Abstract
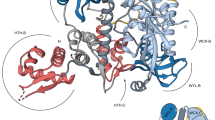
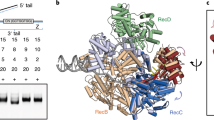
The bacterial SOS response plays a key role in adaptation to DNA damage, including genomic stress caused by antibiotics. SOS induction begins when activated RecA*, an oligomeric nucleoprotein filament that forms on single-stranded DNA, binds to and stimulates autoproteolysis of the repressor LexA. Here, we present the structure of the complete Escherichia coli SOS signal complex, constituting full-length LexA bound to RecA*. We uncover an extensive interface unexpectedly including the LexA DNA-binding domain, providing a new molecular rationale for ordered SOS gene induction. We further find that the interface involves three RecA subunits, with a single residue in the central engaged subunit acting as a molecular key, inserting into an allosteric binding pocket to induce LexA cleavage. Given the pro-mutagenic nature of SOS activation, our structural and mechanistic insights provide a foundation for developing new therapeutics to slow the evolution of antibiotic resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The cryo-EM maps and associated atomic model for this study have been deposited to the Electron Microscopy Data Bank (EMD-41579) and Protein Data Bank (8TRG). The earlier maps and model used as a comparison in this work are available on the Electron Microscopy Data Bank (EMD-34152) or the Protein Data Bank (1JHE, 3JSP, 8GMS). Source data are provided with this paper. All other data necessary to evaluate the claims in the paper is present in the text or Supplementary Information. Plasmids for LexA or RecA variants are available upon request.
References
Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 21, 280–295 (2023).
Culyba, M. J., Mo, C. Y. & Kohli, R. M. Targets for combating the evolution of acquired antibiotic resistance. Biochemistry 54, 3573–3582 (2015).
Pribis, J. P., Zhai, Y., Hastings, P. J. & Rosenberg, S. M. Stress-induced mutagenesis, gambler cells, and stealth targeting antibiotic-induced evolution. mBio 13, e0107422 (2022).
Maslowska, K. H., Makiela‐Dzbenska, K. & Fijalkowska, I. J. The SOS system: a complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 60, 368–384 (2019).
Recacha, E. et al. Quinolone resistance reversion by targeting the SOS response. mBio 8, e00971-17 (2017).
Mo, C. Y. et al. Systematically altering bacterial SOS activity under stress reveals therapeutic strategies for potentiating antibiotics. mSphere 1, 163 (2016).
Cirz, R. T. et al. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 3, e176 (2005).
Courcelle, J., Khodursky, A., Peter, B., Brown, P. O. & Hanawalt, P. C. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158, 41–64 (2001).
Bell, J. C. & Kowalczykowski, S. C. RecA: regulation and mechanism of a molecular search engine. Trends Biochem. Sci. 41, 491–507 (2016).
Sankar, T. S., Wastuwidyaningtyas, B. D., Dong, Y., Lewis, S. A. & Wang, J. D. The nature of mutations induced by replication–transcription collisions. Nature 535, 178–181 (2016).
Cox, M. M. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 42, 41–63 (2007).
Butala, M., Zgur-Bertok, D. & Busby, S. J. The bacterial LexA transcriptional repressor. Cell. Mol. Life Sci. 66, 82–93 (2009).
Mo, C. Y., Birdwell, L. D. & Kohli, R. M. Specificity determinants for autoproteolysis of LexA, a key regulator of bacterial SOS mutagenesis. Biochemistry 53, 3158–3168 (2014).
Luo, Y. et al. Crystal structure of LexA: a conformational switch for regulation of self-cleavage. Cell 106, 585–594 (2001).
Little, J. W. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie 73, 411–421 (1991).
Butala, M. et al. Interconversion between bound and free conformations of LexA orchestrates the bacterial SOS response. Nucleic Acids Res. 39, 6546–6557 (2011).
Neher, S. B., Flynn, J. M., Sauer, R. T. & Baker, T. A. Latent ClpX-recognition signals ensure LexA destruction after DNA damage. Genes Dev. 17, 1084–1089 (2003).
Friedman, N., Vardi, S., Ronen, M., Alon, U. & Stavans, J. Precise temporal modulation in the response of the SOS DNA repair network in individual bacteria. PLoS Biol. 3, e238 (2005).
Culyba, M. J., Kubiak, J. M., Mo, C. Y., Goulian, M. & Kohli, R. M. Non-equilibrium repressor binding kinetics link DNA damage dose to transcriptional timing within the SOS gene network. PLoS Genet. 14, e1007405 (2018).
