Abstract
This dataset contains biogeochemical samples from the Barents Sea and Arctic region analyzed by the Plankton Chemistry Laboratory at the Institute of Marine Research (IMR). Number of surveys and stations visited in the Barents Sea and Arctic has varied over the last 30 years. One major effort is the annual Ecosystem Survey in the fall, with multiple trawl surveys, net tows and CTD water sampling. Additionally, two transects are visited multiple times each year (Fugløya-Bjørnøya and Vardø-North). Only samples collected from water bottles are reported here. Bottle samples from each CTD cast were collected for dissolved inorganic nutrients (nitrate, nitrite, phosphate, silicate) and phytoplankton chlorophyll-a and phaeopigments (ChlA, PHAEO) at predetermined depths and for later analysis at IMR. On occasion, short-term projects have performed Winkler dissolved oxygen titrations (DOW) and particulate organic carbon and nitrogen (POC, PN) determinations. This unique data set has seen limited use over the years but is a great contribution towards global ocean research and climate change investigations.
Measurement(s) | dissolved inorganic nutrients (nitrate, nitrite, phosphate, silicate) • cellular pigments (chlorophyll-a, phaeopigments) • dioxygen dissolved in marine water • particulate organic carbon and nitrogen |
Technology Type(s) | nutrients autoanalyser • fluorometer • Winkler titration • CHN analyzer |
Factor Type(s) | nitrate • nitrite • phosphate • silicate • chlorophyll-a • phaeopigment • dissolved oxygen • particulate organic carbon • particulate nitrogen |
Sample Characteristic - Organism | phytoplankton |
Sample Characteristic - Environment | sea water |
Sample Characteristic - Location | Barents Sea and Arctic Ocean |
Similar content being viewed by others
Background & Summary
The failing capelin fisheries in the Barents Sea initiated the Open Ocean Time-series Program (Havovervåkingen) at IMR in the late nineteen eighties, starting with a number of large-scale short-term surveys in the Barents Sea region. PRO MARE and other shorter-term projects had demonstrated the significance of seasonal cycling of phytoplankton blooms to zooplankton biomass, and how critical these sources of food can be for fish larvae1. In 1990, the MARE NOR program was launched to forecast marine resource developments in order to improve fisheries management1. In 2003, annual joint Russian-Norwegian fisheries surveys were combined into one investigation and became the annual Ecosystem Survey of the Barents Sea2.
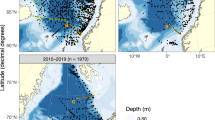
The number of surveys and stations visited annually in the Barents Sea has varied over the 30 years reported here (Fig. 1). To this day, the annual Ecosystem Survey is a Russian-Norwegian effort, and 2–3 research vessels from IMR will cover the entire Norwegian side of the Barents Sea with trawl surveys, net tows and CTD water sampling. As the summer ice edge has receded further north in recent years, the continental slope to the Polar Basin (north of the Barents Sea) has been added to these surveys. In addition to the annual Ecosystem Survey two trans-sectional core surveys, the Fugløya-Bjørnøya and the Vardø-Nord transects (Fig. 1), are still conducted 4–6 times each year. Water samples are also collected on short-term sampling projects (ships of opportunity) by all major ships operated by the IMR (Table 1). On these cruises, each CTD cast is sampled for dissolved inorganic nutrients (nitrate, nitrite, phosphate, silicate) and phytoplankton pigments (chlorophyll-a, phaeopigments) at predetermined depths (Table 2) for later analysis. Occasionally, short-term projects have also performed Winkler DO titrations (DOW) and particulate organic carbon and nitrogen (POC, PN) determinations. This set of quality-controlled data, from 30 years of ocean surveys conducted by the IMR, has never been published in its entirety.
Distribution of sampling stations over three decades in the Barents Sea and the adjoining Arctic Ocean (closed circles). The red lines show the two seasonal transects in the Open Ocean Monitoring Survey (a = Fugløya-Bjørnøya; b = Vardø-North) and total number of stations visited (n) is summarized for each decade.
