Abstract
Most people with mental health disorders cannot receive timely and evidence-based care despite billions of dollars spent by healthcare systems. Researchers have been exploring using digital health technologies to measure behavior in real-world settings with mixed results. There is a need to create accessible and computable digital mental health datasets to advance inclusive and transparently validated research for creating robust real-world digital biomarkers of mental health. Here we share and describe one of the largest and most diverse real-world behavior datasets from over two thousand individuals across the US. The data were generated as part of the two NIMH-funded randomized clinical trials conducted to assess the effectiveness of delivering mental health care continuously remotely. The longitudinal dataset consists of self-assessment of mood, depression, anxiety, and passively gathered phone-based behavioral data streams in real-world settings. This dataset will provide a timely and long-term data resource to evaluate analytical approaches for developing digital behavioral markers and understand the effectiveness of mental health care delivered continuously and remotely.
Measurement(s) | depression • real-world behavior • anxiety • Depressed Mood • physical mobility • daily smartphone usage |
Technology Type(s) | Smartphone Application • GPS navigation system |
Factor Type(s) | depression level, socioeconomic indicators, demographics |
Sample Characteristic - Organism | Homo sapiens |
Sample Characteristic - Environment | real world setting |
Sample Characteristic - Location | contiguous United States of America |
Similar content being viewed by others
Background & Summary
Although effective treatments exist, depression is one of the leading causes of disability worldwide1. A challenge in eradicating this burdensome and costly illness is the poor access to timely diagnosis and treatment. Individuals with depression often lack adequate and timely care, and even when care is available, it is often not evidence-based, and outcomes are not measured consistently1,2,3. In addition, there are known barriers such as time commitment, regular follow-ups in behavioral therapy4, and limited availability of highly trained professionals, especially in rural and lower-income areas5. Some populations also do not access care because of the stigma associated with mental health problems6. In particular, there are known socio-technical challenges in recruiting underserved communities7 such as the Hispanic/Latino population, in traditional research studies8,9 in the US. Asian, Hispanic, and Black people are less likely to receive access to and utilize mental healthcare services than their White counterparts10,11,12. As a result, our understanding of optimal support and treatment options for underserved and minoritized populations remains limited.
In response to such challenges, there is broad interest in using digital health technology to provide individuals with novel preventative and therapeutic solutions in a timely and scalable manner compared to traditional mental health services13. Current applications of digital technology in mental health include the remote deployment14 of guided interventions (e.g., cognitive behavioral therapy15). Due to heterogeneity in diagnoses of mental illnesses, researchers are beginning to explore the use of smartphones and wearables16 in understanding personalized day-to-day factors and their severity impacting individuals’ mental health. Multimodal data gathered from connected devices can help understand individualized real-world behavior, aiding the development of digital phenotypes17. Once validated, these digital signatures can ultimately help track individual physiological symptoms that can be used to tailor and augment the treatment of mental illnesses18 as well as guide the development of novel treatments for mental health disorders.
In the last ten years, technology developers and researchers have begun to explore the potential of smartphone apps as an economical and scalable means for assessing and delivering mental health care. Over 10,000 smartphone apps for mental health and well-being are available on the App and Play stores. However, there is little information about their utilization, efficacy, and effectiveness19. When assessing the quality and efficacy of smartphone-based interventions as a supplement to treatments or compared to other therapeutic interventions, there have been varying results across treatment groups20. There have also been notable concerns regarding the use of mobile apps, potentially excluding groups who may not have access to technology due to cost, digital literacy, location, or availability21. Many individuals also express concerns over potential privacy and consent issues regarding access to personal mental health-related data22 and apps storing sensitive information such as account information and demographics, which may discourage their use23. Essential questions regarding the utility of digital mental health apps are (1) who downloads these apps, (2) do people who use these apps use them as designed, (3) are they effective in collecting valid and long-term behavioral data, (4) for whom are they effective and for how long, and (5) are these tools easy to access and as effective in under-represented minorities?
