Abstract
Speech production is an intricate process involving a large number of muscles and cognitive processes. The neural processes underlying speech production are not completely understood. As speech is a uniquely human ability, it can not be investigated in animal models. High-fidelity human data can only be obtained in clinical settings and is therefore not easily available to all researchers. Here, we provide a dataset of 10 participants reading out individual words while we measured intracranial EEG from a total of 1103 electrodes. The data, with its high temporal resolution and coverage of a large variety of cortical and sub-cortical brain regions, can help in understanding the speech production process better. Simultaneously, the data can be used to test speech decoding and synthesis approaches from neural data to develop speech Brain-Computer Interfaces and speech neuroprostheses.
Measurement(s) | Brain activity |
Technology Type(s) | Stereotactic electroencephalography |
Sample Characteristic - Organism | Homo sapiens |
Sample Characteristic - Environment | Epilepsy monitoring center |
Sample Characteristic - Location | The Netherlands |
Similar content being viewed by others
Background & Summary
Brain-Computer Interfaces (BCIs)1 that directly decode speech from neural activity have recently gained large attention as they could provide an intuitive means of communication for patients who lost the ability to speak2,3,4,5,6,7.
The creation of a speech neuroprosthesis depends on a firm understanding of the speech production process in the brain, the particular timing of brain regions involved and where to best decode them. Despite a number of existing models on the speech production process8,9, the precise role of all areas involved has yet to be understood. Recent advances highlight that deeper brain structures, such as the hippocampus10,11,12 and thalamus13,14, are also involved in language in general and speech production specifically. A dataset providing accessible data for a simple speech production task in cortical and deeper brain structures could help to further understand this intricate process. This understanding may additionally aid functional language mapping prior to resective surgery in patients suffering from pharmaco-resistant epilepsy15.
Despite the fact that a full understanding of speech production is currently lacking, great advances have been made in the field of speech neuroprostheses recently. The decoding of a textual representation by decoding phonemes16,17, phonetic18 or articulatory19 features, words20, full sentences21,22,23,24 or spotting of speech keywords25 is possible from neural recordings during actual speech production. Results are becoming robust enough for first trials in speech impaired patients7. To facilitate more natural communication, some studies aimed at directly synthesizing an audio waveform of speech from neural data recorded during speech production26,27,28,29. Initial results indicate that the decoding of speech processes is possible from imagined speech production from offline data30,31,32 and in real-time33,34.
Most of these recent advances employ electrocorticography (ECoG), an invasive recording modality of neural activity that provides high temporal and spatial resolution and high signal-to-noise ratio35. Additionally, ECoG is less affected by movement artifacts than non-invasive measures of neural activity. Other studies have used intracortical microarrays to decode speech36,37,38,39 or a neurotrophic electrode40 to synthesize formant frequencies41,42 from the motor cortex. An alternative measure of intracranial neural activity is stereotactic EEG (sEEG), in which electrode shafts are implanted into the brain through small burr holes43. sEEG is considered to be minimally invasive, as a large craniotomy is not necessary and the infection risk is therefore smaller44. Additionally, the method of implanting the electrodes is very similar to that used in Deep Brain Stimulation (DBS), a method that has been used in the treatment of Parkinson’s Disease for several decades. In DBS, electrodes routinely remain implanted for many years, giving hope for the potential of sEEG for long-term BCIs.
Similar to ECoG, sEEG is used in the monitoring of epilogenic zones in the treatment of refractory epilepsy. Between 5 and 15 electrode shafts are typically implanted covering a large variety of cortical and sub-cortical brain areas. Here lies one of the main differences to ECoG: instead of high density coverage of specific regions, sEEG provides sparse sampling of multiple regions. This sparse sampling could provide great potential for various BCI applications as most of them involve processes in deep (sub-cortical or within sulci) and spatially disparate, bilateral, brain regions45. For example, besides the primary motor cortex, movement can also be decoded well from the basal ganglia46 and supramarginal gyrus47, amongst others. With sEEG, these areas can be recorded simultaneously, leveraging multiple sources of potential information.
