) with multiple applications or a specific use, e.g., as nematicide, molluscicide, avicide, or as herbicide safener. 80 compounds are considered obsolete, and 49 pesticides are also used as veterinary substances. Pure biocides are rare because most compounds with biocidal action also serve as industrial chemicals, or have an application in the agricultural sector as pesticide. The group of pharmaceuticals can be further distinguished into various detailed use groups, with psychotropic drugs, antibiotics, antihypertensive, antihistamine, and anti-inflammatory drugs being the most prominent. There is a large overlap between the use groups of pharmaceuticals and drugs of abuse, e.g., for doping agents used in sports. Otherwise, the use group of drugs of abuse contains mostly cannabinoids and stimulating, psychedelic or hallucinogenic substances. Finally, even with additional literature research efforts, 27 compounds could not be assigned to a specific application sector and remain with an unknown use group. Consequently, these are the ones for which an assignment to parent and TP was not possible.
Mode of action
The amount of information available on MoAs varied significantly between use groups and chemicals. MoA information covers molecular targets (e.g., action on a specific receptor), a pathway (e.g., photosynthesis inhibition), and/or general information about a disturbance (e.g., endocrine disruption).
Based on the collected data and information and on our previous work3,4, we aimed to provide systematic and harmonized broad MoA categories but also additional and more specific information about the biological actions of the chemicals. Therefore, we started to note down whether there are target and/or known non-target species for the respective chemicals, their biological molecular targets and/or specific ways of action as well as additional MoA information. We sorted the retrieved information, which was available for more than half of the compounds, and established harmonized terms for specific MoAs and the broad MoA categories systematically. We grouped chemicals that act on the same target or the same biological signaling pathway into a common “MoA_specific” also considering terms established by the HRAC, IRAC and FRAC databases. Different specific MoAs that could be assigned to a broader biological system, such as, e.g., the nervous system or the endocrine system, were then grouped into common “MoA_broad” categories (Table C, columns “MoA_broad” and “MoA_specific”, available on Zenodo)37. Abbreviations for biological molecules used within these terms, such as, e.g., AChE for acetylcholine esterase, are explained in the following columns that contain information on molecular targets and further MoA details. The contents of these additional columns were only partly harmonized (Table C, columns “molecular_target” and “further_MoA_info”, available on Zenodo)37. Finally, many compounds act on multiple molecular targets and can be assigned to more than one specific or broad MoA group. In these cases, all MoAs are listed with “,” in the respective columns.
MoA information could be retrieved for 2,172 compounds (64%) for at least one of the three levels (broad, specific, or molecular target) (Table S3). Of those, 1,975 were assigned to one of the 32 broad MoA categories, 174 to two or more categories and 1,238 (including 337 TPs) could not be assigned to a broad MoA category. Considering compounds with one broad MoA, chemicals with a neuroactive mode of action build the most prominent group (579 compounds), followed by compounds acting on neuromuscular (200), endocrine (154), and cardiovascular systems (136) as exemplarily shown in Fig. 2A. While, for example, the MoA category ‘Antibiotics’ and ‘Synthetic auxins’ contain chemicals of only one-use group, pharmaceuticals and pesticides, respectively, most MoA categories contain chemicals of different use groups. 15 broad MoA categories could be discriminated within the group of industrial chemicals, led by the categories ‘Nucleic acid damage’ and ‘Endocrine’ with 55 and 38 chemicals, respectively. 25 broad MoA categories were identified among the pharmaceuticals/drugs, with ‘Neuroactive’ (458) and ‘Cardiovascular system’ (150) being those with the most assigned chemicals. 23 MoA broad categories were identified for the pesticides/biocides, with the largest number of chemicals known to act on the ‘Neuromuscular system’ (124) and as ‘Neuroactive’ compounds (85). Only for 10% of all TPs, a broad MoA category could be assigned (Fig. 2B). Although a transformation product is sometimes the actual acting chemical, MoA information is in most cases described for the parent compound. Therefore, details on the biological action of TPs are widely lacking. We did assign the MoA of a parent compound also to its TP only in cases, where the respective action of the TP was clearly indicated in a respective information sources, which is also given Table C (column “source”, available on Zenodo)37.
(A) Curated broad mode of action (MoA) categories with the number of assigned compounds in the dataset; colors indicate the different use group categories, chemicals with multiple or unknown MoA broad are not considered in this figure; (B) Number of chemicals distributed across the ten most common broad MoA categories per use group in our dataset, among the parent compounds (left), and the transformation products (right).
