Abstract
Benthic fauna refers to all fauna that live in or on the seafloor, which researchers typically divide into size classes meiobenthos (32/64 µm–0.5/1 mm), macrobenthos (250 µm–1 cm), and megabenthos (>1 cm). Benthic fauna play important roles in bioturbation activity, mineralization of organic matter, and in marine food webs. Evaluating their role in these ecosystem functions requires knowledge of their global distribution and biomass. We therefore established the BenBioDen database, the largest open-access database for marine benthic biomass and density data compiled so far. In total, it includes 11,792 georeferenced benthic biomass and 51,559 benthic density records from 384 and 600 studies, respectively. We selected all references following the procedure for systematic reviews and meta-analyses, and report biomass records as grams of wet mass, dry mass, or ash-free dry mass, or carbon per m2 and as abundance records as individuals per m2. This database provides a point of reference for future studies on the distribution and biomass of benthic fauna.
Measurement(s) | marine benthic feature • organic material • mass density |
Technology Type(s) | digital curation |
Factor Type(s) | geographic location • water depth • organism size class |
Sample Characteristic - Organism | meiobenthos • macrobenthos • megabenthos |
Sample Characteristic - Environment | marine benthic biome • ocean |
Sample Characteristic - Location | Atlantic Ocean • Pacific Ocean • Arctic Ocean • Southern Ocean • Indian Ocean |
Machine-accessible metadata file describing the reported data: https://doi.org/10.6084/m9.figshare.12481985
Similar content being viewed by others
Background & Summary
Benthic fauna, the fauna living in (infauna) or on the seafloor (epifauna), includes size classes known as metazoan meiobenthos, metazoan macrobenthos, and megabenthos. Metazoan meiobenthos passes through a 500 µm or 1 mm1 mesh and is retained on a sieve with a mesh size of 32 µm (deep-sea meiobenthos) or 63 µm (shallower water depth). Frequently, however, no upper sieve is used. Meiobenthos can actively rework sediment particles and build microscale burrows in the sediment2. Additionally, it represents a food source for juvenile and small adult fish3,4. Meiobenthos also contributes to organic matter mineralization and nutrient regeneration by stimulating the microbial community5. Metazoan macrobenthos passes through a 1 cm mesh and is retained on sieves with a mesh size of 250 or 300 or 500 µm (depending on the study). Macrobenthos is an important bioturbator that reworks the sediment (bioturbation sensu6), and in doing so alters the texture of the sediment7 and reduces slope failure8. It can be ecosystem engineers, i.e., organisms that alter the physical environment to change directly or indirectly the availability of resources to other organisms9, and modify hydrodynamics. Megabenthos or fauna larger than 1 cm includes organisms such as scleractinian corals or sponges that form biological structures and thus provide new habitats for associated fauna10,11. Other examples of megabenthos assemblages are oyster reefs and mussel beds that create biogeochemical hotspots for the burial of organic matter and the recycling of nutrients12,13,14. Additionally, mussels, cockles, oysters, but also sea cucumbers, are part of the human diet.
Despite their ecological importance, benthic ecosystems face increasing pressures from fishing, pollution and litter disposal, gas and oil exploration, extraction of minerals, development of coastlines, shipping, tourism, invasive species, and wind farms15,16,17. Sea-level rise can force intertidal habitats, such as salt marshes and tidal flats, to migrate landwards where they may be squeezed against artificial coastal structures18. This “coastal squeeze” leads to the loss of intertidal habitats and macrobenthic biomass19,20. Furthermore, ocean acidification will strongly impact tropical and cold-water coral reefs21,22,23 and calcareous fauna such as bivalves, gastropods, bryozoans, echinoderms, and foraminifera24. A combination of changes in pH, temperature, and oxygenation will even affect the export flux of particulate organic carbon (POC) to the seafloor25 and subsequently result in decreased benthic biomass26.
Evaluation of the severity of these threats and climate change for the benthic ecosystem on a global scale requires quantifying the role of benthos and its biomass and density in particular. Here, we introduce the open access “BenBioDen database”27 that, in comparison to previous databases by, e.g., Rex et al.28 and Wei et al.29, makes the benthic biomass and abundance records freely available and describes the data selection procedure transparently. Furthermore, this database includes records from the whole globe and not only from specific geographic regions, like the MarLIN – The Marine Life Information Network database (https://www.marlin.ac.uk/) or the OSPAR Data & Information Management System (ODIMS) database (https://odims.ospar.org/).
The “BenBioDen database” reports 1,445 benthic biomass and 2,085 benthic density studies and datasets identified following standardized procedures for systematic reviews and meta-analyses30. As a result, we extracted 11,792 georeferenced records of benthic biomass (1,240 metazoan meiobenthos records, 9,292 macrobenthos records, and 1,260 invertebrate megabenthos records) and 51,559 georeferenced records of benthic densities (4,129 metazoan meiobenthos records, 46,389 macrobenthos records, and 1,041 invertebrate megabenthos records) from 384 and 600 selected studies, respectively. We report benthic biomass as g wet mass (WM) m−2, as g dry mass (DM) m−2, as g ash-free dry mass (AFDM) m−2 or as g carbon (C) m−2. All biomass and density data records include further information about the mesh size used to separate meiobenthos from macrobenthos and megabenthos, and macrobenthos from megabenthos, and the sampling gear. In this way, researchers can decide whether they wish to exclude specific studies that do not match organism size criteria or sampling gear criteria. The database provides an important point of reference for future studies on the distribution and biomass of benthos and may also stimulate future sampling campaigns by indicating undersampled locations and water depth.