Invasive recordings of neural activity are usually obtained during a seizure localization procedure or glioma resection surgery and are therefore not available to many researchers working on traditional speech decoding or speech synthesis technologies. These researchers are part of an active research community investigating the potential of non-invasive brain measurement technologies for speech neuroprostheses. Techniques include scalp-electroencephalography (EEG)48,49,50,51,52,53,54, providing high temporal resolution, especially the Kara One database55 provides the foundation for many studies; Magnetoencephalography56,57, providing more localized information than EEG due to a larger amount of sensors; and Functional Near Infrared Spectroscopy58,59,60,61, providing localized information of cortical hemoglobin levels. The advances made by this community could also benefit invasive speech neuroprostheses, a dataset that is provided to everyone could be used to evaluate and leverage their approaches.
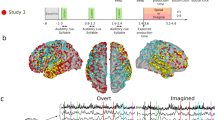
To facilitate an increased understanding of the speech production process in the brain, including deeper brain structures, and to accelerate the development of speech neuroprostheses, we provide this dataset of 10 participants speaking prompted words aloud while audio and intracranial EEG data are recorded simultaneously (Fig. 1).
Methods
Participants
A total of 10 participants suffering from pharmaco-resistant epilepsy participated in our experiment (mean age 32 years (range 16–50 years); 5 male, 5 female). Participants were implanted with sEEG electrodes (Table 1) as part of the clinical therapy for their epilepsy. Electrode locations were purely determined based on clinical necessity. All participants joined the study on a voluntary basis and gave written informed consent. Experiment design and data recording were approved by the Institutional Review Boards of both Maastricht Unviversity and Epilepsy Center Kempenhaeghe. Data recording was conducted under the supervision of experienced healthcare staff. All participants were native speakers of Dutch. Participants’ voices were pitch-shifted with a randomized offset between 1 and 3 semitones up or down that was constant over the entire recording to ensure anonymity.
Experimental design
In this study, participants were asked to read aloud words that were shown to them on a laptop screen (Fig. 1). One random word from the stimulus library (the Dutch IFA corpus62 extended with the numbers one to ten in word form) was presented on the screen for a duration of 2 seconds during which the participant read the word aloud once. This relatively large window accounts for differences in word length and pronunciation speed. After the word, a fixation cross was displayed for 1 second. This was repeated for a total of 100 words, resulting in a total recording time of 300 seconds for each participant. The presented stimuli and timings were saved for later processing, hereafter referred to as stimulus data.
Data acquisition
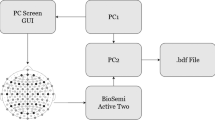
Participants were implanted with platinum-iridium sEEG electrode shafts (Microdeep intracerebral electrodes; Dixi Medical, Beçanson, France) with a diameter of 0.8 mm, a contact length of 2 mm and a inter-contact distance of 1.5 mm. Each electrode shaft contained between 5 and 18 electrode contacts.
Neural data was recorded using two or more Micromed SD LTM amplifier(s) (Micromed S.p.A., Treviso, Italy) with 64 channels each. Electrode contacts were referenced to a common white matter contact. Data were recorded at either 1024 Hz or 2048 Hz and subsequently downsampled to 1024 Hz. We used the onboard microphone of the recording notebook (HP Probook) to record audio at 48 kHz. Audio data was subsequently pitch-shifted to ensure our participants’ anonymity using LibRosa63. We used LabStreamingLayer64 to synchronize the neural, audio and stimulus data.
Anatomical labeling
Electrode locations (Fig. 2) were detected using the img_pipe Python package65 for anatomical labeling of intracranial electrodes. Within the package, for each participant, a pre-implantation anatomical T1-weighted Magnetic Resonance Imaging (MRI) scan was parcellated using Freesurfer (http://surfer.nmr.mgh.harvard.edu/), a post-implantation Computer Tomography (CT) scan was co-registered to the MRI scan and electrode contacts were manually localized. The anatomical location label of each contact was automatically extracted from the Destrieux atlas66 based parcellation.