Although most chemicals could be assigned to one broad MoA category, more specific considerations showed that they still can act on multiple molecular targets in the same or across species. Up to eight molecular targets or specific MoAs were found per compound. There are considerable differences between the numbers of specific MoAs or molecular targets within one broad MoA category. ‘Endocrine’ acting chemicals were found across all use groups (including industrial chemicals). This broad MoA category contains 48 specific MoAs, with action on the glucocorticoid, androgen, and estrogen receptors being the most abundant ones (Fig. 3A). The broad MoA category ‘Endocrine’ accounts for all organisms which includes action on the hormone system of animals or humans, e.g., androgen or estrogen receptors, as well as the plant hormone syntheses, e.g., the inhibition of the gibberellin biosynthesis. This indicates that the broad MoA categories help to summarize the biological actions exerted by chemicals in the environment, but for detailed analysis or species-specific assessments the specific MoAs need to be considered. Indeed, single MoA broad categories are overlapping, e.g., the category ‘Neuromuscular system’ is related to the category of ‘Neuroactive’ compounds. In Fig. 3B it is shown that these two categories alone comprise more than 100 specific MoAs. Again, it becomes obvious that some seem to be use group-specific (e.g., serotonin receptor antagonists are all pharmaceuticals) while other specific MoAs contain chemicals of different use groups (e.g., the specific MoA with the largest number of chemicals in this category: ‘Acetylcholinesterase inhibition’).
Number of chemicals assigned to specific MoAs within two broad MoA categories in the dataset (A) ‘Endocrine’ and (B) ‘Neuroactive’ and ‘Neuromuscular system’ colored according to use groups. MoA specific categories with less than two chemicals in (A) and nine chemicals in (B) are, respectively, not shown.
Finally, multiple pharmaceuticals, e.g., cortisol, serve as pro-drugs and are only active at a specific target when transformed to their active metabolites. Even though the prodrug itself is inactive, we decided to include the MoA of the respective active metabolite in the dataset. Furthermore, a unique assignment of a molecular target to a single broad MoA category is often challenging. For instance, cannabinoids act on the endocannabinoid system and are declared as neuroactive in the dataset, but effects on the immune system have also been proposed for the cannabinoid receptor 2. Therefore, the classification of broad MoA categories serves as a guidance and can be further discussed and developed in the future.
Target/non-target taxa and additional information
Data about action on target species (e.g., insects in the case of insecticides) and non-target species (e.g., other aquatic species or humans) were included into the dataset in case such information occurred during the curation. However, we did not systematically search for non-target effects and information of all compounds. A target taxon could be assigned for pesticides, e.g., insects, plants, fungi, or nematodes as these chemicals are designed, respectively. Moreover, for most pharmaceuticals and drugs of abuse, humans can be declared as target species, while in the case of veterinary substances, the target group can be extended to mammals or even vertebrates. The target taxa of antibiotics, antiviral and antifungal pharmaceuticals were specified as bacteria, viruses and fungi in humans, respectively. No target species was determined for natural compounds, food additives, metals or industrial chemicals, with a few exceptions, such as disinfectants or antifungals with target taxa of bacteria or fungi.
While MoAs of pesticides and pharmaceuticals are intentionally designed for specific target taxa, their toxicological MoA on vertebrates as non-target species might be entirely different. For most of the chemicals, little information about the mechanisms of action in non-target species was identified. However, in cases where such information was found during our research, alerts for non-target species were included into the dataset. Risks of pesticides for non-target species were retrieved from the PPDB38 database, risks of potential carcinogenic compounds for humans were retrieved from the International Agency for Research on Cancer (IARC)39 of the World Health Organization, and information on endocrine disruptors were retrieved from the endocrine disruptor lists of ECHA35. Therefore, Table C also contains the columns “nontarget_taxa” and “nontarget_taxa_alert” available on Zenodo37.
Chemical data
Data compilation and retrieval
The list of chemicals was compiled based on existing collections of known environmental contaminants and amended by chemical information retrieved from US EPA Chemical Dashboard40 and PubChem41. The data were linked by unequivocal identifiers (i.e. InChiKey, PubChem CID, and DTXSID).
Curation of chemical structures
To improve the quality of the chemical structures (i.e., SMILES) and QSAR modeling, curation of all structures was applied. It has been proven that deploying the pure and neutralized chemical structures enhances the quality of machine learning based QSAR predictions15,42. The curation included, for example, the canonicalization of the SMILES code, the desalting, de-aromatization, removal of stereochemistry, solving hyper valency, and other transformations to generate QSAR-ready SMILES described elsewhere utilizing OPERA 2.715.
Prediction of log Sw values
The water solubility at 25 °C of all included was predicted applying OPERA 2.7 based on QSAR-ready SMILES42. OPERA exports the values in csv format. The unit of log Sw is mol/L. The Sw in mg/L was calculated using the average molecular mass in g/mol. The solubility was needed to estimate the solubility domain class of the ecotoxicity data described below.