Methods
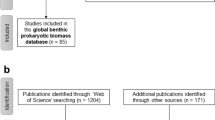
In April and May 2019, we compiled the “BenBio” part of the “BenBioDen database” following the “Preferred Reporting Items for Systematic reviews and Meta-Analyses” (PRISMA) Statement for systematic reviews and meta-analyses30 (Fig. 1a). In the first PRISMA step, the “Identification” step, we identified 1,373 articles in the Web of Science using the key words “marine meiofauna biomass”, “marine macrofauna biomass”, “marine megafauna biomass”, “marine meiobenth* biomass”, “marine macrobenth* biomass”, “marine megabenth* biomass”, “nematode biomass”, and “benthic ‘standing stock’”. We located an additional 201 publications based on expert knowledge. A search of the PANGAEA(R) Data Publisher (https://www.pangaea.de/) identified 1,488 datasets representing 148 publications using the key words “meiofauna biomass”, “macrofauna biomass” and “megafauna biomass”. Further 30 datasets were found in the EOL data archive (http://data.eol.ucar.edu/), through citations in review papers, and based on expert knowledge. After removing duplicates, we screened the titles and abstracts of 1,445 studies (Online-only Table 1) in PRISMA step 2 (“Screening”; Fig. 1a). This step excluded 951 studies because they did not report biomass values. In the Eligibility step (step 3; Fig. 1a), we assessed full texts of 494 studies for eligibility and excluded 110 studies because they did not report biomass, the publications or data were not accessible, or they did not report benthic biomass in appropriate units (g WW m−2, g DW m−2, g AFDW m−2, g or mol C m−2). Further reasons for excluding full texts included combining benthic biomass for several size classes, reporting benthic biomass for particular taxa rather than the whole size class, presenting biomass for faunal assemblages and/or a group of sampling stations rather than for individual stations, not presenting primary research or lacking geographical details about sampling stations. We also excluded studies that estimated benthic biomass using modelling approaches, that conducted manipulative experiments, or did not report benthic biomass as single values, means or median values, but instead as ranges. The final “BenBio” part included 384 studies from which we extracted 11,792 georeferenced benthic biomass entries (Online-only Table 1; Fig. 1a).
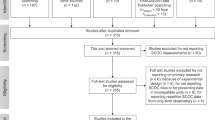
The Benthos Density, i.e. “BenDen”, part of the “BenBioDen” database was established in July and August 2019 following the PRISMA Statement for systematic reviews and meta-analyses30 (Fig. 1b). In the Identification step, we found 2,515 articles in the Web of Science using the key words “meiofauna abundance”, “meiobenthos abundance”, “macrofauna abundance”, “macrobenthos abundance”, “megafauna abundance”, “megabenthos abundance”, “meiofauna Arctic Ocean”, “meiofauna Atlantic Ocean”, “meiofauna Black Sea”, “meiofauna Gulf of Mexico”, “meiofauna Indian Ocean”, “meiofauna Mediterranean Sea”, “meiofauna Pacific Ocean”, “meiofauna Southern Ocean”, “meiofauna Red Sea”, “meiofauna Pacific Ocean”, “megafauna Southern Ocean”, “megafauna Red Sea”, “megafauna Pacific Ocean”, “megafauna Mediterranean Sea”, “megafauna Indian Ocean”, “megafauna Black Sea”, “megafauna Gulf of Mexico”, “megafauna Atlantic Ocean”, “megafauna Arctic Ocean”, “macrofauna Arctic Ocean”, “macrofauna Atlantic Ocean”, “macrofauna Black Sea”, “macrofauna Southern Ocean”, “macrofauna Red Sea”, “macrofauna Pacific Ocean”, “macrofauna Gulf of Mexico”, “macrofauna Indian Ocean”, and “macrofauna Mediterranean Sea”. Expert knowledge identified a further 232 publications. Consulting PANGAEA(R) Data Publisher (https://www.pangaea.de/) identified 1,549 datasets from 172 publications using the key words “meiofauna abundance”, “macrofauna abundance” and “megafauna abundance”. Expert knowledge or unpublished datasets added a further 21 datasets. After removal of duplicates, the “Screening” step filtered 2,086 titles and abstracts (Online-only Table 1; Fig. 1b) and excluded 1,133 studies because they did not report benthic densities. The third PRISMA step (“Eligibility”; Fig. 1b) assessed 953 studies and excluded 353 studies because they did not report metazoan meiobenthic, macrobenthic, or invertebrate megabenthic densities or they combined multiple size classes or sampling stations. We excluded other studies in the database that reported experimental studies, were inaccessible, or reported densities in a unit other than ind. m−2 or a unit that could be converted to ind. m−2, or reported densities for specific taxa instead of the entire size class. Studies were also excluded when they reported meta-studies or reviews rather than primary research, presented results of models, lacked sufficient geographical detail about sampling locations, or reported fauna associated with whale falls. The final “BenDen” part consisted of 600 studies from which we extracted 51,559 georeferenced benthic density records (Online-only Table 1; Fig. 1b).