By far the most electrodes are located in white matter (40.3%) and unknown areas (12.6%). Unknown areas are contacts that were not able to be labelled through the Freesurfer parcellation, for example a contact located just outside of the cortex. Thereafter, electrodes are predominantly located in the superior temporal sulcus, hippocampus and the inferior parietal gyrus. See Fig. 3 for a full breakdown of anatomical regions and the number of electrodes implanted in those areas.
Data Records
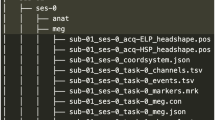
The SingleWordProductionDutch-iBIDS dataset67 is available at https://doi.org/10.17605/OSF.IO/NRGX6. The raw data files (XDF format) were converted to Neurodata Without Borders (NWB; https://www.nwb.org/) format and organised in the iBIDS68 data structure format using custom Python scripts. The NWB format allows for compact storage of multiple data streams within a single file. It is compatible to the iBIDS structure, a community-driven effort to improve the transparency, reusibility and reproducibility of iEEG data.
The data is structured following the BIDS version 1.7.0 specification (https://bids-specification.readthedocs.io/en/stable/). The root folder contains metadata of the participants (participants.tsv), subject specific data folders (i.e., sub-01) and a derivatives folder. The subject specific folders contain .tsv files with information about the implanted electrode coordinates (_electrodes.tsv), recording montage (_channels.tsv) and event markers (_events.tsv). The _ieeg.nwb file contains three raw data streams as timeseries (iEEG, Audio and Stimulus), which are located in the acquisition container. Descriptions of recording aspects and of specific .tsv columns are provided in correspondingly named .json files (i.e., participants.json). The derivatives folder contains the pial surface cortical meshes of the right (_rh_pial.mat) and left (_lh_pial.mat) hemisphere, the brain anatomy (_brain.mgz), the Destrieux atlas (_aparc.a2009s + aseg.mgz) and a white matter atlas (_wmparc.mgz) per subject, derived from the Freesurfer pipeline. The description column in the _channels.tsv file refers to the anatomical labels derived from the Destrieux atlas66.
The iBIDS dataset passed a validation check using the BIDS Validator (https://bids-standard.github.io/bids-validator/) and manual inspection of each datafile.
Technical Validation
We validate the recorded data by demonstrating that a spectral representation of speech can be reconstructed from the neural recordings using a simple linear regression model. This analysis is similar to a previous analysis in ECoG69.
Checking for acoustic contamination
Acoustic contamination of neural recordings has been reported by Roussel et al.70. To check the presented dataset for acoustic contamination in the neural timeseries, we apply the method provided by the authors and correlate spectral energy between audio and neural data. We do not find any significant correlations (p > 0.01) on the diagonal of the contamination matrix for any of the participants. The risk for falsely rejecting the hypothesis of no contamination is therefore smaller than 1%.
Feature extraction
We extract the Hilbert envelope of the broadband high-frequency activity (70–170 Hz) for each contact using an IIR bandpass filter (filter order 4). To attenuate the first two harmonics of the 50 Hz line noise, we used two IIR bandstop filters (filter order 4). All filters were applied forward and backward so that no phase-shift is introduced. We averaged the envelope over 50 ms windows with a frameshift of 10 ms. To include temporal information into the decoding process, non-overlapping neighboring windows up to 200 ms into the past and future were stacked. Features are normalized to zero mean and unit variance using the mean and standard deviation of the training data. The same transform is then applied to the evaluation data.
The audio data is first downsampled to 16 kHz. To extract features for the audio data, we subsequently calculated the Short-Term-Fourier-Transform in windows of 50 ms with an frameshift of 10 ms. As the frameshift between neural and audio data is the same, there is a correspondence between audio and neural feature vectors. The resulting spectrogram is then compressed into a log-mel representation71 using 23 triangular filter banks.