The limited availability of real-world behavioral datasets curated using FAIR24 data sharing and management principles is a critical bottleneck that hampers digital mental health research advancement. A recent systematic review25 of real-world behavior sensing studies highlighted substantial challenges such as small sample sizes and inconsistent reporting of analytical results that affect the reproducibility of findings. And as a result, it impacts the development of robust and transparently validated digital biomarkers of mental health using real-world data (RWD). Irreproducibility of research findings remains the top challenge stymieing the real-world application of biomedical research. Recent meta-studies have shown that up to 50% of reviewed studies were not reproducible, costing taxpayers billions of dollars every year26,27. NIH has recently instituted a mandate requiring researchers to make their research data accessible for all NIH-funded research starting January 202328.
We describe and share one of the most extensive longitudinal real-world behavioral datasets to enable a broader ecosystem of digital health datasets accessible to the biomedical research community and curated using FAIR principles24. We believe this dataset will provide a significant long-term value for the researchers interested in digital mental health across various use cases, such as (1) cross-study data integration and comparisons of findings, (2) benchmarking analytical approaches for predicting functional and behavioral outcomes, (3) specific app use signatures and their relationship to outcomes, (4) modeling individualized behavioral trajectories over time, (5) the impact of being in traditional treatment while using the app on outcomes, (6) discovery depression subtypes based on baseline characteristics and how they relate to: i) adherence with care, ii) changes in mood and sleep patterns, and iii) stability of outcomes.
Methods
Brighten V1 and V2 studies
The two randomized clinical trials, Brighten (Bridging Research Innovations for Greater Health in Technology, Emotion, and Neuroscience) V1 study29, (henceforth V1) and Brighten V27, (henceforth V2), have been used to investigate: 1) feasibility of conducting fully remote decentralized clinical trials7,29, 2) the relative effectiveness of digital health apps in improving mood30, 3) utility of real-world behavioral data collected passively from smartphones in predicting changes in mood over time at the population and individual level31 and 4) factors impacting participant recruitment and retention in decentralized health research32. Both studies deployed three app-based remote interventions and gathered real-world data (active: remote surveys and passive: smartphone usage) related to depression shared and described here.
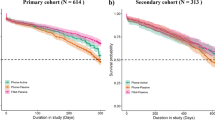
Overall, 7,850 participants from across the US were screened, with 2,193 participants meeting the eligibility criteria and consenting to enroll in the two studies (Figs. 1, 2; Table 1). The individual-level engagement data analysis showed significant variation in participant retention linked to demographic and socioeconomic factors further described within the two studies7,32. Study participants’ average depression severity (assessed using PHQ-9 survey33) at onboarding indicated moderate depression symptoms (V1:14 ± 5.1, V2:14.3 ± 5.4). A notable proportion of the cohort indicated suicidal ideation (non-zero response to 9th item of PHQ-9) at onboarding (V1:29.4%, V2:35.6%).
There were significant differences in race/ethnicity between the two study cohorts (Hispanic/Latino V1:13%, V2:38%) primarily driven by underlying differences in recruitment approaches between studies. Using targeted recruitment approaches (further described here7), we enriched the V2 study cohort for Hispanics/Latinos to help investigate the feasibility of using digital mental health tools in this target population. See Table 2 for further details and a descriptive summary of the overall cohort stratified by V1 and V2 studies.
Study design
Brighten studies (V1 and V2) aimed to assess the feasibility of conducting fully remote smartphone-based randomized clinical trials to evaluate and deliver digital interventions to individuals with depression. While both trials were open to any individual meeting the eligibility criteria, Brighten V2 focused on increasing the participation of Hispanic/Latino populations. Ethical oversight and approval for both trials (V1:NCT00540865, V2:NCT01808976) were granted by the Institutional Review Board of the University of California, San Francisco. Participants in both studies were randomized to one of three intervention apps and prospectively observed for 12 weeks. The three intervention apps were: 1.) iPST - an app based on problem-solving therapy34,35, an evidence-based treatment for depression, 2.) Project Evo - a therapeutic video game meant to improve cognitive skills associated with depression36, and 3.) Health Tips - an app that provides information about strategies to improve mood.
Participant recruitment & onboarding
Participants had to speak English or Spanish (in V2), be 18 years or older, and own either an iPhone with Wi-Fi or 3 G/4 G/LTE capabilities or an Android phone with an Apple iPad version 2.0 or newer device. In both studies, participants had to experience clinically significant symptoms of depression, as indicated by either a score of 5 or higher on PHQ-9 or a score of 2 or greater on PHQ item 10.