For 12% (BioBen part) and 4% (BioDen part) of all data records, no exact sampling location in geographical coordinates (latitude, longitude) was indicated. For these cases, we approximated the coordinates of the sampling locations using Google Maps based on information about sampling area or based on maps presented in the original publications. We labelled these data records as ‘approximated location’.
For studies that presented biomasses in several units, such as WM and DM, we report the data only once (preferred units: WM > DM > AFDM > C). The authors of this study intended to report all data records in the ‘raw’ units in which benthic fauna was measured initially. Whenever unknown conversion factors precluded calculating biomass back to ‘raw’ units, we noted this issue in the database using the label ‘converted data’ and listed references for the individual biomass conversion factors in the database. Furthermore, we prepared Table 1 that reports all literature used by the authors of the original studies to convert their biomasses size-class dependent to WM, DM, AFDM, and C content.
The authors of the various studies compiled in this database sometimes used different lower and upper limits (in mm) for mesh sizes of nets and/or sieves to define the size class. Whenever an original study reported a lower and/or upper limit mesh size, we included this information in the database as ‘sieve mesh size (mm) lower limit’ and ‘sieve mesh size (mm) upper limit’. Studies lacking this information were scored as NA.
For those studies that reported data as mean or median ± error terms, we incorporated only mean or median values into the database. In all cases that did not report benthic biomasses and/or densities in the text or in tables, but presented them in figures, we extracted biomass and/or density values from these figures using ImageJ31.
Data Records
The BenBioDen database is openly accessible in the Dryad Digital Repository27 and includes two txt.files, i.e. the List of studies for BenBio database file and the List of studies for BenDen database file, and two csv.files, i.e., the BenBio database file and the BioDen database file. The List of studies files list all 3,531 studies alphabetically (benthic biomasses: 1,445 studies; benthic densities: 2,086 studies) which we identified in the “Identification” step of the systematic review after removing all duplicates. Each data entry in the BioBenDen database contains information about the region where the biomasses and/or densities were sampled and the corresponding ocean, the geographical location (latitude, longitude), whether geographic location was exactly known or approximated, water depth (in m), and a depth range following Dunne et al.32. Dunne and co-authors divided the ocean in near-shore areas that stretch to 50 m water depth, continental shelves from > 50 to 200 m water depth, continental slopes from > 200 to 2,000 m water depth, and continental rises/abyssal plains > 2,000 m water depth. The database indicates whether we determined the biomasses as WM, DM, AFDM, or C content; densities are reported as ind. m−2. The database also reports the specific size class (metazoan meiobenthos, macrobenthos, invertebrate megabenthos), the mesh size of the sieves used by the authors of the studies to separate the different size classes and the sampling gear.
Technical Validation
Geographical and water depth bias
In the database, 60% of all meiobenthic biomass records were sampled in the Atlantic Ocean (including the Gulf of Mexico and the Mediterranean Sea), 22% in the Pacific Ocean, and 12% in the Arctic Ocean (Fig. 2). Most macrobenthic samples were collected in the Atlantic Ocean (including the Gulf of Mexico and the Mediterranean Sea; 56%), with additional sampling in the Arctic Ocean (26%), and the Pacific Ocean (15%) (Fig. 2). In contrast, most megafaunal biomass data compiled in the BioBen database originated from the Arctic Ocean (50%) and the Atlantic Ocean (21%) (Fig. 2). All three benthic size classes were predominantly sampled in the northern hemisphere north of 1°N (meiobenthos: 82%, macrobenthos: 95%, megabenthos: 90%), and macrobenthos in particular was seriously undersampled south of 1°S (5% of all samples) (Figs. 3, 4). Almost no biomass samples were taken in the Indian Ocean (meiobenthos: 2%, macrobenthos: 1%, megabenthos: 0%) and the Southern Ocean (meiobenthos: 3%, macrobenthos: 1%, megabenthos: 1%) (Fig. 4). Additionally, the Pacific Ocean that represents 56% to the global ocean’s area33 is comparatively undersampled for macrobenthos (15% of all macrobenthos) (Fig. 4).
Benthic biomass (g m−2) of metazoan meiobenthos (upper panel), macrobenthos (middle panel), and invertebrate megabenthos (lower panel) along a latitudinal gradient. Each dot corresponds to a single biomass record and the dashed line indicates the equator. Notice the logarithmic scale on the x-axis. Abbreviations: AFDM = ash-free dry mass, DM = dry mass, C = carbon, WM = wet mass.
Sampling effort of meiobenthos, macrobenthos, and megabenthos as % samples taken in relation to % surface area of the different oceans. The dashed 1:1 line indicates the equal distribution of samples over all oceans. All samples above the diagonal indicate oversampling of specific faunal biomass and/or density records, whereas all samples below the diagonal indicate undersampling.