Decoding model
To reduce the dimensionality of our decoding problem, we compress the feature space to the first 50 principal components. Principal components are estimated for each fold individually on the training data. The first 50 principal components explain between 29% and 76% of the variance depending on the participant.
We reconstruct the log-mel spectrogram from the high-frequency features using linear regression models. In these models, the high-frequency feature vector is multiplied with a weight matrix to reconstruct the log-mel spectrogram. The weights are determined using a least-squares approach.
As a baseline, we chose 1000 random split points, at least 10% distant from the beginning and end of the data, and swapped the audio spectrogram on this split point. In this procedure, which is also called random circular shift, the temporal structure and auto-regressive properties of speech are maintained. We then correlated these spectrograms with the original spectrogram to estimate a distribution of chance correlation coefficients.
Waveform reconstruction
The log-mel spectrogram does not contain the phase information anymore and an audio waveform can thus not be reconstructed directly. We utilize the method by Griffin and Lim72 for waveform reconstruction, in which the phase is initialized with noise and then iteratively modified. For a good algorithmic discription of the method, see73.
Results
All results are obtained in a non-shuffled 10-fold cross validation in which 9 folds are used for training and the remaining fold is used for evaluation. This process is repeated until each fold has been used for evaluation exactly once.
Spectrograms can be reconstructed
We evaluate the spectral reconstructions in terms of Pearson correlation coefficient between the spectral coefficients of the spectrogram of the original speech and the reconstructed spectrogram. For all 10 participants, speech spectrograms can be reconstructed from the neural data using linear regression (Fig. 4a) with higher correlations than all 1000 randomizations. Reconstruction results were consistent across all 23 mel-scaled spectral coefficients (Fig. 4b) and consistently above all randomization in all frequency ranges. Inspecting the spectrograms further (Fig. 5a), it can be seen that the results are mostly driven by the accurate reconstruction of speech versus silence. The spectral variations within speech are not captured by the linear regression approach. The Pearson correlation is not a perfect evaluation metric as this lack of detail during speech does not have a large impact on the score. We utilize the Pearson correlation here as a better metric has yet to be identified. By providing this open dataset, we hope that researchers developing more advanced metrics, such as the Spectro-Temporal Glimpsing Index (STGI)74 or the extended Short-Time Objective Intelligibility (eSTOI)75, will have the means to address this problem. Similarly, we hope that the dataset will be useful for developing and evaluating models that improve the quality of the reconstructed speech, such as those that are more informed about speech processes (e.g. Unit Selection29) or neural network approaches with enough trainable parameters to produce high-quality speech26,27,76,77,78.
Results for the spectral reconstruction. (a) Mean correlation coefficients for each participant across all spectral bins and folds. Reconstruction of the spectrogram is possible for all 10 participants. Whiskers indicate standard deviations. Results of individual folds are illustrated by points. (b) Mean correlation coefficients for each spectral bin. Correlations are stable across all spectral bins. Shaded areas show standard errors.
Waveforms of speech can be reconstructed
Using the method by Griffin and Lim72, we can recreate an audio waveform from the reconstructed spectrograms (Fig. 5b). The timing between original and reconstructed waveforms is very similar, but listening to the audio shows that a substantial part of the audio quality is lost due to the synthesis approach. This is particularly clear when listening to the audio recreated from the original spectrogams (_orig_synthesized.wav). State-of-the-art synthesis approaches, such as WaveGlow79 or WaveNet80, may be applied to the current dataset to evaluate an improvement in the reconstruction quality.
Usage Notes
iBIDS data
Scripts to handle the data can be obtained from our Github repository: https://github.com/neuralinterfacinglab/SingleWordProductionDutch.
Data loading and feature extraction
Neural traces, synchronized audio and experimental markers can be loaded using the provided extract_features.py script. High-frequency features are subsequently extracted and aligned to logarithmic mel-scaled spectrograms. Electrode channel names are also loaded.
Spectrogram reconstruction & waveform synthesis
Reconstruction of the spectrogram as well as the resynthesis to an audio waveform is performed in the reconstruction_minimal.py script.