Ecotoxicity data
US EPA ECOTOXicology Knowledgebase data
Environmental risk assessment requires exposure as well as effect concentrations and is often challenged by the variety of experimentally determined effect concentrations that differ between species, endpoints, and experimental settings. In this study, we aimed to provide one value per compound and BQE (algae, crustaceans, and fish according to the WFD), derived from all available data in the ECOTOXDB10 using carefully selected and developed criteria that ensure that comparable data is selected and merged, and a robust and representative effect concentration for each respective species group is derived. The whole procedure of data retrieval and processing was performed using the R package REcoTox43 version 0.4.1 (https://github.com/tsufz/REcoTox/tree/0.4.1). The software and workflow is described briefly below and in detail in the vignette document44.
Data retrieval, processing, and curation
The ASCII file version ecotox_ascii_03_10_2022.zip of the ECOTOXDB10 was downloaded from US EPA website (https://cfpub.epa.gov/ecotox). The database files were unzipped and the tables tests, results, species, chemicals, and references were selected for further processing. Data from the selected tables were joined and further filtered by the IDs test_id, cas_number, species_number, and reference_number, and under consideration of the following criteria:
-
type of dosing (only water-based dosing was considered, e.g., mg/L); dosing_group = “water_concentration“
-
time unit of endpoint (e.g., d for days, h for hours); duration_d = c(“d“, “dph“, “dpf“); duration_h = c(“h“, “ht“, “hph“, “hpf“, “hv“)
-
duration range of the exposure (e.g., minimum and maximum hours); duration_m = mi; min_d = 0; min_h = 0; min_m = 0; max_d = 5; max_h = 120; max_m = 7200
-
species (e.g., species group (ecotoxgroup) such as algae, habitat, subsets of species like standard species); ecotoxgroup = c(“Algae“, “Crustacean“, “Fish“); habitat = “Water“; species_selection = “all“ for “Algae“; species_selection = “standard_test_species“ for “Crustacean“ and “Fish“
-
effects and measurements; effects = c(“MOR“, “GRO“, “POP“, “REP“, “MPH“, “DEV“) for “Algae“ and “Fish“; effects = c(“MOR“, “GRO“, “POP“, “REP“, “MPH“, “DEV“, “ITX“) for “Crustacean“
The definitions of the ECOTOXDB terms are detailed elsewhere (ECOTOX Help. https://cfpub.epa.gov/ecotox/help.cfm?sub=term-appendix).
According to Busch et al.3, the selected endpoints were cover the whole distribution of the concentration-effect-relationships. This includes all effect or lethal levels recorded in the ECOTOXDB namely: effect concentrations - EC1–99, effective doses - ED1–99, effect levels - EL1–99, inductive concentrations - IC1–99, lethal concentrations - LC1–99, lethal doses - LD1–99, lethal levels - LL1–99, lethal thresholds - LT1–99, lowest observed effect concentrations - LOEC, and lowest observed lethal concentrations - LETC.
Afterwards, the different dosing units were standardized to mg/L. In cases where mol/L are used in the database, the table ecotoxgroup_mol_weight.csv was applied to recalculate concentrations to mg/L.
In this study, the percentile value was set to 0.05 (quantile = 0.05) to calculate the 5th percentile of the included effect data. Additionally, the average, the geometric average, the median, the minimum, and the maximum effect concentrations were calculated and rounded to four significant digits. The estimated values can exceed the solubility of the single compounds. In older studies, often the nominal concentration was reported without measurement of the real concentrations in the bioassays. Some reported values might be thus overestimated. To mitigate the risk of false positive results, the so-called solubility domain was calculated according to the ideas realized in ChemProp45 (https://www.ufz.de/index.php?en=34593).
The solubility domain has three classes:
Where ECx is the respective ecotoxicity value and S is the solubility value in mg/L. The basis of log (S) is 10. The classes of the solubility domain were calculated for each of the aggregated values (e.g., geometrical mean) and amended to the results table. Furthermore, all raw ecotoxicity values, endpoints, effects, measurements, species, durations, and reference IDs are collapsed in one single field per category for later review.
Prediction of effect concentrations
Measured effect concentrations were successfully derived and curated for a maximum of 25% of all compounds. Therefore, we additionally predicted effect concentrations for all chemicals for which the respective QSAR models15,16,42 were applicable. It was not possible to predict values for inorganic and metal-organic compounds, and complexes because existing QSAR models do not handle such structures. In addition, mixtures were excluded. The VEGA QSAR models16 (https://www.vegahub.eu/portfolio-item/vega-qsar) were applied to QSAR-ready SMILES, including these QSAR models:
-
Algae acute [EC50] toxicity model (IRFMN) v. 1.0.1
-
Daphnia magna acute [EC50] toxicity model (IRFMN) v. 1.0.1
-
Fish acute [EC50] toxicity model (IRFM) v. 1.0.1
The IRFMN models report the toxicity values in -log(mmol/L). In addition, an a-dimensional predicted value is reported. Since the employed version of the models contained a software bug related to molecular weight estimation, the a-dimensional value was used to calculate correct predicted values (email correspondence with IRFMN). The correction requires a box-cox transformation of the a-dimensional values of the algae and fish models utilizing equations
Summary of ecotoxicity dataset
The distributions of measured (retrieved from the ECOTOXDB) and QSAR-predicted effect concentrations for the biological quality elements (BQE)46,47 algae, crustaceans, and fish are shown in Fig. 4 and Table 2. The measured values, which were considered in this dataset, cover 586 (17%), 858 (25%), and 855 (25%) out of the 3,387 chemicals included here, respectively, for algae, crustacean, and fish. The experimental data is based on 6,156, 9,760, and 19,416 data points with a mean of 5 ± 3, 4 ± 3, and 7 ± 4 data points per chemical, respectively, for algae, crustacean, and fish (Table 3).