Meiobenthos biomasses were quantified mostly on the continental slope (35%) and on the continental rise and abyssal plains (31%) that collectively encompass 95% of the ocean seafloor32 (Fig. 5). In contrast, near-shore areas (29%) and continental shelves (21%; Fig. 5) dominated macrobenthic biomass samples, although these areas collectively encompass < 5% of the global seafloor32. In 35% of the cases no sampling depth was given in the original publications. Also 47% of all megabenthos biomass records came from areas < 50 m water depth, whereas only 10% of all megabenthos biomass samples were taken in the largest part of the seafloor, the continental rise and abyssal plains (Fig. 5). Hence, not surprisingly the benthic biomass database is biased towards shallow waters (<200 m) in the northern hemisphere, particularly, in the North Atlantic.
Meiobenthic density samples were mainly taken in the Atlantic Ocean (including the Gulf of Mexico and the Mediterranean Sea; 59%) and in the Pacific Ocean (22%) (Figs. 6, 7), whereas macrobenthic density was dominantly sampled in the Atlantic Ocean (including the Gulf of Mexico and the Mediterranean Sea; 87%) (Figs. 6, 7). Megabenthic densities originated from the Arctic Ocean (53%), the Atlantic Ocean (including the Gulf of Mexico and the Mediterranean Sea; 26%), and the Pacific Ocean (14%) (Figs. 6, 7). More than 83% of all samples were taken in the northern hemisphere (>1°N), in case of macrobenthos, even 98% of all density samples were taken > 1°N (Fig. 8).
Meiobenthic and megabenthic densities were sampled to 65% and 56% at the continental slope and at the continental rise and abyssal plain (Fig. 7), whereas information about sampling depth was missing for 82% of the macrobenthos samples that originated predominantly from the North Atlantic. When these records are not taken into account, most of the macrobenthic density samples were collected in near-shore areas (38%) and at the continental shelf (33%). Hence, benthic density samples are biased towards the northern hemisphere and in particular towards the North Atlantic and the Arctic Ocean (Fig. 4).
Differences in size ranges of meiobenthos, macrobenthos, and megabenthos
Metazoan meiobenthos usually includes organisms that pass through 500 μm to 1 mm mesh size and are retained on sieves with 44 μm mesh size1, though deep-sea biologists often use a lower mesh size limit of 32 μm for metazoan meiobenthos34. In our database, however, the lower mesh size limit for metazoan meiobenthos ranges from 20 μm to 74 μm, and the upper mesh size limit spans from 100 μm to 2 mm because of the different mesh sizes chosen by the authors of the original studies. Hence, some metazoan meiobenthos records include organisms that might be allocated to microbenthos, and other records that group them with macrobenthos.
Macrobenthos refers to organisms retained on a mesh of 0.5 cm, though different studies used mesh sizes between 0.5 mm and 2 mm35. In our database, however, authors of different studies sieved macrobenthos samples with meshes ranging from 0.25 mm to 20 mm in size. This implies, that depending on the size range used for macrobenthos, some macrobenthic records might include also be metazoan meiobenthos.
Invertebrate megabenthos are larger than macrobenthos and defined as invertebrates visible in bottom photographs (> 1 cm or > 3 cm36). Most megabenthic biomass and density records in the BioBenDen database lack specific information about minimum size (82% of all megabenthic biomass records and 79% of all megabenthic density records), but the studies that report a minimum size used a minimum animal length between 0.5 cm and 2 cm. Consequently, part of the megabenthic biomass and density data unavoidably might include some macrobenthos.
Therefore, researchers should consider the lower and upper sieve mesh sizes when using data from this database to ensure that the data coincide with their size requirements.
References
Giere, O. Meiobenthology - The Microscopic Motile Fauna of Aquatic Sediment. (Springer-Verlag, 2009).
Schratzberger, M. & Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Bio. Ecol. 502, 12–25 (2018).
Carpentier, A., Como, S., Dupuy, C., Lefrançois, C. & Feunteun, E. Feeding ecology of Liza spp. in a tidal flat: Evidence of the importance of primary production (biofilm) and associated meiofauna. J. Sea Res. 92, 86–91 (2014).
Schückel, S. et al. Meiofauna as food source for small-sized demersal fish in the southern North Sea. Helgol. Mar. Res. 67, 203–218 (2013).
Coull, B. C. Role of meiofauna in estuarine soft-bottom habitats. Aust. J. Ecol. 24, 327–343 (1999).
Meysman, F. J. R., Middelburg, J. J. & Heip, C. H. R. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol. Evol. 21, 688–695 (2006).
Meadows, P. S. & Tait, J. Modification of sediment permeability and shear strength by two burrowing invertebrates. Mar. Biol. 101, 75–82 (1989).
Shaikh, M. A., Meadows, A. & Meadows, P. S. Biological control of avalanching and slope stability in the intertidal zone. In Sedimentary Processes in the Intertidal Zone (eds. Black, K. S., Paterson, D. M. & Cramp, A.) vol. 139, 309–329 (Geological Society, 1998).