Anatomical data
Electrode locations can be found in the participant folder (_electrodes.tsv) and then be visualized using the cortical meshes (_lh_pial.mat and _rh_pial.mat) within the derivatives folder. These mesh files contain vertices coordinates and triangles, which are described by indices corresponding to vertex numbers. The T1-weighted brain anatomy, the Destrieux parcellation and a white matter parcellation can also be found in the derivatives folder.
References
Wolpaw, J., Birbaumer, N., McFarland, D., Pfurtscheller, G. & Vaughan, T. Brain–computer interfaces for communication and control. Clinical neurophysiology 113, 767–791 (2002).
Rabbani, Q., Milsap, G. & Crone, N. E. The potential for a speech brain–computer interface using chronic electrocorticography. Neurotherapeutics 16, 144–165 (2019).
Schultz, T. et al. Biosignal-based spoken communication: A survey. IEEE/ACM Transactions on Audio, Speech and Language Processing 25, 2257–2271, https://doi.org/10.1109/TASLP.2017.2752365 (2017).
Chakrabarti, S., Sandberg, H. M., Brumberg, J. S. & Krusienski, D. J. Progress in speech decoding from the electrocorticogram. Biomedical Engineering Letters 5, 10–21 (2015).
Herff, C. & Schultz, T. Automatic speech recognition from neural signals: a focused review. Frontiers in neuroscience 10 (2016).
Bocquelet, F., Hueber, T., Girin, L., Chabardès, S. & Yvert, B. Key considerations in designing a speech brain-computer interface. Journal of Physiology-Paris 110, 392–401 (2016).
Moses, D. A. et al. Neuroprosthesis for decoding speech in a paralyzed person with anarthria. New England Journal of Medicine 385, 217–227 (2021).
Tourville, J. A. & Guenther, F. H. The diva model: A neural theory of speech acquisition and production. Language and cognitive processes 26, 952–981 (2011).
Hickok, G. Computational neuroanatomy of speech production. Nature Reviews Neuroscience 13, 135 (2012).
Piai, V. et al. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proceedings of the National Academy of Sciences 113, 11366–11371 (2016).
Duff, M. C. & Brown-Schmidt, S. The hippocampus and the flexible use and processing of language. Frontiers in human neuroscience 6, 69 (2012).
Covington, N. V. & Duff, M. C. Expanding the language network: Direct contributions from the hippocampus. Trends in Cognitive Sciences 20, 869–870 (2016).
Hebb, A. O. & Ojemann, G. A. The thalamus and language revisited. Brain and Language 126, 99–108 (2013).
Klostermann, F. & Ehlen, F. Functional roles of the thalamus for language capacities. Frontiers in systems neuroscience 7, 32 (2013).
Brunner, P. et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy & Behavior 15, 278–286 (2009).
Mugler, E. M. et al. Direct classification of all american english phonemes using signals from functional speech motor cortex. Journal of neural engineering 11, 035015 (2014).
Ramsey, N. F. et al. Decoding spoken phonemes from sensorimotor cortex with high-density ECoG grids. Neuroimage 180, 301–311 (2018).
Lotte, F. et al. Electrocorticographic representations of segmental features in continuous speech. Frontiers in human neuroscience 9 (2015).
Mugler, E. M. et al. Differential representation of articulatory gestures and phonemes in precentral and inferior frontal gyri. Journal of Neuroscience 38, 9803–9813 (2018).
Kellis, S. et al. Decoding spoken words using local field potentials recorded from the cortical surface. Journal of Neural Engineering 7 (2010).
Herff, C. et al. Brain-to-text: decoding spoken phrases from phone representations in the brain. Frontiers in neuroscience 9 (2015).
Moses, D. A., Mesgarani, N., Leonard, M. K. & Chang, E. F. Neural speech recognition: continuous phoneme decoding using spatiotemporal representations of human cortical activity. Journal of neural engineering 13, 056004 (2016).
Moses, D. A., Leonard, M. K. & Chang, E. F. Real-time classification of auditory sentences using evoked cortical activity in humans. Journal of neural engineering 15, 036005 (2018).