Distributions of measured and predicted effect concentrations for the three BQE (algae, crustacean, and fish) according to the data availability listed in Table 1.
While the applied QSARs predict to which extent the processed chemical structure covers the application domains of the models, the quality and accuracy of the experimental data is difficult to discover. The QSAR derived application domain classes assigned to each chemical included in this dataset are listed in the respective Tables D-F (available on Zenodo)37.
Data compilation
The final tables were processed using an R script. The different tables are linked by an internal identifier (ID). The dataset includes mixtures or compounds without InChiKeys, PubChem CIDs and/or DTXSIDs, and thus no other unequivocal identifier was applicable.
Data Records
All data is available on ZENODO37 (https://doi.org/10.5281/zenodo.10071824). The ZENODO repository contains the following six tables in csv format and one table in xlsx format, containing all six csv files. The first table contains explanations for all columns of the dataset:
Table A_column_descriptions_for_Tables B to F.csv
This table contains all column headers of the following tables B to F and respective explanations and descriptions.
Table B_chemical_information.csv
Table B contains chemical names and common identifiers, such as CAS numbers, PubChem IDs, DTXSIDs, InCHI and Smiles codes for all 3387 chemicals considered in this study.
Table_C_use_groups_and_mode_of_action_information_for_chemicals.csv
Table C contains the collected and curated information on the usage domains of all chemicals and their biological modes of action. The curation process and all data sources are described in the methods part.
Table_D_ecotoxicity_data_algae.csv
Table D contains measured and predicted acute toxicity data for algae species curated and derived for all chemicals as described in detail in the methods part.
Table_E_ecotoxicity_data_algae.csv
Table E contains measured and predicted acute toxicity data for the species group of crustaceans curated and derived for all chemicals as described in detail in the methods part.
Table_F_ecotoxicity_data_fish.csv
Table F contains measured and predicted acute toxicity data for fish species curated and derived for all chemicals as described in detail in the methods part.
Table_X_Tables_A_to_F_chemicals_MoAs_ecotoxdata.xlsx
Table X is provided as additional service and contains all csv tables in an Excel spreadsheet.
Technical Validation
Toxicity data and chemical solubility
Using and curating experimental data is always biased by the availability of the data itself. There are different reasons for variations in the availability of experimental data for different chemicals: i) not all chemicals have been tested in bioassays, ii) some chemicals were only tested in a single study, in a single bioassay, with only one species, or with one specific exposure setting, iii) newer chemicals have been screened less often than older (legacy) compounds. For example, this dataset included 475 individual data points for the (legacy in Europe) herbicide atrazine tested in an algae bioassay, but only one datapoint for the herbicide mecoprop, which is currently used.
For filling such data gaps, we used QSARs that are state-of-the art and compliant with the recommendations of the Organization for Economic Co-operation and Development (OECD)48. The QSAR predicted data cover 3,318 (98%) compounds for the three species groups. In theory, the property of each chemical could be predicted using QSARs. However, QSARs do not cover all structures or compounds (e.g., organometallic or inorganic compounds cannot be predicted) and some included substances in our research are mixtures which are as well not covered by our QSAR analysis. The distribution of effect concentrations in Fig. 4 are broader for the experimental data compared with the predicted values by one to two log orders of magnitude, while the median effect concentrations across all chemicals are similar between the measured and predicted data.
The quality of the experimental and computational ecotoxicity data was validated using a solubility domain classification to identify and annotate possible outliers in the dataset.