Methods
Sample collection and analysis
Seawater samples are collected from Niskin-type water bottles at predetermined depths (Table 2) triggered by a CTD (Conductivity Temperature Depth) unit mounted on a rosette. Two types of CTD have been used on our survey ships over the years (Table 3); the Neil-Brown MK3B and MK3C systems (Commonwealth Scientific and Industrial Research Organisation [CSIRO], Division of Marine Research, Australia) were most common up until the millennium, when it was replaced with Sea-Bird Scientific (SBE) 911plus units (Sea-Bird Scientific, USA). Total numbers of stations sampled has varied over the years but IMR has, in addition to more random visits to the region on short-term projects, been visiting each of the two transects approximately 4–5 times each year (Fig. 1). Additionally, an annual, full-scale survey covering major parts of the Barents Sea has been conducted each year since 2003, usually in concert with one or two other IMR ships and a Russian ship covering the Russian sector.
Dissolved inorganic nutrients (nitrite, nitrate, phosphate, silicate)
After three rinses, each water sample (20 mL) was collected in a polyethylene vial. Up until the turn of the century (1999–2002) most nutrient samples were analysed in real time onboard the research vessels and without chloroform additions. As the research cruise activity expanded, the number of automated analysers could no longer match the number of ships operating simultaneously (Table 3) and nutrient samples were added chloroform (200 µL) to retard biological activity and stored at + 4 °C for analysis in the home laboratory, usually within 1–6 weeks. Prior to analysis, the samples were acclimated to room temperature as the evaporating chloroform was evacuated by vacuum prior to analysis on an Automated Analysis (AA) system. A number of automated systems have been in use over the years (Table 4) and up until recently, all nutrients were run on homemade Skalar and Alpkem hybrid systems. The latest upgrade was in 2017 when a complete AA system was purchased from Skalar Analytical B.V. (The Netherlands) for the first time. Colorimetric determinations of dissolved inorganic nutrients are based on the methods first described by Bendschneider & Robinson3 and Grasshof4 with a number of minor adjustments suggested by the manufacturers (Alpkem, Skalar). The AA system measures nitrate, nitrite, phosphate and silicate. Briefly, nitrate in seawater is reduced to nitrite coupled to a diazonium ion and, in the presence of aromatic amines, the resulting blue azo-dye is determined spectrophotometrically at 540 nm. The nitrate concentration is corrected for ambient nitrite (same analytical method as for nitrate, but without cadmium reduction) measured concurrently. Phosphate reacts with molybdate at low pH and the resulting phosphomolybdate is reduced with ascorbic acid to a blue complex measured spectrophotometrically at 810 nm. With the new Skalar AA purchased in 2017 (Table 4) the phosphomolybdate peak is now measured at 880 nm. Silicate (silicic acid) is reacting to molybdate at low pH and the resulting silicomolybdate is reduced by ascorbic acid to a blue dye measured spectrophotometrically at 810 nm.
Chlorophyll-a and phaeopigment samples (ChlA, Phaeo)
A standard volume (265 mL) is collected from each depth (Table 2), filtered onto a 25 mm GFF filter and stored at −20 °C until analysis in the land-based laboratory. Historically, pigment samples were transported home by one of the cruise participants, as hand-luggage in a cooler with frozen cooler-elements. These days, pigment samples are brought back in specially designed coolers, with an internal temperature recorder, that is rated for −20 °C for a minimum of 3 days. In the laboratory, the samples are thawed in 90% acetone, and stored at +4 °C overnight before analysis on a Turner Design 10 AU fluorometer. Phaeopigments (Phaeo) are measured separately from ChlA, in a second reading of the sample after adding 3 drops of a weak acid (5% HCl). The fluorometer is standardized regularly using a solid standard with known fluorescence, and in accordance with Holm-Hansen & Riemann5 and the manufacturer6. Up until the 2008 the drift in the light-source was monitored annually but from 2009 the solid standard has been recorded every time the fluorometer is used.
Particulate organic carbon and nitrogen (POC, PN) samples
A standard volume (265 mL) is collected from each depth (Table 2) and filtered onto a pre-combusted 25 mm GFF filter (+450 °C, min. 4 h). Each sample is stored frozen (−20 °C) in a pre-combusted glass tube until analysis in the land-based laboratory. Preparations of samples and analysis of elemental C and N is described in detail in Grasshof et al.7. Briefly, the dried filter-samples are fumed in acid (conc. HCl) in a desiccator for 4–12 h, before they are dried again and packed in a tin-foil capsule. Analysis of POC and PN is performed on an elemental analyzer (see Table 4 for details) and in accordance with the manufacturer’s recommendations.