Makin, J. G., Moses, D. A. & Chang, E. F. Machine translation of cortical activity to text with an encoder–decoder framework. Tech. Rep., Nature Publishing Group (2020).
Milsap, G. et al. Keyword spotting using human electrocorticographic recordings. Frontiers in neuroscience 13, 60 (2019).
Anumanchipalli, G. K., Chartier, J. & Chang, E. F. Speech synthesis from neural decoding of spoken sentences. Nature 568, 493–498, https://doi.org/10.1038/s41586-019-1119-1 (2019).
Angrick, M. et al. Speech synthesis from ECoG using densely connected 3D convolutional neural networks. Journal of neural engineering (2019).
Wang, R., Wang, Y. & Flinker, A. Reconstructing speech stimuli from human auditory cortex activity using a wavenet approach. In 2018 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), 1–6 (IEEE, 2018).
Herff, C. et al. Generating natural, intelligible speech from brain activity in motor, premotor, and inferior frontal cortices. Frontiers in Neuroscience 13, 1267 (2019).
Martin, S. et al. Decoding spectrotemporal features of overt and covert speech from the human cortex. Frontiers in neuroengineering 7 (2014).
Martin, S. et al. Word pair classification during imagined speech using direct brain recordings. Scientific reports 6, 25803 (2016).
Proix, T. et al. Imagined speech can be decoded from low-and cross-frequency intracranial EEG features. Nature communications 13, 1–14 (2022).
Angrick, M. et al. Real-time synthesis of imagined speech processes from minimally invasive recordings of neural activity. Communications biology 4, 1–10 (2021).
Meng, K. et al. Implementation of a closed-loop BCI system for real-time speech synthesis under clinical constraints. In 2022 10th International Winter Conference on Brain-Computer Interface (BCI), 1–6 (IEEE, 2022).
Parvizi, J. & Kastner, S. Promises and limitations of human intracranial electroencephalography. Nature neuroscience 21, 474–483 (2018).
Stavisky, S. D. et al. Decoding speech from intracortical multielectrode arrays in dorsal “arm/hand areas” of human motor cortex. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 93–97 (IEEE, 2018).
Stavisky, S. D. et al. Neural ensemble dynamics in dorsal motor cortex during speech in people with paralysis. Elife 8, e46015 (2019).
Stavisky, S. D. et al. Speech-related dorsal motor cortex activity does not interfere with iBCI cursor control. Journal of Neural Engineering 17, 016049 (2020).
Wilson, G. H. et al. Decoding spoken english phonemes from intracortical electrode arrays in dorsal precentral gyrus. bioRxiv (2020).
Bartels, J. et al. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. Journal of neuroscience methods 174, 168–176 (2008).
Brumberg, J., Wright, E., Andreasen, D., Guenther, F. & Kennedy, P. Classification of intended phoneme production from chronic intracortical microelectrode recordings in speech motor cortex. Frontiers in Neuroscience 5, 65, https://doi.org/10.3389/fnins.2011.00065 (2011).
Guenther, F. H. et al. A wireless brain-machine interface for real-time speech synthesis. PloS one 4, e8218 (2009).
van der Loo, L. E. et al. Methodology, outcome, safety and in vivo accuracy in traditional frame-based stereoelectroencephalography. Acta neurochirurgica 159, 1733–1746 (2017).
Iida, K. & Otsubo, H. Stereoelectroencephalography: indication and efficacy. Neurologia medico-chirurgica 57, 375–385 (2017).
Herff, C., Krusienski, D. J. & Kubben, P. The potential of stereotactic-EEG for brain-computer interfaces: Current progress and future directions. Frontiers in Neuroscience 14, 123 (2020).
Mamun, K. et al. Movement decoding using neural synchronization and inter-hemispheric connectivity from deep brain local field potentials. Journal of neural engineering 12, 056011 (2015).
Wandelt, S. K. et al. Decoding grasp and speech signals from the cortical grasp circuit in a tetraplegic human. Neuron 110, 1777–1787 (2022).