Naiman, K., Philippin, G., Fuchs, R. P. & Pagès, V. Chronology in lesion tolerance gives priority to genetic variability. Proc. Natl Acad. Sci. USA 111, 5526–5531 (2014).
Fuchs, R. P. Tolerance of lesions in E. coli: chronological competition between translesion synthesis and damage avoidance. DNA Repair 44, 51–58 (2016).
Merrikh, H. & Kohli, R. M. Targeting evolution to inhibit antibiotic resistance. FEBS J. 287, 4341–4353 (2020).
Podlesek, Z. & Žgur Bertok, D. The DNA damage inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Front. Microbiol. 11, 1785 (2020).
Mo, C. Y. et al. Type III-A CRISPR immunity promotes mutagenesis of staphylococci. Nature 592, 611–615 (2021).
Marx, P. et al. Environmental stress perception activates structural remodeling of extant Streptococcus mutans biofilms. NPJ Biofilms Microbiomes 6, 17 (2020).
Soares, A., Alexandre, K. & Etienne, M. Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: molecular mechanisms, antibiotic strategies and therapeutic perspectives. Front. Microbiol. 11, 2057 (2020).
Chen, Z., Yang, H. & Pavletich, N. P. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature 453, 489–494 (2008).
Adikesavan, A. K. et al. Separation of recombination and SOS response in Escherichia coli RecA suggests LexA interaction sites. PLoS Genet. 7, e1002244 (2011).
Kovacic, L. et al. Structural insight into LexA–RecA* interaction. Nucleic Acids Res. 41, 9901–9910 (2013).
Cory, M. B. et al. Engineered RecA constructs reveal the minimal SOS activation complex. Biochemistry 61, 2884–2896 (2022).
Yu, X. & Egelman, E. H. The LexA repressor binds within the deep helical groove of the activated RecA filament. J. Mol. Biol. 231, 29–40 (1993).
Gao, B. et al. Structural basis for regulation of SOS response in bacteria. Proc. Natl Acad. Sci. USA 120, e2217493120 (2023).
Egelman, E. H. & Stasiak, A. Structure of helical RecA–DNA complexes. Complexes formed in the presence of ATP-gamma-S or ATP. J. Mol. Biol. 191, 677–697 (1986).
Giese, K. C., Michalowski, C. B. & Little, J. W. RecA-dependent cleavage of LexA dimers. J. Mol. Biol. 377, 148–161 (2008).
Hostetler, Z. M., Cory, M. B., Jones, C. M., Petersson, E. J. & Kohli, R. M. The kinetic and molecular basis for the interaction of LexA and activated RecA revealed by a fluorescent amino acid probe. ACS Chem. Biol. 15, 1127–1133 (2020).
Zhang, A. P., Pigli, Y. Z. & Rice, P. A. Structure of the LexA–DNA complex and implications for SOS box measurement. Nature 466, 883–886 (2010).
Mo, C. Y. et al. Inhibitors of LexA autoproteolysis and the bacterial SOS response discovered by an academic–industry partnership. ACS Infect. Dis. 4, 349–359 (2018).
Cory, M. B., Hostetler, Z. M. & Kohli, R. M. Kinetic dissection of macromolecular complex formation with minimally perturbing fluorescent probes. Methods Enzymol. 664, 151–171 (2022).
Hörtnagel, K. et al. Saturation mutagenesis of the E. coli RecA loop L2 homologous DNA pairing region reveals residues essential for recombination and recombinational repair. J. Mol. Biol. 286, 1097–1106 (1999).
Shepley, D. P. & Little, J. W. Mutant LexA proteins with specific defects in autodigestion. Proc. Natl Acad. Sci. USA 93, 11528–11533 (1996).
McGrew, D. A. & Knight, K. L. Molecular design and functional organization of the RecA protein. Crit. Rev. Biochem. Mol. Biol. 38, 385–432 (2003).
Hostetler, Z. M. et al. Systematic evaluation of soluble protein expression using a fluorescent unnatural amino acid reveals no reliable predictors of tolerability. ACS Chem. Biol. 13, 2855–2861 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Naydenova, K. & Russo, C. J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. 8, 629 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 74, 814–840 (2018).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods 17, 665–680 (2020).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Canutescu, A. A. & Dunbrack, R. L. J. Cyclic coordinate descent: a robotics algorithm for protein loop closure. Protein Sci. 12, 963–972 (2003).