Participants were recruited using a combination of three different approaches - traditional methods (ads placed in local media), social media-based advertising (ads on Facebook and Craigslist), and online targeted recruitment campaigns (in partnership with Trialspark.com for the V2 study). The onboarding process involved learning about the study through the study website. A video highlighting the research study’s goals and procedures and the risks and benefits of participation was also made available.
After viewing the video and reading the online study consent, participants had to correctly answer three questions to confirm their understanding that participation was voluntary, not a substitute for treatment, and that they were randomized to treatment conditions. Each question had to be answered correctly before a participant could consent to participate, at which point they could move on to the baseline assessment and randomization. Participants’ consent to participate in the study was asked before their enrollment eligibility could be established. This was done to assess their baseline depression state (via PHQ-9 survey), which was required to meet the study eligibility criteria and could not be collected without the participant’s consent. In the consent form signed by participants, there was a clause describing that other organizations and researchers may use these data for research and quality assurance. This follows the National Institute of Mental Health (NIMH) data sharing policy (NOT-MH-19-033), designed to share data at the end of the award period or upon the research findings being published.
Once confirmed, participants were sent a link to download the study intervention app they were randomly assigned. The V2 study translated the recruitment material into Spanish for Spanish speakers. Participants were compensated for their time in both studies. Each participant received up to $75 gift vouchers for completing all assessments over the 12-week study period: US $15 after completing the initial baseline assessment and US $20 for each subsequent assessment at the 4-, 8-, and 12-week time points.
Data collection included participant self-reported data using a combination of validated survey instruments and short ecological momentary assessments. We also collected passive sensor data from smartphones related to participants’ daily activities as described below.
Baseline assessment
All consented participants in V1 and V2 studies provided the same baseline information. The demographic survey included age, race/ethnicity, marital and employment status, income, and education. To assess the mental health status of participants at baseline, we used the following scales: The Patient Health Questionnaire (PHQ-9)33 for measuring depression symptoms, Sheehan disability scale (SDS)37 for assessing functional impairment, and Generalized anxiety disorder (GAD-7) for anxiety. The enrollment system flagged any participant indicating suicidal ideation (non-zero response on the 9th item of the PHQ-9 survey). Such participants were suggested that this study may not be the best fit for them and that they should consider reaching out to their healthcare provider. We also provided links to online resources, including a 24-hour suicide help hotline, to receive immediate help.
All participants were evaluated for a history of mania, psychosis, and alcohol consumption using a four-item mania and psychosis screening instrument38 and the four-item National Institute on Alcohol Abuse and Alcoholism (NIAAA) Alcohol Screening Test. We also asked study participants about smart device ownership, use of health apps, and mental health services, including the use of medications and psychotherapy. Participants were asked to rate their health on a scale of excellent to poor. All baseline assessments were collected from eligible participants before interventions were provided, and all questions were optional for participants to answer. For further descriptions of the clinical assessments and other surveys administered in the V1 and V2 studies, please visit the Brighten study data portal (www.synapse.org/brighten).
Longitudinal data collection
The participants answered daily and weekly questions using the study app and continual passive data collection. The study app did not collect private information such as the content of text messages, emails, or voice calls.
Active tasks
Participants completed primary outcome assessments using PHQ-9 and SDS surveys once a week for the first four weeks and then every other week for the duration of the study (12 weeks). To assess participants’ mood and anhedonia (core symptoms of depression), we used the PHQ-2 survey administered daily in the morning. Participants also completed other secondary measures (described below) at daily, weekly, or biweekly intervals. These included sleep assessment with three questions - time taken to fall asleep, duration of sleep, and awake time during the night. Each question used a multiple-choice Likert scale of time (e.g., 0–15 minutes, 16–30 minutes, 31–60 minutes, etc.) to answer these sleep questions. Participants were automatically notified every 8 hours, for 24 hours, if they had not completed a survey within 8 hours of its original delivery. An assessment was considered missing if it was not completed within this 24-hour time frame.