Wang, L., Zhang, X., Zhong, X. & Zhang, Y. Analysis and classification of speech imagery EEG for BCI. Biomedical signal processing and control 8, 901–908 (2013).
Sereshkeh, A. R., Trott, R., Bricout, A. & Chau, T. Online EEG classification of covert speech for brain–computer interfacing. International journal of neural systems 27, 1750033 (2017).
Garca-Salinas, J. S., Villaseñor-Pineda, L., Reyes-Garca, C. A. & Torres-Garca, A. A. Transfer learning in imagined speech EEG-based BCIs. Biomedical Signal Processing and Control 50, 151–157 (2019).
Cooney, C., Folli, R. & Coyle, D. Mel frequency cepstral coefficients enhance imagined speech decoding accuracy from EEG. In 2018 29th Irish Signals and Systems Conference (ISSC), 1–7 (IEEE, 2018).
Krishna, G., Tran, C., Carnahan, M. & Tewfik, A. Advancing speech recognition with no speech or with noisy speech. In 2019 27th European Signal Processing Conference (EUSIPCO), 1–5 (IEEE, 2019).
Sharon, R. A., Narayanan, S. S., Sur, M. & Murthy, A. H. Neural speech decoding during audition, imagination and production. IEEE Access 8, 149714–149729 (2020).
Islam, M. M. & Shuvo, M. M. H. DenseNet based speech imagery EEG signal classification using Gramian Angular Field. In 2019 5th International Conference on Advances in Electrical Engineering (ICAEE), 149–154, https://doi.org/10.1109/ICAEE48663.2019.8975572 (2019).
Zhao, S. & Rudzicz, F. Classifying phonological categories in imagined and articulated speech. In 2015 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), 992–996 (IEEE, 2015).
Wang, J., Kim, M., Hernandez-Mulero, A. W., Heitzman, D. & Ferrari, P. Towards decoding speech production from single-trial magnetoencephalography (MEG) signals. In 2017 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), 3036–3040 (IEEE, 2017).
Dash, D., Ferrari, P. & Wang, J. Decoding imagined and spoken phrases from non-invasive neural (MEG) signals. Frontiers in Neuroscience (2020).
Sereshkeh, A. R., Yousefi, R., Wong, A. T. & Chau, T. Online classification of imagined speech using functional near-infrared spectroscopy signals. Journal of neural engineering 16, 016005 (2018).
Herff, C., Heger, D., Putze, F., Guan, C. & Schultz, T. Cross-subject classification of speaking modes using fNIRS. In International Conference on Neural Information Processing, 417–424 (Springer, 2012).
Liu, Y. & Ayaz, H. Speech recognition via fNIRS based brain signals. Frontiers in neuroscience 12, 695 (2018).
Rezazadeh Sereshkeh, A., Yousefi, R., Wong, A. T., Rudzicz, F. & Chau, T. Development of a ternary hybrid fNIRS-EEG brain–computer interface based on imagined speech. Brain-Computer Interfaces 6, 128–140 (2019).
Van Son, R. J., Binnenpoorte, D., Heuvel, H. v. d. & Pols, L. The IFA corpus: a phonemically segmented dutch ”open source” speech database. In 7th European Conference on Speech Communication and Technology (Aalborg, Denmark, 2001).
McFee, B. et al. librosa: Audio and music signal analysis in python. In Proceedings of the 14th python in science conference, vol. 8, 18–25 (Citeseer, 2015).
Kothe, C. Lab streaming layer (LSL). https://github.com/sccn/labstreaminglayer26, 2015 (2014).
Hamilton, L. S., Chang, D. L., Lee, M. B. & Chang, E. F. Semi-automated anatomical labeling and inter-subject warping of high-density intracranial recording electrodes in electrocorticography. Frontiers in Neuroinformatics 11, 62 (2017).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1–15, https://doi.org/10.1016/j.neuroimage.2010.06.010 (2010).