Acknowledgements
This work was supported by the National Institutes of Health (grant no. R01-GM127593 to R.M.K. and E.J.P.). R.M.K. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. The National Institutes of Health also provided training grants (grant nos. T32-AI141393 for M.B.C. and T32-GM133398 for C.M.H. and R.M.P.) and mass spectrometry instrumentation support (grant no. S10-OD030460). Structural data collection was performed with the help of the Institute of Structural Biology, the Electron Microscopy Resource Laboratory and the Beckman Center for Cryo-EM at the University of Pennsylvania (RRID: SCR_022375). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.B.C., E.J.P. and R.M.K. conceived of the experiments. M.B.C. designed the overall experimental plan. M.B.C., A.L., C.M.H., Z.M.H. and Y.V. designed and executed biochemical experiments. M.B.C., A.L., P.J.C., R.A.P. and K.G. designed structural biology experiments, collected associated data and performed analysis. R.M.P. and X.L. performed computational modeling experiments. M.B.C., E.J.P. and R.M.K. wrote the manuscript. All authors were involved in editing and reviewing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Michael Cox, Edward Egelman and Yu Feng for their contribution to the peer review of this work. Dimitris Typas was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM analysis pipeline.
The flow of data from the collected and filtered micrographs through the final local refinement is shown. Each labeled step includes relevant information for the partitioning of data at each junction. For each refinement and reconstruction step, the FSC curve generated by CryoSPARC is shown.
Extended Data Fig. 2 Characteristics of the EM density and model.
a) Final sharpened map colored by the estimated local resolution using Relion. The entire complex is shown at top, with the relevant sub-complex components at the bottom with RecA* and LexA labeled and colored relative to grayed out other components. b) Orientation distribution of the final particle stack as determined by cryoEF. Orientation efficiency, Eod is given below. c) Closeup of the ATP binding pocket at the interface of two RecA protomers within the filament, showing the coordinated Mg2+ ion in green. Density from the two independent half maps and the corresponding full map at a contour level of 0.203 and 0.172 respectively shown in mesh. d) Closeup of the bound ssDNA within the filament. Density from the two independent half maps and the corresponding full map at a contour level of 0.203 and 0.172 respectively shown in mesh.
Extended Data Fig. 3 Comparison to prior models.
a) Global comparison of this current model of full-length LexA bound to RecA* (8TRG, blue and pink) to prior published model of a RecA* dimer bound to the CTD-only LexA (8GMS, gold and sea green). Global RMSD is between Cα atoms of residues present in both models, RMSD of the L2 pocket is all-atom of the residues shown by sticks. b) Overlay of the current model of the SOS complex with native, full-length LexA into the cryoEM density from Gao et al (Ref. 32). The displayed density is that derived from 8GMS, and is colored to match the model colors from A (LexA in gold, RecA* in sea green), including the density from a second LexA CTD (dark orange). The ribbon structures show our fit model, colored accordingly (8TRG, blue and pink). The overlay demonstrates that full-length LexA containing the NTD is not compatible with the symmetrically decorated filament previously studied with CTD-only LexA. c) Molecular dynamics was performed to build potential poses for the missing NTD from the unbound LexA subunit. Five distinctive poses were selected in this overlay, represented by different colors in ribbons, with the cryo-EM density-derived model shown as a surface.
Extended Data Fig. 4 Discrimination between operator-bound LexA and free LexA by RecA*.
a) SDS-PAGE gel of autoproteolysis of fluorescent LexA-CF variant at pH 7.5 in the absence of operator or in the presence of either 20 bp or 40 bp consensus operator (single replicate). b) Fluorescence anisotropy of LexA-δ with various in vitro binding partners. Each data point represents a single replicate. The various contributing species to the observed anisotropic signal are given to the right. c) Equilibrium endpoint anisotropy titration of either Ec or Mtb LexA with FAM-labeled 40-mer consensus operator. Data shows a single replicate and the solid line is a fit to a quadratic equation, using a fixed [operator] of 1 nM. d) SDS-PAGE analysis of RecA*-dependent cleavage of E. coli LexA with either an Ec or Mtb inter-domain linker when incubated with consensus operator (single replicate). e) Structural overlay of our modeled SOS complex (8TRG, blue and pink) with the crystal structure of the DNA-bound LexA dimer (3JSP, yellow). Steric clashes are colored in red. Lower left panel shows a close-up view of one of the modeled bound LexA NTD alongside the corresponding DNA-bound NTD, demonstrating the distinctive orientations of the NTDs in the two different structures. The relative numbering of each alpha helix in the NTD is numbered according to the topological diagram on the left side of the panel.