Passive data collection
For the V1 study, we deployed an app in collaboration with Ginger.io that collected passive data. The application automatically launched the background data gathering processes once the user logged in. After the initial logon, the process was automatically launched whenever it was not already running (on phone restart or other events that terminate the process). The data collected through the device include communication metadata (e.g., time of call/SMS, call duration, SMS length) and mobile data such as activity type and distance traveled. The study app did not collect personal and identifiable communication data such as the actual text of SMS or the phone numbers for incoming and outgoing calls.
In the V2 study, we developed an in-house app named Survetory to help collect more fine-grained behavioral data. The passive data streams included a daily descriptive summary of phone-based communications (e.g., number of calls and texts) and intraday GPS-based locations. If location services (GPS) were available, the study app recorded the latitude and longitude every ten minutes or a movement more than 100 meters from the previously recorded spot. While the raw GPS data from participants cannot be shared broadly due to data privacy, we are sharing granular intraday level summaries of features derived from the raw GPS data. To featurize the raw GPS data, we developed an open-source pipeline, gSCAP-Geospatial Context Analysis Pipeline (https://github.com/aid4mh/gSCAP). Briefly, the gSCAP pipeline uses raw and sensitive individual-level GPS data to generate individual-level geospatial semantics that is not identifiable in the same way as GPS coordinates. Some examples of these contextual features include overall daily mobility (time and distance walking, biking, etc.), average daily weather (temperature, dew, cloud) at the participant’s location, and type of places visited in a day (coffee shop, park, shopping mall, etc.). However, the pipeline does not extract and store the location of the different places visited).
Further details about study design, participant recruitment, onboarding, and data collection are available in Brighten V129 and V27 papers, respectively.
Data storage and security
The initial raw data from each study were collected and stored on HIPAA-compliant servers located in the Department of Neurology at the University of California, San Francisco. The study servers were configured to UCSF Minimum Security Standards for Electronic Information Resources standards and policies.
Data Records
The data gathered from study participants during the 12-week observation period (as described above) can be accessed by qualified researchers39 via the study data portal (www.synapse.org/brighten). The study portal has detailed data descriptions for each active and passive data stream. The detailed steps for accessing the study data can be found on the study portal under the “Accessing the Brighten Study data” page and highlighted in the usage notes below.
Please refer to Table 1 for a record of surveys completed by the participants and Table 3 for passive behavioral data. Participants’ demographics and clinical characteristics at onboarding are summarized in Table 2.
Technical Validation
The two Brighten studies collected multimodal mental health and behavioral data in fully remote real-world settings of over two thousand participants and should be treated in that context. All shared data has been fully described on the study data portal as part of the data annotation and curation process. During the data cleaning and harmonization process, column names in the data files were aligned. The study portal also stores the provenance of upstream data cleaning and curation steps.
To preserve participants’ privacy and in consultation with data governance experts, we removed the self-reported zip code of study participants and the raw GPS data gathered in the V2 study. However, granular intraday GPS-derived features40 including the open-source pipeline gSCAP used to featurize the GPS data is freely available41. To optimize the parameters of the gSCAP pipeline for clustering GPS data, we took an iterative and visual approach to select parameters. Further details on pipeline parameters are available through the GitHub page (https://github.com/aid4mh/gSCAP).
Since both studies had monetary incentives for study participants to engage with the study apps over the 12 weeks, we paid particular attention to mitigating the possibility of individuals looking to take advantage of this study to acquire the research payment in several ways. First, the maximum compensation of $75 was spread over 12 weeks, reducing the motivation to “game the system.” Second, participants had to have both a valid email and phone number to successfully enroll in the study, reducing the ability to create multiple accounts. The study team also monitored for any duplicate records. Additionally, if an individual made multiple attempts to enroll using the same email address and each time did not properly answer the enrollment questions, the individual would not be enrolled. This prevents the individual from receiving access to any of the study tools or compensation. And finally, the link to download subject-specific study apps was only valid for a single user, as a unique password was required to view the download page.
Limitations
There is active ongoing research to efficiently develop and deploy large-scale, fully remote research studies to collect health data at scale. While the early evidence shows substantial potential42,43,44 for the use of digital health technology in healthcare settings, several challenges42,43,45 have also emerged, such as the underrepresentation of certain populations who may lack access to the technology or the technological literacy to use the apps. These issues will need to be addressed so that the collected health-related data is representative of the target population and remains balanced over time.