To reveal the data quality of the measured and predicted data, we calculated the solubility-based application domain classes based on the ideas realized in ChemProp45. In theory, a chemical cannot be tested above its intrinsic water solubility in bioassays. In older studies, only the nominal concentrations (the initially dosed concentrations), but not the real concentrations in the test media, were reported. Thus, the nominal effect concentration may overestimate the effect if the effect value is above the solubility or saturation of the chemical in the test medium. Though, in cases, a solvation agent was used, or the test medium improves solvation, the solubility can be enhanced. Neither the ECOTOXDB nor most of the QSARs address the solubility issue comprehensively. Figure 5 shows the number of chemicals of our dataset within respective solubility domain classes. The majority of chemicals fall into class 3. This means that the determined effect concentrations are below the calculated solubility limits, and, therefore, valid to be used. Class 2 refers to effect concentrations that lay between the solubility limit and a half log step above. Class 2 compounds can be considered valid according to the solvation criterion mentioned above. No chemicals fall into class 1 and only few into class 0. The latter values should be maintained with caution and considered in cases where such chemicals are high-ranking as toxicity drivers in risk assessments based on QSAR predictions. Such effect concentrations might only be valid after experimental confirmations.
Curated modes of chemical action in comparison to pre-existing data
Modes of chemical action were defined and categorized differently during the last decades. While Verhaar et al.17 proposed four categories, namely, ‘inert’, ‘less inert’, ‘reactive’, and ‘specifically acting’, based on the composition of chemical structures, later approaches included more categories that were either of general nature (e.g., ‘narcotic action’) or more specifically related to the interaction with a certain biological enzyme or receptor (e.g., ‘acetylcholinesterase inhibition’)49,50. In this study, we did not build categories upfront but summarized the existing knowledge into meaningful groups based on biological modes of action. These groups, the broad and specific MoA categories as well as use groups were harmonized and standardized in their names. Duplicates and typos were removed, and harmonization was cross-checked by counting the number of chemicals per term. With this, we provide the largest and most systematic dataset on MoA categories for environmental chemicals. Figure 6 illustrates this by showing all 32 broad MoA categories with respective specific categories within. The comprehensiveness was confirmed by comparing chemicals and assigned MoA categories with results obtained with the tool provided by Firman et al.51. We found that some MoAs are predicted correctly for many chemicals, e.g., ‘acetylcholinesterase inhibition’ or ‘fatty acid biosynthesis inhibition’ while other MoAs such as interactions of chemicals with endocrine nuclear receptors or biological structures of the nervous system are not well covered by the prediction tool. Hence, the here provided data and the resulting MoA categories can be used to extend tools and approaches that aim to predict MoAs based on chemical structure information as summarized in Firman et al.51 and Kienzler et al.52. In turn, the mentioned tools might be applied and evidence generated, especially for those chemicals for which no MoA could be assigned in this study.
Overview of the 32 broad MoA categories, with their specific sub-categories sorted according to numbers of chemicals within a category. This figure was created using the “SankeyMatic” online tool (https://sankeymatic.com, accessed 15 May 2023).
Usage Notes
Data can be used with each software that reads csv. CSV can be imported to excel via the “data/import csv function”.
Recommendations for the use of the dataset
The dataset was carefully compiled and curated. Quality measures were applied to amend the dataset with quality control tags (i.e. the application domains) to ensure a good data quality and re-usability. The provided dataset should be applied in a scientific and/or regulatory context by experienced assessors or under supervision of an experienced person. To our knowledge, this is the first curation of its kind which provides a starting point and structure that could and should be updated repeatedly as experimental data as well as prediction models constantly evolve53. The provided workflow and dataset fulfil the FAIR principles31 and therefore enable comprehensive and transparent risk assessments with harmonized data for the aquatic environment as full transparency and reproducibility was not given in so far published large-scale chemical risk assessments for aquatic environments (e.g., Malaji et al.54, Rorije et al.55). Furthermore, next to the identification of risk driving compounds or sites at risk due to mixture exposures, the comparison of the available effect data with environmental monitoring data can also provide guidance for the prioritization of chemicals for toxicity testing.
The curated collection of MoAs represents the state of knowledge and is definitely not complete but provides guidance and evidence for risk assessors and scientists. Chemicals can be grouped according to their similar ways of action, novel compounds might be assigned to MoAs via read-across approaches, and machine learning or artificial intelligence approaches might use the data for model training. This means, the collection of MoAs and molecular biological targets of chemicals provided in this study provides guidance for the development of in vitro and in silico methods that should be applied to identify MoAs of novel future chemicals in high throughput screening approaches prior to animal testing. In this regard, especially methods for the detection of MoAs indicative of chronic toxicity, such as neuroactivity or endocrine action of chemicals need to be further developed as we show here that a substantial number of environmentally relevant chemicals can act respectively. The date collected in this study also indicates that chemicals of diverse use groups can act via the same MoA. However, so far, joint exposures to chemicals of the same use group or with the same MoA belonging to different regulatory silos (legislative sectors) are not considered in risk assessments. Currently, initiatives are under way to change this and consider chemicals in common assessment groups or introduce risk factors for mixture exposures27,30,56. The former approach, proposed by EFSA, aims to apply hazard-driven criteria for grouping of chemicals into assessment groups using MoA information on toxicity27. Furthermore, ECHA applies MoA information within risk assessments in the frame of read-across and grouping of chemicals28. The here provided dataset supports all of these efforts as it can be used i) for read-across studies with novel compounds, ii) for the definition of meaningful MoAs and MoA categories for the grouping of chemicals, and iii) for qualitative and quantitative mixture risk assessments based on data of joint chemical occurrence. Future efforts need to integrate data on more species as are currently curated, e.g., within the EU project PrecisionTox57, and further harmonize environmental and human risk assessment frameworks across regulatory silos as it is anticipated, e.g., in the European Partnership for the Assessment of Risks of Chemicals (PARC)58.