Dissolved oxygen samples by winkler titrations (DOW)
Samples for dissolved oxygen are collected in volume-calibrated glass BOD bottles (approximate volume 125 mL) and filled from bottom up using a Tygon-tubing. The sample is let to overflow approximately 3 times the volume and great care is taken to avoid small air bubbles at the inside of the sample bottle. Thiosulfate titrations of dissolved oxygen are still done as first described by Winkler8 but the method has seen some updates and improvements over the years9,10,11. Grasshof et al.7 is describing in detail the current method of sample collection, pretreatment and coulometric titrations of Winkler samples. Briefly, dissolved oxygen is reacting with an alkaline solution (Reagent 1) to form a manganese-hydroxy-complex. Under alkaline conditions, the Mn-complex is reacting with the iodide solution (Reagent 2) and let to precipitate at the bottom of the sample bottle. The sample is added 10 N sulphuric acid to dissolve the iodide precipitate (pH = 1–2.5) and the yellow iodine is titrated by thiosulfate to a clear solution. The titrant is standardized by a known concentration of potassium iodate (KIO3) as described by Grasshof et al.7.
Data Records
Data from the Barents Sea region and the adjoining Arctic Ocean has been submitted to the SeaDataNet (SDN) services (https://www.seadatanet.org/), and can be downloaded as a 1990–2019 compilation12. SeaDataNet is the standard publication format for biogeochemical data at IMR and the specifics of the data file format chosen here (netCDF) can be found in chapter 4.5.1 of the current version (v.1.22) at https://archimer.ifremer.fr/doc/00454/56547/. Key features of SeaDataNet include rigorous use of the Natural Environment Research Council (NERC, UK) libraries, including measurement techniques (P01) and use of data units (P06). The file format is self-contained for both datasets and variables, and we have chosen variable names most familiar to the ocean science community. All data in this paper are considered public domain and, as such, are also integrated with the Copernicus Marine Service (https://marine.copernicus.eu/) and the EMODnet Chemistry (https://www.emodnet-chemistry.eu/) platforms.
Distribution and number of stations visited has changed over the three decades presented here (Fig. 1). The first decade (1990–1999) had sampling stations more scattered and collections were made both in Norwegian and Russian sector of the Barents Sea region (total number of stations = 2730). The following decade (2000–2009) saw more stations sampled (total number = 3131), but largely in the Norwegian sector of the Barents Sea. Due to the receding ice during summer, this is also the decade where increasing number of stations were sampled north of Svalbard, in the adjoining Arctic Ocean (Fig. 1). During the last decade (2010–2019) number of stations are similar to 1990–1999 levels (total number = 2776) but the exact same station locations are sampled repeatedly each year, and mostly in the Norwegian sector. Distribution of stations visited within a year (Fig. 2) shows a maximum coverage in August-September during all three decades. Each month is not always visited each year (n < 10 in Fig. 2) but September, the month of the Ecosystem Survey, shows maximum number of stations that has been sampled each year through all three decades (n = 10).
This data set (Fig. 3) contains nutrient measurements (nitrate, nitrite, phosphate and silicate), pigments (ChlA and Phaeo), particulate organic carbon and nitrogen (POC, PN), and Winkler titrations of dissolved oxygen (DOW). Peak number of samples range between 3000–4000 for nutrients and 2000–3000 pigment samples analyzed each year (Fig. 3). The lowest number of samples were collected at the very beginning of the times-series, and during selected years (1996–1997, 2002–2004, 2010–2012, 2015–2017) associated with reduced number of cruises to the region (Fig. 3). Only on one cruise were POC and PN samples collected from the region (Fig. 3) and only on a minor number cruises were DOW measured by Winkler titrations. All data were quality controlled (QC) by analysts using quality flags 0–5 (Table 5) in accordance with Jaccard et al.13 and the OceanSITES Data Format Reference Manual V.1.4 (http://www.oceansites.org/docs/oceansites_data_format_reference_manual.pdf), and is available at the Norwegian Marine Data Center (NMDC) home page at IMR (http://metadata.nmdc.no/metadata-api/landingpage/b6ce5b610e4ba316bce2cae9ec2aed61).