Jones, C. G., Lawton, J. H. & Shachak, M. Organisms as ecosystem engineers. Oikos 69, 373–386 (1994).
Buhl-Mortensen, L. et al. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar. Ecol. 31, 21–50 (2010).
Beaulieu, S. E. Life on glass houses: Sponge stalk communities in the deep sea. Mar. Biol. 138, 803–817 (2001).
Chambers, L. G. et al. How well do restored intertidal oyster reefs support key biogeochemical properties in a coastal lagoon? Estuaries and Coasts 41, 784–799 (2018).
Norling, P. & Kautsky, N. Structural and functional effects of Mytilus edulis on diversity of associated species and ecosystem functioning. Mar. Ecol. Prog. Ser. 351, 163–175 (2007).
Largaespada, C., Guichard, F. & Archambault, P. Meta-ecosystem engineering: Nutrient fluxes reveal intraspecific and interspecific feedbacks in fragmented mussel beds. Ecology 93, 324–333 (2012).
Ramírez-Llodrà, E. et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 7, 2851–2899 (2010).
Harris, P. T. Anthropogenic threats to benthic habitats. in Seafloor Geomorphology as Benthic Habitat 39–60 (Elsevier). https://doi.org/10.1016/B978-0-12-385140-6.00003-7 (2012).
Ramírez-Llodrà, E. et al. Man and the last great wilderness: Human impact on the deep sea. PLoS One 6, e22588 (2011).
Birchenough, S. N. R. et al. Climate change and marine benthos: A review of existing research and future directions in the North Atlantic. Wiley Interdiscip. Rev. Clim. Chang. 6, 203–223 (2015).
Raw, J. L. et al. Salt marsh elevation and responses to future sea-level rise in the Knysna Estuary, South Africa. African J. Aquat. Sci. 5914 (2020).
Fujii, T. & Raffaelli, D. Sea-level rise, expected environmental changes, and responses of intertidal benthic macrofauna in the Humber estuary, UK. Mar. Ecol. Prog. Ser. 371, 23–35 (2008).
Langdon, C. & Atkinson, M. J. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. C Ocean. 110, 1–16 (2005).
Smith, J. N. et al. Shifts in coralline algae, macroalgae, and coral juveniles in the Great Barrier Reef associated with present-day ocean acidification. Glob. Chang. Biol. 26, 2149-2160 (2020).
Turley, C. M., Roberts, J. M. & Guinotte, J. M. Corals in deep-water: Will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448 (2007).
Andersson, A. J., Mackenzie, F. T. & Gattuso, J.-P. Effects of ocean acidification on benthic processes, organisms, and ecosystems. in Ocean Acidification 122–153 (Oxford University Press, 2011).
Sweetman, A. K. et al. Major impacts of climate change on deep-sea benthic ecosystems. Elem. - Sci. Anthr. 5, 4 (2017).
Jones, D. O. B. et al. Global reductions in seafloor biomass in response to climate change. Glob. Chang. Biol. 20, 1861–1872 (2014).
Stratmann, T. et al. The BenBioDen database, a global database for meio-, macro- and megabenthic biomass and densities. Dryad Digital Repository https://doi.org/10.5061/dryad.gb5mkkwm6 (2020).
Rex, M. A. et al. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar. Ecol. Prog. Ser. 317, 1–8 (2006).
Wei, C.-L. et al. Global patterns and predictions of seafloor biomass using random forests. PLoS One 5, e15323 (2010).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, e1000097 (2009).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Dunne, J. P., Sarmiento, J. L. & Gnanadesikan, A. A synthesis of global particle export from the surface ocean and cycling through the ocean interior and on the seafloor. Global Biogeochem. Cycles 21, GB4006 (2007).
Eakins, B. W. & Sharman, G. F. Volumes of the World’s Oceans from ETOPO1. NOAA National Geophysical Data Center (2010).
Soltwedel, T. Metazoan meiobenthos along continental margins: a review. Prog. Oceanogr. 46, 59–84 (2000).
Eleftheriou, A. & Moore, D. C. Macrofauna Techniques. in Methods for the Study of Marine Benthos (eds. Eleftheriou, A. & McIntyre, A.) vol. 16 160–228 (Blackwell Science Ltd, 1984).
Rex, M. A. & Etter, R. J. Deep-sea biodiversity. (Harvard University Press, 2010).
Stratmann, T. et al. The BenBioDen database, a global database for meio-, macro- and megabenthic biomass and densities - R code. Zenodo, https://doi.org/10.5281/zenodo.3843149 (2020).
Andrassy, I. Die Rauminhalts- und Gewichtsbestimmung der Fadenwürmer (Nematoden). Acta Zool. 2, 1–15 (1956).
Feller, R. J. & Warwick, R. M. Energetics. in Introduction to the Study of Meiofauna 181–196 (Smithonian Institution Press, 1988).
Jensen, P. Measuring carbon content in nematodes. Helgoländer Meeresuntersuchungen 38, 83–86 (1984).
Wieser, W. Benthic studies in Buzzard Bay. II. The meiofauna. Limnol. Oceanogr. 5, 121–137 (1960).
Wieser, W. Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Ark. für Zool. 4, 439–484 (1953).