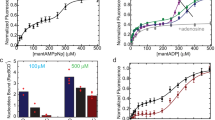
Extended Data Fig. 5 Alkaline autoproteolysis rates of each tested LexA variant.
Data were fit to a single exponential decay (solid line) with 95% confidence intervals shown (shaded region). The best-fit value for the decay rate is shown on each graph. Data represents the mean from three replicates, with error bars denoting standard deviation.
Extended Data Fig. 6 Analysis of potential charge-charge interactions between RecA* and LexA.
a–c) Three different sections within the interaction interface that provide potential charge-charge interactions. Each of the RecA protomers within the three consecutive RecA units providing a majority of the contacts are highlighted and labeled in shades of pink to purple. The bound LexA monomer is shown in blue. Insets highlight the distances between interacting residues in green and show the sharpened map density as a wire-mesh surface. The panels highlight A) the ‘CTD Patch’ of interaction residues between RecA* and LexA. B) the ‘L2 Stabilizing Patch’ interaction residues, and C) the ‘NTD Patch’ interaction residues. d) Top: Representative SDS-PAGE analysis of RecA*-dependent cleavage of CTD patch residues in isolation or in combination. Bottom: Quantified LexA cleavage of CTD mutants expressed as a percentage of WT LexA rate (normalized to 100% shown by the dotted line; derived from n = 10 independent WT cleavage replicates). Each bar represents the mean from replicates (grey circles; n = 7 independent cleavage experiments for single mutants and n = 4 for QM) and error bars denote standard deviation. Below the graph, the posterior likelihoods of being either less than WT or greater than QMCTD are given via pairwise Bayesian comparisons of sample means, assuming unequal variance between samples. e) Top: Representative SDS-PAGE analysis of RecA*-dependent cleavage of L2-stabilizing patch residues. Bottom: Quantified LexA cleavage of L2-stabilizing mutants in isolation or in combination, expressed as a percentage of WT LexA rate (normalized to 100% shown by the dotted line; derived from n = 10 independent WT cleavage replicates). Each bar represents the mean from replicates (grey circles; n = 7 independent cleavage experiments for single mutants and n = 3 for QM) and error bars denote standard deviation. Below the graph, the posterior likelihoods of being either less than WT or greater than DML2 are given via pairwise Bayesian comparisons of sample means, assuming unequal variance between samples.
Extended Data Fig. 7 Allosteric binding pocket on LexA and species variation.
a) Surface (left) and cartoon (right) representations of the SOS complex model, as shown in Fig. 2. RecA F203 (pink) is bound to LexA (blue) within the hydrophobic pocket formed by the highlighted LexA residues (purple). The map density is shown on the right as a mesh surface. b) Sequence alignment of LexA and RecA proteins from select different species, showing the LexA hydrophobic pocket residues (left) and a subsection of the RecA L2 loop (right). F203 is highlighted in pink.
Extended Data Fig. 8 Biochemical analysis of RecA3x mutant filamentation and LexA binding.
a) RecA3x mutant filamentation expressed as a percentage of RecA3x WT anisotropy (normalized to 100% shown by the dotted line; derived from n = 3 independent RecA3x filamentation experiments) in the FAM-ssDNA binding assay. Data show the means of three replicates, with error bars denoting standard deviation. b) RecA3x mutant binding to LexA-δ expressed as a percentage of RecA3x WT anisotropy (normalized to 100% shown by the dotted line; derived from n = 3 independent RecA3x binding experiments) in the LexA binding assay. Data show the means of three replicates, with error bars denoting standard deviation. All samples had >0.999 posterior likelihood (via pairwise Bayesian comparisons of sample means, assuming unequal variance between samples) of being less than WT, and the posterior likelihoods of each successive mutant being less than the prior mutant is shown.
Extended Data Fig. 9 LexA and RecA constructs.
Shown is an SDS-PAGE gel including various LexA constructs evaluated in this study with the molecular weights of the ladder labeled at left (single replicate).
Supplementary information
Source data
Source Data Fig. 3
Unprocessed SDS–PAGE gels with labels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed SDS–PAGE gels with labels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed SDS–PAGE gels with labels.
Source Data Extended Data Fig. 4
Gels for Extended Data Fig. 4.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed SDS–PAGE gels with labels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed SDS–PAGE gels with labels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed SDS–PAGE gels with labels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cory, M.B., Li, A., Hurley, C.M. et al. The LexA–RecA* structure reveals a cryptic lock-and-key mechanism for SOS activation. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01317-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01317-3