Limitations of the dataset
MoA information was curated and researched systematically by using external data sources. For many chemicals, no MoA information was found in those sources, which does not mean that this information is not known or provided elsewhere. We tried to consider MoA knowledge for more than one species group. However, each chemical may act via other MoAs in other species that were not considered here. To our knowledge, there is no ontology for MoAs available and annotations of MoAs are less harmonized than biological molecules, for example. We tried to start MoA annotations considering existing knowledge. However, this dataset is far from being complete and, dependent on the context, the grouping of chemicals into use groups and MoA categories could also be done in other ways.
Despite the careful curation of the ecotoxicity dataset, the input data was retrieved from an external database or QSAR estimations. The underlying data might contain data with quality issues (e.g., bad experimental design, or wrong rating due to reported nominal concentrations). Furthermore, in some cases, only a few data points are available, and thus the predictive power is lacking due to the scarcity of data. This also applies to QSAR models because a limited number of chemicals with similar structural features in the training dataset results in low reliability of the predictions.
Peer review
The data descriptor was peer reviewed in 2023 based on the data version 2 available on Zenodo37.
Code availability
The R package REcoTox43 version 0.4.1 for processing US EPA ECOTOX Knowledgebase files is available on GitHub (https://github.com/tsufz/REcoTox/releases/tag/0.4.1) under AGPL-3.0 license. The REcoTox and graphics processing scripts are available on Zenodo44 under AGPL-3.0 license.
References
Persson, L. et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 56, 1510–1521 (2022).
Wang, R., Jones, K. C. & Zhang, H. Monitoring Organic Pollutants in Waters Using the Diffusive Gradients in the Thin Films Technique: Investigations on the Effects of Biofouling and Degradation. Environ. Sci. Technol. 54, 7961–7969 (2020).
Busch, W. et al. Micropollutants in European rivers: A mode of action survey to support the development of effect-based tools for water monitoring. Environ. Toxicol. Chem. 35, 1887–1898 (2016).
Finckh, S. et al. A risk based assessment approach for chemical mixtures from wastewater treatment plant effluents. Environ. Int. 164, 107234 (2022).
Alygizakis, N. et al. Evaluation of chemical and biological contaminants of emerging concern in treated wastewater intended for agricultural reuse. Environ. Int. 138, 105597 (2020).
Scheringer, M., Johansson, J. H., Salter, M. E., Sha, B. & Cousins, I. T. Stories of Global Chemical Pollution: Will We Ever Understand Environmental Persistence? Environ. Sci. Technol. 56, 17498–17501 (2022).
Machate, O., Schmeller, D. S., Schulze, T. & Brack, W. Review: mountain lakes as freshwater resources at risk from chemical pollution. Environ. Sci. Eur. 35, 3 (2023).
Joerss, H., Menger, F., Tang, J., Ebinghaus, R. & Ahrens, L. Beyond the Tip of the Iceberg: Suspect Screening Reveals Point Source-Specific Patterns of Emerging and Novel Per- and Polyfluoroalkyl Substances in German and Chinese Rivers. Environ. Sci. Technol. 56, 5456–5465 (2022).
Dulio, V. et al. The NORMAN Association and the European Partnership for Chemicals Risk Assessment (PARC): let’s cooperate! Environ. Sci. Eur. 32, 100 (2020).
Olker, J. H. et al. The ECOTOXicology Knowledgebase: A Curated Database of Ecologically Relevant Toxicity Tests to Support Environmental Research and Risk Assessment. Environ. Toxicol. Chem. 41, 1520–1539 (2022).
Cherkasov, A. et al. QSAR Modeling: Where Have You Been? Where Are You Going To? J. Med. Chem. 57, 4977–5010 (2014).
Mayer, F. L., Krause, G. F., Ellersieck, M. R., Lee, G. & Buckler, D. R. Predicting chronic lethality of chemicals to fishes from acute toxicity test data: Concepts and linear regression analysis. Environ. Toxicol. Chem. 13, 671–678 (1994).
Raevsky, O. A., Grigor’ev, V. Y., Weber, E. E. & Dearden, J. C. Classification and Quantification of the Toxicity of Chemicals to Guppy, Fathead Minnow and Rainbow Trout: Part 1. Nonpolar Narcosis Mode of Action. QSAR Comb. Sci. 27, 1274–1281 (2008).