The Brighten data shows similar limitations. First, potential participants with iOS and Android phones could join the study. Participants with android devices were also required to have an iOS-based tablet to use one of the interventional apps. This additional requirement impacted the cohort’s diversity, particularly in the V2 study, where significantly more participants had an iPhone. Second, both studies recruited a population not representative of the general population. For example, close to three-fourths of the enrolled participants were females and are known to be twice as likely depressed as males46. Third, the V2 study tried to enrich the study cohort for Hispanics/Latinos to investigate the use of digital mental health tools within that target population. This led to significant differences in the underlying race/ethnicity between the two studies and should be factored in the downstream analysis. Fourth, despite both studies providing participation incentives, we saw significant participant attrition rates, especially in the V2 study that has been further discussed in our previous work7,32. And finally, the passive data gathered from smartphones was impacted by underlying system-level differences in iOS and Android operating systems, along with different apps used to collect passive data in V1 and V2 studies. We suggest any downstream analysis account for such underlying differences in real-world data collection.
Usage Notes
Brighten data have been donated by many thousands of research participants to advance our understanding of mental health. Due to the sensitive nature of the data, we implemented the following data access governance structure that respects the desire of participants to share their data while also protecting the confidentiality and privacy of research participants. Researchers interested in accessing these data need to complete the following steps:
-
1.
Become a Synapse Certified User with a validated user profile
-
2.
Submit a 1–3 paragraph Intended Data Use statement to be posted publicly on Synapse
-
3.
Agree to comply with the data-specific Conditions for Use when prompted.
These include a commitment to keep the data secure and confidential, not attempt to re-identify research participants for any reason, abide by the guiding principles for responsible research use and the Synapse Awareness and Ethics Pledge(to only use the data as intended, not use the data for any commercial advertisement), and commit to reporting any misuse or data release, intentional or inadvertent to the Synapse Access Compliance Team within five business days. In addition, users must agree to publish findings in open access publications and acknowledge the research participants as data contributors and the study investigators on all publications or presentations resulting from using these data as follows: “These data were contributed by the participants of the Brighten Studies, which were funded by the National Institute of Mental Health with scientific oversight from Pat Arean, Ph.D. and Joaquin A. Anguera, Ph.D., as described in Synapse: https://www.synapse.org/brighten. See the full instructions for requesting data access on the Accessing the Brighten Data page (https://www.synapse.org/brighten)
Code availability
The studies used a combination of open-source and proprietary apps to collect observational data and deploy remote interventions via three apps (described above). The source code for tools used for the data collection, such as the passive data collection app (Survetory - used in the V2 study), iPST, and HealthTips (two of the three randomized interventions), is available on the study Github archive (https://github.com/aid4mh/Brighten_Studies_Archive). The gSCAP pipeline used to featurize the raw GPS data from the V2 study is available here (https://github.com/aid4mh/gSCAP). The data cleaning and curation pipeline is also available on GitHub (https://github.com/apratap/BRIGHTEN-Data-Release).
References
Depression. at https://www.who.int/news-room/fact-sheets/detail/depression.
Scott, K. & Lewis, C. C. Using Measurement-Based Care to Enhance Any Treatment. Cogn. Behav. Pract. 22, 49–59 (2015).
Lewis, C. C. et al. Implementing Measurement-Based Care in Behavioral Health: A Review. JAMA Psychiatry 76, 324–335 (2019).
Cuijpers, P., Quero, S., Dowrick, C. & Arroll, B. Psychological Treatment of Depression in Primary Care: Recent Developments. Curr. Psychiatry Rep. 21, 129 (2019).
Alavi, N. et al. Feasibility and Efficacy of Delivering Cognitive Behavioral Therapy Through an Online Psychotherapy Tool for Depression: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 10, e27489 (2021).
Andrade, L. H. et al. Barriers to mental health treatment: results from the WHO World Mental Health surveys. Psychol. Med. 44, 1303–1317 (2014).
Pratap, A. et al. Using Mobile Apps to Assess and Treat Depression in Hispanic and Latino Populations: Fully Remote Randomized Clinical Trial. J. Med. Internet Res. 20, e10130 (2018).