Only QC flags 1, 2, and 5 are available from these data records. “Good data” (flag = 1) are data that passed QC, “probably good data” (flag = 2) are data that may seem unexpected but (based on information from sampling and analysis) we have no reason to exclude them; “value changed” data (flag = 5) are obvious errors based on notes from the sample sheet, where analytically correct data has been relocated to another depth (e.g. where the entire nutrient profile has been mislabelled and logged in “up-side-down”). In the cases where single samples appear mislabelled, exhibit analytical errors, or appear to fall outside expected QC envelope, such values are discarded as “bad data” (flags = 3 and 4 are dedicated to doubtful and erroneous data, respectively). All data (Table 6) are hosted on an OPeNDAP server at IMR and available both as netCDF4 and csv files. The netCDF file (Table 7) follow the SeaDataNet format for profile data (https://www.seadatanet.org/Standards/Data-Transport-Formats).
Technical Validation
The Plankton Chemistry Laboratory at IMR maintain quality control of precision and accuracy by daily assessments of analytical standard curves of internal standards. As large data sets are becoming increasingly available for a wider, global array of users, it is important that the scientific community agree on a set of common practices that will lead to a common QC. Therefore, our laboratory find it crucial to maintain contacts with other laboratories through regional intercalibration studies such as QUASIMEME (http://www.quasimeme.org/) and in global intercalibrations such as IOCCP (https://repository.oceanbestpractices.org/handle/11329/883).
Our 30-year time-series of seawater nutrients are one of the most complete sets of data to date. However, handling and storage of, for instance, nutrient samples have varied considerably over this time as in the rest of the scientific community (see recent overview by 14). Early in our time-series (the first decade) seawater nutrients were commonly analyzed at sea using an onboard, automated nutrient analyzer. There are considerable challenges to maintaining high quality and analytical precision on a moving ship at sea, as well as the need for qualified people to follow the ship on all cruises. During the last decades the number of samples analyzed by the Plankton Chemistry Laboratory, and the number of vessels used simultaneously in different oceans, have increased dramatically. Therefore, we are currently analyzing a total of 70,000–110,000 nutrient samples each year. Mostly due to these cost-effective and practical considerations two decades ago, the laboratory decided to continue with one single, land-based analytical unit with high throughput of samples poisoned with chloroform. The laboratory-based unit is manned by highly skilled analysts that can take turns and work uninterrupted and continuously over long periods of time. This arrangement also means that samples are now stored at sea for periods of time and transported to the land-based laboratory prior to analysis (see Storage of nutrient-samples below). Filter-collection and storage of frozen particulate samples (ChlA, Phaeo, POC, PN), and onboard Winkler titrations of DOW samples, do follow internationally recognized guidelines for time-series measurements (e.g.11,14).
Storage of nutrient-samples
Our collection of unfiltered seawater samples, added small aliquots of chloroform, is not commonplace in ocean research and the choice of various storage techniques have been debated for decades15. However, when ship-based analysis is no longer an option (see section above), there is a need to poison or preserve the samples prior to analysis. Adding preserving agents may contaminate the samples, so great care must be taken and only analytical grade additives can be used for this purpose. If you chose to prefilter the samples before storing them frozen at −20 °C, on the other hand, the very act of prefiltration may also be a source of contamination14. An added drawback with frozen samples, is that recovery after thawing is not always complete at high silicate concentrations16. Therefore, the Plankton Chemistry Laboratory decided early on to poison the nutrient samples with analytical grade chloroform and store them at +4 °C until analysis (see Methods for details). Our nutrient samples are collected in 20 mL polyethylene vials (PE scintillation vials), added 200 µL chloroform to prevent further biological activity, and stored (up until 3 months) in the dark at +4 °C until analysis. The PE vials are used directly from the package without being acid-washed. Initially, we had some concerns that the PE vials would contaminate the nutrient samples. However, several comparative studies, between acid-washed and unwashed PE-bottles, did not show any discernable difference for any of the 4 nutrients (data not shown here).