Aalizadeh, R., von der Ohe, P. C. & Thomaidis, N. S. Prediction of acute toxicity of emerging contaminants on the water flea Daphnia magna by Ant Colony Optimization–Support Vector Machine QSTR models. Environ. Sci. Process. Impacts 19, 438–448 (2017).
Mansouri, K., Grulke, C. M., Judson, R. S. & Williams, A. J. OPERA models for predicting physicochemical properties and environmental fate endpoints. J. Cheminformatics 10, 10 (2018).
Pedretti, A., Mazzolari, A., Gervasoni, S., Fumagalli, L. & Vistoli, G. The VEGA suite of programs: an versatile platform for cheminformatics and drug design projects. Bioinformatics 37, 1174–1175 (2021).
Verhaar, H. J. M., van Leeuwen, C. J. & Hermens, J. L. M. Classifying environmental pollutants. Chemosphere 25, 471–491 (1992).
Russom, C. L., Anderson, E. B., Greenwood, B. E. & Pilli, A. ASTER: an integration of the AQUIRE data base and the QSAR system for use in ecological risk assessments. Sci. Total Environ. 109–110, 667–670 (1991).
Barron, M. G., Lilavois, C. R. & Martin, T. M. MOAtox: A comprehensive mode of action and acute aquatic toxicity database for predictive model development. Aquat. Toxicol. 161, 102–107 (2015).
Kienzler, A., Barron, M. G., Belanger, S. E., Beasley, A. & Embry, M. R. Mode of Action (MOA) Assignment Classifications for Ecotoxicology: An Evaluation of Approaches. Environ. Sci. Technol. 51, 10203–10211 (2017).
Sumpter, J. P., Johnson, A. C. & Runnalls, T. J. Renewing and improving the environmental risk assessment of chemicals. Sci. Total Environ. 845, 157256 (2022).
European Commission. Communication From The Commission To The European Parliament, The Council, The European Economic And Social Committee And The Committee Of The Regions Chemicals Strategy for Sustainability Towards a Toxic-Free Environment. (2020).
Sun, H., Xia, M., Austin, C. P. & Huang, R. Paradigm Shift in Toxicity Testing and Modeling. AAPS J. 14, 473–480 (2012).
Ankley, G. T. et al. Adverse Outcome Pathway Network–Based Assessment of the Interactive Effects of an Androgen Receptor Agonist and an Aromatase Inhibitor on Fish Endocrine Function. Environ. Toxicol. Chem. 39, 913–922 (2020).
OECD. Guidance Document for the Use of Adverse Outcome Pathways in Developing Integrated Approaches to Testing and Assessment (IATA). (Organisation for Economic Co-operation and Development, 2017).
Van der Stel, W. et al. New approach methods (NAMs) supporting read-across: Two neurotoxicity AOP-based IATA case studies. ALTEX - Altern. Anim. Exp. 38, 615–635 (2021).
EFSA Scientific Committee. et al. Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals. EFSA J. 19, e07033 (2021).
ECHA. Guidance on Information RequiremeGuidance on information requirements and chemical safety assessment Appendix R.6-1. for nanomaterials applicable to the Guidance on QSARs and Grouping of Chemicalsnts and Chemical Safety Assessment - ECHA. https://doi.org/10.2823/273911 (European Chemicals Agency (ECHA), 2019).
EFSA Panel on Plant Protection Products and their Residues (PPR). Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters. EFSA J. 11, 3290 (2013).
Backhaus, T. The Mixture Assessment or Allocation Factor: conceptual background, estimation algorithms and a case study example. Resarch Square, https://doi.org/10.21203/rs.3.rs-1986611/v1 (2022).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Taha, H. M. et al. The NORMAN Suspect List Exchange (NORMAN-SLE): facilitating European and worldwide collaboration on suspect screening in high resolution mass spectrometry. Environ. Sci. Eur. 34, 104 (2022).
Beckers, L.-M. et al. Unraveling longitudinal pollution patterns of organic micropollutants in a river by non-target screening and cluster analysis. Sci. Total Environ. 727, 138388 (2020).
Brack, W. Solutions for present and future emerging pollutants in land and water resources management. Policy briefs summarizing scientific project results for decision makers. Environ. Sci. Eur. 31, 74 (2019).
Loos, R. et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 17, 6475–6487 (2013).
Loos, R. et al. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 157, 561–568 (2009).
Kramer, L. et al. Curated mode-of-action data and effect concentrations for chemicals relevant for the aquatic environment. Zenodo. https://doi.org/10.5281/zenodo.10071824 (2023).
Lewis, K. A., Tzilivakis, J., Warner, D. J. & Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 22, 1050–1064 (2016).