Arevalo, M. et al. Mexican-American perspectives on participation in clinical trials: A qualitative study. Contemp Clin Trials Commun 4, 52–57 (2016).
Mental Health: Culture, Race, and Ethnicity: Executive Summary: a Supplement to Mental Health: a Report of the Surgeon General. (2001).
Cook, B. L., Trinh, N.-H., Li, Z., Hou, S. S.-Y. & Progovac, A. M. Trends in Racial-Ethnic Disparities in Access to Mental Health Care, 2004–2012. Psychiatr. Serv. 68, 9–16 (2017).
Thomeer, M. B., Moody, M. D. & Yahirun, J. Racial and Ethnic Disparities in Mental Health and Mental Health Care During The COVID-19 Pandemic. J Racial Ethn Health Disparities, https://doi.org/10.1007/s40615-022-01284-9 (2022).
Harris, K. M., Edlund, M. J. & Larson, S. Racial and ethnic differences in the mental health problems and use of mental health care. Med. Care 43, 775–784 (2005).
Wies, B., Landers, C. & Ienca, M. Digital Mental Health for Young People: A Scoping Review of Ethical Promises and Challenges. Front Digit Health 3, 697072 (2021).
Lattie, E. G., Stiles-Shields, C. & Graham, A. K. An overview of and recommendations for more accessible digital mental health services. Nature Reviews Psychology 1, 87–100 (2022).
Nordh, M. et al. Therapist-Guided Internet-Delivered Cognitive Behavioral Therapy vs Internet-Delivered Supportive Therapy for Children and Adolescents With Social Anxiety Disorder: A Randomized Clinical Trial. JAMA Psychiatry 78, 705–713 (2021).
Griffin, B. & Saunders, K. E. A. Smartphones and Wearables as a Method for Understanding Symptom Mechanisms. Front. Psychiatry 10, 949 (2019).
Jain, S. H., Powers, B. W., Hawkins, J. B. & Brownstein, J. S. The digital phenotype. Nat. Biotechnol. 33, 462–463 (2015).
Moshe, I. et al. Digital interventions for the treatment of depression: A meta-analytic review. Psychol. Bull. 147, 749–786 (2021).
Larsen, M. E. et al. Using science to sell apps: Evaluation of mental health app store quality claims. NPJ Digit Med 2, 18 (2019).
Goldberg, S. B., Lam, S. U., Simonsson, O., Torous, J. & Sun, S. Mobile phone-based interventions for mental health: A systematic meta-review of 14 meta-analyses of randomized controlled trials. PLOS Digit Health 1 (2022).
Seabrook, E. M. & Nedeljkovic, M. The evolving landscape of digital mental health: implications for research and practice. Clin. Psychol. 25, 121–123 (2021).
Huckvale, K., Torous, J. & Larsen, M. E. Assessment of the Data Sharing and Privacy Practices of Smartphone Apps for Depression and Smoking Cessation. JAMA Netw Open 2, e192542 (2019).
Lustgarten, S. D., Garrison, Y. L., Sinnard, M. T. & Flynn, A. W. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol 36, 25–31 (2020).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3, 160018 (2016).
De Angel, V. et al. Digital health tools for the passive monitoring of depression: a systematic review of methods. NPJ Digit Med 5, 3 (2022).
Freedman, L. P., Cockburn, I. M. & Simcoe, T. S. The Economics of Reproducibility in Preclinical Research. PLoS Biol. 13, e1002165 (2015).
Stupple, A., Singerman, D. & Celi, L. A. The reproducibility crisis in the age of digital medicine. NPJ Digit Med 2, 2 (2019).
Kozlov, M. NIH issues a seismic mandate: share data publicly. Nature 602, 558–559 (2022).
Anguera, J. A., Jordan, J. T., Castaneda, D., Gazzaley, A. & Areán, P. A. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innov 2, 14–21 (2016).
Arean, P. A. et al. The Use and Effectiveness of Mobile Apps for Depression: Results From a Fully Remote Clinical Trial. J. Med. Internet Res. 18, e330 (2016).
Pratap, A. et al. The accuracy of passive phone sensors in predicting daily mood. Depress. Anxiety 36, 72–81 (2019).