Widbom, B. Determination of average individual dry weights and ash-free dry weights in different sieve fractions of marine meiofauna. Mar. Biol. 84, 101–108 (1984).
Zeng, Q., Huang, D., Lin, R. & Wang, J. Deep-sea metazoan meiofauna from a polymetallic nodule area in the Central Indian Ocean Basin. Mar. Biodivers. 48, 395–405 (2018).
Grove, S. L., Probert, P. K., Berkenbusch, K. & Nodder, S. D. Distribution of bathyal meiofauna in the region of the Subtropical Front, Chatham Rise, south-west Pacific. J. Exp. Mar. Bio. Ecol. 330, 342–355 (2006).
Zhang, Z., Zhou, H., Guo, Y. & Mu, F. Comparative study on the nematode community structure in the submarine delta of Huanghe river estuary and its adjacent waters. Oceanol. Limnol. Sin. 32, 436–444 (2001).
Nodder, S. D., Pilditch, C. A., Probert, P. K. & Hall, J. A. Variability in benthic biomass and activity beneath the Subtropical Front, Chatham Rise, SW Pacific Ocean. Deep-Sea Res. I 50, 959–985 (2003).
Pfannkuche, O. The deep-sea meiofauna of the Porcupine Seabight and abyssal plain (NE Atlantic): Population structure, distribution, standing stocks. Oceanol. Acta 8, 343–353 (1985).
Soetaert, K. An ecological-systematical study of the deep-sea meiofauna and nematode communities in the western Mediterranean Sea. PhD Thesis. (Ghent University, 1989).
Vanhove, S., Beghyn, M., Van Gansbeke, D., Bullough, L. & Vincx, M. A seasonally varying biotope at Signy Island, Antarctic: Implications for meiofaunal structure. Mar. Ecol. Prog. Ser. 202, 13–25 (2000).
Vanhove, S. et al. Deep-sea meiofauna communities in Antarctica: Structural analysis and relation with the environment. Mar. Ecol. Prog. Ser. 127, 65–76 (1995).
Xiaoshou, L. et al. Abundance and biomass of meiobenthos in the spawning ground of anchovy (Engraulis japanicus) in the southern Huanghai Sea. Acta Oceanol. Sin. 24, 94–104 (2005).
Heip, C. H. R., Vincx, M. & Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. An Annu. Rev. 23, 399–489 (1985).
Gray, J. S. The fauna of the polluted river Tees estuary. Estuar. Coast. Mar. Sci. 4, 653–676 (1976).
Grelet, Y. Peuplements méiobenthiques et structure de la Nématofaune du Golfe d’Aqaba (Jordanie-Mer Rouge). PhD Thesis. (Université Aix-Marseille II, 1984).
Witte, J. & Zijlstra, J. The meiofauna of a tidal flat in the western part of the Wadden Sea and its role in the benthic ecosystem. Mar. Ecol. Prog. Ser. 14, 129–138 (1984).
Faubel, A. Determination of individual meiofauna dry weight values in relation to definite size classes. Cah. Biol. Mar. 23, 339–345 (1982).
Lin, R. et al. Abundance and distribution of meiofauna in the Chukchi Sea. Acta Oceanol. Sin. 33, 90–94 (2014).
Shirayama, Y. Size structure of deep-sea meio- and macrobenthos in the western Pacific. Int. Rev. der gesamten Hydrobiol. und Hydrogr. 68, 799–810 (1983).
Warwick, R. M. & Gee, J. Community structure of estuarine meiobenthos. Mar. Ecol. Prog. Ser. 18, 97–111 (1984).
Higgins, R. P. & Thiel, H. Introduction to the Study of Meiofauna. (Smithonian Institution Press, 1988).
Rutgers van der Loeff, M. M. & Lavaleye, M. S. S. Sediments, fauna and the dispersal of radionuclides at the N.E. Atlantic dumpsite for low-level radioactive wast. Report of the Dutch DORA program. (1984).
Vanaverbeke, J., Soetaert, K., Heip, C. H. R. & Vanreusel, A. The metazoan meiobenthos along the continental slope of the Goban Spur (NE Atlantic). J. Sea Res. 38, 93–107 (1997).
Dinet, A. Répartition quantitative et écologie du méiobenthos de la plaine abyssale Atlantique. PhD Thesis. (University Aix-Marseille II, 1980).
Baguley, J. G., Hyde, L. J. & Montagna, P. A. A semi-automated digital microphotographic approach to measure meiofaunal biomass. Limnol. Oceanogr. Methods 2, 181–190 (2004).
Baguley, J. C. Meiofauna community structure and function in the northern Gulf of Mexico deep sea. PhD Thesis. (University of Texas at Austin, 2004).
McIntyre, A. D. & Warwick, R. M. Meiofauna techniques. in Methods for the Study of Marine Benthos (Blackwell, 1984).
Warwick, R. M. & Price, R. Ecological and metabolic studies on free-living nematodes from an estuarine mud-flat. Estuar. Coast. Mar. Sci. 9, 257–271 (1979).