We also had some concerns that the biological inhibitor (chloroform) could disrupt microplankton cells and add intracellular nutrients to the pool of inorganic nutrients in a sample vial, so we compared filtered and unfiltered nutrient samples. It is however more commonplace, as in large scale open ocean time-series programs, to store pre-filtered nutrient samples frozen14,15. Therefore, we combined these two issues and compared the two treatments; unfiltered seawater samples added chloroform and stored at + 4 °C (our standard mode of sampling and storage), and filtered seawater samples stored frozen at −20 °C. We used a 10 µm nylon filter disc since gravitational filtration did not work efficiently on smaller pore sizes in our samples from the Norwegian Sea. Nitrate (Fig. 4a) did show slightly elevated concentrations in the filtered samples stored frozen, but we found no difference between levels of nitrite, phosphate, and silicate in the two treatments (Fig. 4b–d). The difference between frozen and chloroform treated samples analyzed for nitrate however, was not beyond analytical error (10%) for this analysis (Fig. 4a). Our findings are comparable to Dore et al.16, who found no difference between frozen samples and natural seawater that was analyzed immediately in a lower range of nutrient concentrations from the Pacific Ocean, and we concluded that samples added chloroform and stored at + 4 °C worked equally well to frozen samples.
Comparison of nutrient samples that were filtered and stored at −20 °C for 3 months, and natural seawater samples added chloroform and stored equally long at +4 °C. Each panel shows lower level of detection for Skalar-D (Table 4) used for these analyses (horizontal and vertical broken lines) and estimated analytical error (dotted lines) associated with nitrate (a), nitrite (b), phosphate (c) and silicate (d) measurements.
Usage Notes
All data in this publication are available in accordance with Creative Commons rules (https://creativecommons.org/licenses/by/4.0/) and users are free to share, copy and redistribute the material in any medium or format. The data can be adapted and built upon at users own volition as long as the data source is appropriately accredited.
Quality control of large scale, long-term data sets are crucial to account for potential mislabeling, potential errors in storage and handling of the samples leading to contamination, and potential anomalies during analysis of the samples. The Plankton Chemistry Laboratory is currently using a QC-flagging system to account for the quality of the data that is produced (Table 5). Data with flags 1, 2 and 5 are made available for use in this publication, while flags 3 and 4 are deemed compromised and are not included. Due to the many different people participating in the sampling program on IMR cruises, we must accept that minor mistakes can be made. Some of these can be corrected (e.g. a mislabeled depths with a value that clearly belongs somewhere else) and are given Flag = 5, while others are beyond correction (e.g. samples that shows signs of contamination or appears to have been stored too long) and are given flags 3 or 4. An important QC flag is number 2, where we label data points that are outside expected value, but for no apparent reason. Flag number 2 data can be an expression of small term changes that may turn out to be significant over a larger time scales, and this kind of data cannot be discarded in ocean monitoring studies lasting decades to centuries. Unfortunately, data from samples collected prior to 2010 in our time-series used a different QC definition of flag number 2, and these data were excluded. Therefore, flags number 2, 3 and 4 were routinely excluded from data sets during the 1990–2009 period.
Code availability
No custom code was used to generate or process the data described in this paper.
References
Skjoldal, H.R., Hopkins, C., Erikstad, K.E. & Leinaas, H.P. Ecology of fjords and coastal waters (Elsevier, 1995)
Olsen, E., Michaelsen, K.,Ushakov, N. & Zabavnikov, V.B. The Ecosystem Survey (eds. Jakobsen T. & Ozhigin V. K.) The Barents Sea. Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation (Tapir Academic Press, 2011).
Bendschneider, K. & Robinson, R. I. A new spectrophotometric method for the determination of nitrite in seawater. J. Maine Res. 2, 87–96 (1952).
Grasshoff, K. On the automatic determination of phosphate, silicate and fluoride in seawater (ICES Hydrographic Committee Report No. 129, 1965).
Holm-Hansen, O. & Riemann, B. Chlorophyll a determination: improvements in methodology. Oikos 30, 438–447, https://doi.org/10.2307/3543338 (1978).
Turner Designs Model 10-AU-005 Field Fluorometer User’s Manual, Version S1C (Turner Designs, California, USA, 1992)
Grasshoff, K., Ehrhart, M. & Kremling, F. Methods of seawater analysis 2nd edn (Verlag Chemie, Wiley, Weinheim, 1983)
Winkler, L. W. Die Bestimmung des im wasser gelösten Sauerstoffes. Berichte der Deutschen Chemishe Gesellschaft 21, 2843–2855 (1888).
Carpenter, J. H. The Chesapeake Bay Institute. Technique for the Winkler oxygen method. Limnol. Oceanogr. 10, 141–143 (1965).