International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Volumes 1–132 – IARC Monographs on the Identification of Carcinogenic Hazards to Humans. https://monographs.iarc.who.int/agents-classified-by-the-iarc/.
Williams, A. J. et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Cheminformatics 9, 61 (2017).
Kim, S. et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 49, D1388–D1395 (2021).
Mansouri, K., Grulke, C. M., Richard, A. M., Judson, R. S. & Williams, A. J. An automated curation procedure for addressing chemical errors and inconsistencies in public datasets used in QSAR modelling. SAR QSAR Environ. Res. 27, 911–937 (2016).
Schulze, T. REcoTox - a workflow to process US EPA ECOTOX Knowledgebase ASCII files [Software]. Zenodo. https://doi.org/10.5281/zenodo.10071710 (2023).
Schulze, T. REcoTox - a workflow to process US EPA ECOTOX Knowledgebase ASCII files [Vignette]. Zenodo. https://doi.org/10.5281/zenodo.10401324 (2023).
Schüürmann, G., Ebert, R.-U. & Kühne, R. Quantitative Read-Across for Predicting the Acute Fish Toxicity of Organic Compounds. Environ. Sci. Technol. 45, 4616–4622 (2011).
European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Community, European vol. Directive 2000/60/EC 1–72 (2000).
European Union. Directive 2008/105/EC of the European Parliament and the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Official Journal of the European Union vol. Directive 2008/105/EC 84–97 (2008).
OECD. Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)SAR] Models. (Organisation for Economic Co-operation and Development, 2014)
Russom, C. L., Bradbury, S. P., Broderius, S. J., Hammermeister, D. E. & Drummond, R. A. Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 16, 948–967 (1997).
Sapounidou, M. et al. Development of an Enhanced Mechanistically Driven Mode of Action Classification Scheme for Adverse Effects on Environmental Species. Environ. Sci. Technol. 55, 1897–1907 (2021).
Firman, J. W. et al. Chemoinformatic Consideration of Novel Psychoactive Substances: Compilation and Preliminary Analysis of a Categorised Dataset. Mol. Inform. 38, 1800142 (2019).
Kienzler, A. et al. Mode of Action Classifications in the EnviroTox Database: Development and Implementation of a Consensus MOA Classification. Environ. Toxicol. Chem. 38, 2294–2304 (2019).
Gustavsson, L., Hollert, H., Jönsson, S., Bavel, B. & Engwall, M. Reed beds receiving industrial sludge containing nitroaromatic compounds. Environ. Sci. Pollut. Res. - Int. 14, 202–211 (2007).
Malaj, E. et al. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. 111, 9549–9554 (2014).
Rorije, E. et al. Characterization of ecotoxicological risks from unintentional mixture exposures calculated from European freshwater monitoring data: Forwarding prospective chemical risk management. Sci. Total Environ. 822, 153385 (2022).
More, S. J. et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 17, e05634 (2019).
Colbourne, J. K. et al. Toxicity by descent: A comparative approach for chemical hazard assessment. Environ. Adv. 9, 100287 (2022).
Marx-Stoelting, P. et al. A walk in the PARC: developing and implementing 21st century chemical risk assessment in Europe. Arch. Toxicol, https://doi.org/10.1007/s00204-022-03435-7 (2023).
Sparks, T. C. & Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 121, 122–128 (2015).
Wishart, D. S. et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34, D668–672 (2006).
Wishart, D. S. et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 50, D622–D631 (2022).
Dionisio, K. L. et al. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 5, 180125 (2018).
Acknowledgements
This work was carried out in the framework of the European Partnership for the Assessment of Risks from Chemicals (PARC) and has received funding from the European Union’s Horizon Europe research and innovation program under Grant Agreement No 101057014. Views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or the Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. The study was funded by the Helmholtz Earth and Environment Program, Topic 9 and the Chemicals in The Environment (CITE) integration fund at the UFZ. T.S. acknowledges funding by Deutsche Forschungsgemeinschaft (441958208) and the Development Bank of Saxony (100669418). The authors thank Ronny Krause for legal advises regarding data use and reuse.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.A.: Conceptualization, Review & Editing. W.B.: Conceptualization, Data Curation, Investigation, Validation, Writing, Review & Editing. J.H.: Conceptualization, Review & Editing. N.K.: Writing, Review & Editing. L.K.: Data Curation, Investigation, Data Analyses, Visualization, Writing. M.K.: Data Curation, Investigation, Review & Editing. T.S.: Conceptualization, Data Curation, Investigation, Data Analyses & Validation, Visualization, Writing, Review & Editing
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kramer, L., Schulze, T., Klüver, N. et al. Curated mode-of-action data and effect concentrations for chemicals relevant for the aquatic environment. Sci Data 11, 60 (2024). https://doi.org/10.1038/s41597-023-02904-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02904-7