Gradinger, R., Friedrich, C. & Spindler, M. Abundance, biomass and composition of the sea ice biota of the Greenland Sea pack ice. Deep-Sea Res. II 46, 1457–1472 (1999).
Riemann, F., Ernst, W. & Ernst, R. Acetate uptake from ambient water by the free-living marine nematode Adoncholaimus thalassophygas. Mar. Biol. 104, 453–457 (1990).
Rudnick, D. T. Seasonality of community structure and carbon flow in Narragansett Bay sediments. PhD Thesis. (University of Rhode Island, 1984).
Ólafsson, E. & Elmgren, R. Seasonal dynamics of sublittoral meiobenthos in relation to phytoplankton sedimentation in the Baltic Sea. Estuar. Coast. Shelf Sci. 45, 149–164 (1997).
Newton, A. C. & Rowe, G. T. The abundance of benthic calcareous foraminifera and other meiofauna at a time series station in the Northeast Water Polynya, Greenland. J. Geophys. Res. 100, 4423–4438 (1995).
Newton, A. C. The distribution and ecology of benthic Foraminifera and associated meiofauna in the northeast Greenland polynya, Greenland. PhD Thesis (Texas A & M University, 1994).
Hughes, D. J. & Gage, J. D. Benthic metazoan biomass, community structure and bioturbation at three contrasting deep-water sites on the northwest European continental margin. Prog. Oceanogr. 63, 29–55 (2004).
Danovaro, R., Tselepides, A., Otegui, A., Della Croce, N. & Bianche, V. B. Dynamics of meiofaunal assemblages on the continental shelf and deep-sea sediments of the Cretan Sea (NE Mediterranean): Relationships with seasonal changes in food supply. Prog. Oceanogr. 46, 367–400 (2000).
Danovaro, R. & Fraschetti, S. Meiofaunal vertical zonation on hard-bottoms: Comparison with soft-bottom meiofauna. Mar. Ecol. Prog. Ser. 230, 159–169 (2002).
Danovaro, R. Methods for the Study of Deep-Sea Sediments, their Functioning and Biodiversity. (CRC Press, 2010).
Zeppilli, D., Bongiorni, L., Cattaneo, A., Danovaro, R. & Serrão Santos, R. Meiofauna assemblages of the Condor Seamount (North-East Atlantic Ocean) and adjacent deep-sea sediments. Deep-Sea Res. II 98, 87–100 (2013).
Snider, L. J., Burnett, B. R. & Hessler, R. R. The composition and distribution of meiofauna and nanobiota in a central North Pacific deep-sea area. Deep-Sea Res. A 31, 1225–1249 (1984).
Rowe, G. T. Biomass and production of the deep-sea macrobenthos. in Deep-Sea Biology (ed. Rowe, G. T.) (John Wiley & Sons, Inc., 1983).
Tselepides, A. & Eleftheriou, A. South Aegean (Eastern Mediterranean) continental slope benthos: Macroinfaunal - Environmental relationships. in Deep-Sea Food Chains and the Global Carbon Cycle 139–156, https://doi.org/10.1007/978-94-011-2452-2_9 (Springer Netherlands). (1992).
Brey, T. Population dynamics in benthic invertebrates. A virtual handbook. (2001).
Eleftheriou, A. & Basford, D. J. The macrobenthic infauna of the offshore northern North Sea. J. Mar. Biol. Assoc. UK 69, 123–143 (1989).
Fradette, P. & Bourget, E. Ecology of benthic epifauna of the Estuary and Gulf of St. Lawrence: Factors influencing their distribution and abundance on buoys. Can. J. Fish. Aquat. Sci. 37, 979–999 (1980).
Bourget, E. & Messier, D. Macrobenthic density, biomass, and fauna of intertidal and subtidal sand in a Magdalen Islands lagoon, Gulf of St. Lawrence. Can. J. Zool. 61, 2509–2518 (1983).
Gascón, S. Estructura i dinàmica del sistema bentònic en llacunes costaneres del saiguamolls de l’Empordà. PhD Thesis. (University of Girona, 2003).
McIntyre, A. D. & Eleftheriou, A. The bottom fauna of a flatfish nursery ground. J. Mar. Biol. Assoc. UK 48, 113–142 (1968).
Brey, T., Müller-Wiegmann, C., Zittier, Z. M. C. & Hagen, W. Body composition in aquatic organisms - A global data bank of relationships between mass, elemental composition and energy content. J. Sea Res. 64, 334–340 (2010).
Ellis, D. V. Marine infaunal benthos in arctic North America. vol. 5 (1960).
Ricciardi, A. & Bourget, E. Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol. Prog. Ser. 163, 245–251 (1998).
Brey, T., Rumohr, H. & Ankar, S. Energy content macrobenthic invertebrates: General conversion factors from weight to energy. J. Exp. Mar. Bio. Ecol. 117, 271–278 (1988).
Rumohr, H., Brey, T. & Ankar, S. A compilation of biometric conversion factors for benthic invertebrates of the Baltic Sea. The Baltic Marine Biologists Publication vol. 9 (1987).
Bluhm, B. Zur Ökologie der regulären Seeigel im nördlichen Barentsmeer. PhD Thesis. (Kiel University, 1997).