Murray, J. N., Riley, J. P. & Wilson, T. R. S. The solubility of oxygen in Winkler reagents used for the determination of dissolved oxygen. Deep-Sea Res. 15, 237–238, https://doi.org/10.1016/0011-7471(68)90046-6 (1968).
Strickland, J.D.H & Parsons, T.R. A Practical Handbook of Seawater Analysis (Fisheries Research Board of Canada, Bulletin 167, 1972).
Gundersen, K. et al. Nutrient Biogeochemistry in the Barents Sea and the adjoining Arctic Ocean, 1990–2019. Institute of Marine Research https://doi.org/10.21335/NMDC-2017043378 (2022).
Jaccard, P. et al. Quality information document for global ocean reprocessed in-situ observations of biogeochemical products, https://doi.org/10.13155/54846 (Issue 2.0, 2018)
Becker, S. et al. GO-SHIP Repeat Hydrography Nutrient Manual: The precise and accurate determination of dissolved inorganic nutrients in seawater, using continuous flow analysis methods. Front. Mar. Sci. https://doi.org/10.3389/fmars.2020.581790 (2020).
Rayner, M. et al. International nutrient inter-comparison voyage, https://doi.org/10.25919/5cc9ecb9bc140 (Oceans & Atmosphere. CSIRO, Australia, 2018).
Dore, J. E. et al. Freezing as a method of sample preservation for the analysis of dissolved inorganic nutrients in seawater. Mar. Chem. 53, 173–185, https://doi.org/10.1016/0304-4203(96)00004-7 (1996).
Acknowledgements
This publication is the result of 30 years of work at sea, aided by a great number of onboard technicians and researchers collecting the biogeochemical samples. We are equally thankful for the great number of colleagues who performed subsequent analysis at sea and in the land-based laboratories. The complete list of analysts is shown according to date of first employment; M. Hagebø (1990–2009), T. Jåvold (1990-today), J. Strømstad Møgster (1990-today), J. Erices (1990–2004), S. Olderkjær (1993), I. Iden (1994), J.E. Søreide (1995–1996), A. Fedøy (1996–1997), V. Biseth (1997–1999), A. Nybakk (2003–2006), L. Fonnes Lunde (2004-today), T. Standal (2007–2008), M. Petersen (2010–2016), I. Tjelfåt (2013–2014), H. Arnesen (2018-today), A.-K. Olsen (2019-today). The monitoring program in its current form was initiated by Drs. F. “Pancho” Rey and L. Føyn. Together with M. Hagebø, they established most of the sample collection routines and analytical procedures that are still maintained to this date. We also thank H. Sagen, S. Ringheim Lid, K. Fjellheim, A. Morvik, Ø. Jakobsson and A. Yamakawa (all at HI Digital at IMR, formerly known as The Norwegian Marine Data Center) for facilitating the on-line access portal to the data. The Open Ocean Monitoring Programs (Snitt-undersøkelsene) at IMR, and the annual Ecological Survey in the Barents Sea in August-September each year (Økosystemundersøkelsen i Barentshavet), have been funded by a number of governmental entities over the years (Norsk Forskningsråd, Fiskeridirektoratet, Miljødirektoratet). The data set also contain a minor number of samples, that were analyzed by the Plankton Chemistry Laboratory at IMR but funded by external grants to other institutions. We are therefore grateful for these data contributions, from the University of Bergen, University of Tromsø, University of Oslo, Norwegian Polar Institute in Tromsø, Norwegian Institute for Water Research and Norwegian University of Science and Technology in Trondheim, and it is our hope that access to all these data will be useful in the common public domain.
Author information
Authors and Affiliations
Contributions
K. Gundersen is primary author and lead of final assembly and quality control of the data sets. J. Strømstad Møgster worked in concert with K. Gundersen and performed final quality control and flagging of all the data. After final quality control and flagging, V. Lien and E. Ershova extracted the regional data sets for further use and display. L. Fonnes Lunde, H. Arnesen and A.-K. Olsen, together with J. Strømstad Møgster, are current members of the analytical team in the Plankton Chemistry Laboratory at IMR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gundersen, K., Møgster, J.S., Lien, V.S. et al. Thirty Years of Nutrient Biogeochemistry in the Barents Sea and the adjoining Arctic Ocean, 1990–2019. Sci Data 9, 649 (2022). https://doi.org/10.1038/s41597-022-01781-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01781-w