Herff, C. & Verwoert, M. Dataset of Speech Production in intracranial Electroencephalography, Open Science Framework, https://doi.org/10.17605/OSF.IO/NRGX6 (2022).
Holdgraf, C. et al. iEEG-BIDS, extending the brain imaging data structure specification to human intracranial electrophysiology. Scientific data 6, 1–6 (2019).
Herff, C. et al. Towards direct speech synthesis from ECoG: A pilot study. In Engineering in Medicine and Biology Society (EMBC), 2016 IEEE 38th Annual International Conference of the, 1540–1543 (IEEE, 2016).
Roussel, P. et al. Observation and assessment of acoustic contamination of electrophysiological brain signals during speech production and sound perception. Journal of Neural Engineering 17, 056028 (2020).
Stevens, S. S., Volkmann, J. & Newman, E. B. A scale for the measurement of the psychological magnitude pitch. The Journal of the Acoustical Society of America 8, 185–190 (1937).
Griffin, D. & Lim, J. Signal estimation from modified short-time fourier transform. IEEE Transactions on acoustics, speech, and signal processing 32, 236–243 (1984).
Bayram, I. An analytic wavelet transform with a flexible time-frequency covering. IEEE Transactions on Signal Processing 61, 1131–1142 (2012).
Edraki, A., Chan, W. Y., Jensen, J. & Fogerty, D. A spectro-temporal glimpsing index (STGI) for speech intelligibility prediction. In 22nd Annual Conference of the International Speech Communication Association, INTERSPEECH 2021, 2738–2742 (International Speech Communication Association, 2021).
Jensen, J. & Taal, C. H. An algorithm for predicting the intelligibility of speech masked by modulated noise maskers. IEEE/ACM Transactions on. Audio, Speech, and Language Processing 24, 2009–2022 (2016).
Angrick, M. et al. Speech synthesis from stereotactic EEG using an electrode shaft dependent multi-input convolutional neural network approach. In 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 6045–6048 (IEEE, 2021).
Kohler, J. et al. Synthesizing speech from intracranial depth electrodes using an encoder-decoder framework. arXiv preprint arXiv:2111.01457 (2021).
Wang, R. et al. Distributed feedforward and feedback processing across perisylvian cortex supports human speech. bioRxiv (2021).
Prenger, R., Valle, R. & Catanzaro, B. Waveglow: A flow-based generative network for speech synthesis. In ICASSP 2019-2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), 3617–3621 (IEEE, 2019).
Van Den Oord, A. et al. Wavenet: A generative model for raw audio. arXiv preprint arXiv:1609.03499 (2016).
Harris, C. R. et al. Array programming with numpy. Nature 585, 357–362 (2020).
Virtanen, P. et al. Scipy 1.0: fundamental algorithms for scientific computing in python. Nature methods 17, 261–272 (2020).
Teeters, J. L. et al. Neurodata without borders: creating a common data format for neurophysiology. Neuron 88, 629–634 (2015).
Pedregosa, F. et al. Scikit-learn: Machine learning in python. the Journal of machine Learning research 12, 2825–2830 (2011).
McKinney, W. et al. Pandas: a foundational python library for data analysis and statistics. Python for high performance and scientific computing 14, 1–9 (2011).
Acknowledgements
CH acknowledges funding by the Dutch Research Council (NWO) for the project ‘DEcoding Speech In SEEG (DESIS)’.
Author information
Authors and Affiliations
Contributions
C.H., P.L.K., A.J.C., L.W. and S.T. designed the experiment. M.V., M.C.O. and C.H. recorded the data. M.V. and S.G. localized the electrodes. M.V. and C.H. analyzed the data and wrote the manuscript. All authors approve of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verwoert, M., Ottenhoff, M.C., Goulis, S. et al. Dataset of Speech Production in intracranial Electroencephalography. Sci Data 9, 434 (2022). https://doi.org/10.1038/s41597-022-01542-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01542-9
This article is cited by
-
A neural speech decoding framework leveraging deep learning and speech synthesis
Nature Machine Intelligence (2024)