Gerlach, S., Hahn, A. & Schrage, M. Size spectra of benthic biomass and metabolism. Mar. Ecol. Prog. Ser. 26, 161–173 (1985).
Lie, U. A quantitative study of benthic infauna in Puget Sound, Washington, USA, in 1963-1964. Fisk. Skr. Ser. Havundersøkelse 14, 229–556 (1968).
Persoone, G. A simple volumeter for small invertebrates. Helgoländer Meeresuntersuchungen 22, 141–143 (1971).
Piepenburg, D. & von Juterzenka, K. Abundance, biomass and spatial distribution pattern of brittle stars (Echinodermata: Ophiuroidea) on the Kolbeinsey Ridge north of Iceland. Polar Biol. 14, 185–194 (1994).
Piepenburg, D. & Schmid, M. K. Distribution, abundance, biomass, and mineralization potential of the epibenthic megafauna of the northeast Greenland shelf. Mar. Biol. 125, 321–332 (1996).
Salonen, K., Sarvala, J., Hakala, I. & Viljanen, M. L. The relation of energy and organic carbon in aquatic invertebrates. Limnol. Oceanogr. 21, 724–730 (1976).
Wacasey, J. W. & Atkinson, E. G. Energy values of marine benthic invertebrates from the Canadian Arctic. Mar. Ecol. Prog. Ser. 39, 243–250 (1987).
Frithsen, J. B., Rudnick, D. T. & Doering, P. H. The determination of fresh organic carbon weight from formaldehyde preserved macrofaunal samples. Hydrobiologia 133, 203–208 (1986).
Galéron, J., Sibuet, M., Mahaut, M.-L. & Dinet, A. Variation in structure and biomass of the benthic communities at three contrasting sites in the tropical Northeast Atlantic. Mar. Ecol. Prog. Ser. 197, 121–137 (2000).
Rice, A. L., Aldred, R. G., Darlington, E. & Wild, R. A. The quantitative estimation of the deep-sea megabenthos; A new approach to an old problem. Oceanol. Acta 5, 63–72 (1982).
Curtis, M. A. Life cycles and population dynamics of marine benthic polychaetes from the Disko Bay area of West Greenland. Ophelia 16, 9–58 (1977).
Lambeck, R. H. D. & Valentijn, P. Distribution, dynamics and productivity of a colonizing (Polydora quadrilobata) and an established (P. Ligni) polydorid polychaete in lake grevelingen: An enclosed estuary in the SW Netherlands. Netherlands. J. Sea Res. 21, 143–158 (1987).
Mahaut, M. L. Modélisation à l’état stable du cycle du carbone dans le réseau trophique profond de la Terrasse de Meriadzek (Golfe de Gascogne). PhD Thesis. (Université de Paris VI, 1991).
Vinogradov, A. P. The elementary chemical composition of marine organisms. J. Am. Chem. Soc. 77, 1712–1713 (1953).
Sibuet, M. & Lawrence, J. M. Organic content and biomass of abyssal holothuroids (Echinodermata) from the Bay of Biscay. Mar. Biol. 65, 143–147 (1981).
Acknowledgements
We thank Heike Link (University of Rostock) for her help during the ArcticNet sampling campaigns in Canada. This study received funding by the European Union under EMODnet Thematic Lot n° 5 – BIOLOGY EASME/EMFF/2016/1.3.1.2- Lot 5/SI2.750022 – Biology and from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 678760 (ATLAS). It was also funded by the JPI Oceans – Impacts of deep-sea nodule mining project “MiningImpact 2” (NWO-ALW grant 856.18.003). DvO received additional financial support from the Dutch Research Council (NWO-VIDI grant no. 864.13.007) and TS was partly funded by the Dutch Research Council within the research programme NWO-Rubicon with project number 019.182EN.012.
Author information
Authors and Affiliations
Contributions
T.S. conceived the idea of the database, performed the literature search, compiled the database, and wrote the manuscript. D.v.O. and K.S. conceived the idea of the database and KS contributed to the compilation of the database. P.M.A., C.-L.W., J.-X.L., M.C., R.A.S., P.A., P.V.R.S., P.A.R.B., B.J.B., E.K., K.G., and R.B. contributed datasets to the database. All authors revised and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Online-only Table
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files associated with this article.
About this article
Cite this article
Stratmann, T., van Oevelen, D., Martínez Arbizu, P. et al. The BenBioDen database, a global database for meio-, macro- and megabenthic biomass and densities. Sci Data 7, 206 (2020). https://doi.org/10.1038/s41597-020-0551-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-020-0551-2
This article is cited by
-
‛Trophic switch’ by catfish community from predation to scavenging modulated by human food discard in an estuarine bay
Environmental Science and Pollution Research (2024)
-
Habitat types and megabenthos composition from three sponge-dominated high-Arctic seamounts
Scientific Reports (2022)
-
Intra- and inter-spatial variability of meiofauna in hadal trenches is linked to microbial activity and food availability
Scientific Reports (2022)
-
Implications of 2D versus 3D surveys to measure the abundance and composition of benthic coral reef communities
Coral Reefs (2021)