Pratap, A. et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med 3, 21 (2020).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Mynors-Wallis, L. M., Gath, D. H., Day, A. & Baker, F. Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. BMJ 320, 26–30 (2000).
Arean, P., Hegel, M., Vannoy, S., Fan, M.-Y. & Unuzter, J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist 48, 311–323 (2008).
Anguera, J. A., Gunning, F. M. & Areán, P. A. Improving late life depression and cognitive control through the use of therapeutic video game technology: A proof-of-concept randomized trial. Depress. Anxiety 34, 508–517 (2017).
Sheehan, D. V., Harnett-Sheehan, K. & Raj, B. A. The measurement of disability. Int. Clin. Psychopharmacol. 11(Suppl 3), 89–95 (1996).
Unützer, J. et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med. Care 39, 785–799 (2001).
Grayson, S., Suver, C., Wilbanks, J. & Doerr, M. Open Data Sharing in the 21st Century: Sage Bionetworks’ Qualified Research Program and Its Application in mHealth Data Release, https://doi.org/10.2139/ssrn.3502410 (2019).
Bionetworks, S. Synapse. at https://www.synapse.org/#!Synapse:syn10848316/wiki/588047.
aid4mh. GitHub - aid4mh/gSCAP: Create contextual geospatial features from longitudinal geolocation data. GitHub at https://github.com/aid4mh/gSCAP.
Omberg, L. et al. Remote smartphone monitoring of Parkinson’s disease and individual response to therapy. Nat. Biotechnol. https://doi.org/10.1038/s41587-021-00974-9 (2021).
Pratap, A. et al. Evaluating the Utility of Smartphone-Based Sensor Assessments in Persons With Multiple Sclerosis in the Real-World Using an App (elevateMS): Observational, Prospective Pilot Digital Health Study. JMIR Mhealth Uhealth 8, e22108 (2020).
Onnela, J.-P. & Rauch, S. L. Harnessing Smartphone-Based Digital Phenotyping to Enhance Behavioral and Mental Health. Neuropsychopharmacology 41, 1691–1696 (2016).
Omberg, L., Chaibub Neto, E. & Mangravite, L. M. Data Science Approaches for Effective Use of Mobile Device-Based Collection of Real-World Data. Clin. Pharmacol. Ther. 107, 719–721 (2020).
Albert, P. R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 40, 219 (2015).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn27082597 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn27082811 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17022655 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17021280 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn27051276 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn27202355 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17022658 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17023313 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17022659 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17022660 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17025058 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17025202 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17020855 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17025500 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17116695 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17114662 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17060502 (2021).
Pratap, A. et al. Synapse https://doi.org/10.7303/syn17061284 (2021).
Acknowledgements
Support for this research was provided by the National Institute of Mental Health (PAA R34MH100466, T32MH0182607, K24MH074717). The authors would also like to thank all the study participants for their time and willingness to share their data. We also acknowledge the contributions of all our industry partners, namely Ginger.IO and Akili Interactive. We would also like to thank Wow Labz for helping build the data collection app for the V2 study.
Author information
Authors and Affiliations
Contributions
P.A. and J.A.A. obtained funding from NIMH and provided overall oversight, including the scientific design of Brighten V1 and V2 studies. AP wrote the first manuscript draft. L.W., A.H. and P.A. made significant contributions in editing the manuscript. J.V. and J.A.A. were involved in the data collection. A.P., L.W. performed the data cleaning, curation, and creation of the study data portal. L.W., A.P. and C.H. developed the gSCAP pipeline used for featurizing the raw GPS data. C.S. Developed the data governance process for sharing the dataset with the broader research community. All authors provided editorial feedback and contributed to the final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pratap, A., Homiar, A., Waninger, L. et al. Real-world behavioral dataset from two fully remote smartphone-based randomized clinical trials for depression. Sci Data 9, 522 (2022). https://doi.org/10.1038/s41597-022-01633-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01633-7
This article is cited by
-
Personalized mood prediction from patterns of behavior collected with smartphones
npj Digital Medicine (2024)
-
Digital and precision clinical trials: innovations for testing mental health medications, devices, and psychosocial treatments
Neuropsychopharmacology (2024)