Abstract
The orphan G protein-coupled receptor (GPCR) GPR161 plays a central role in development by suppressing Hedgehog signaling. The fundamental basis of how GPR161 is activated remains unclear. Here, we determined a cryogenic-electron microscopy structure of active human GPR161 bound to heterotrimeric Gs. This structure revealed an extracellular loop 2 that occupies the canonical GPCR orthosteric ligand pocket. Furthermore, a sterol that binds adjacent to transmembrane helices 6 and 7 stabilizes a GPR161 conformation required for Gs coupling. Mutations that prevent sterol binding to GPR161 suppress Gs-mediated signaling. These mutants retain the ability to suppress GLI2 transcription factor accumulation in primary cilia, a key function of ciliary GPR161. By contrast, a protein kinase A-binding site in the GPR161 C terminus is critical in suppressing GLI2 ciliary accumulation. Our work highlights how structural features of GPR161 interface with the Hedgehog pathway and sets a foundation to understand the role of GPR161 function in other signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Coordinates for the GPR161–miniGs–Gβγ–Nb35 protein complex have been deposited in the RCSB PDB under accession code 8SMV. EM density maps for GPR161–miniGs–Gβγ–Nb35 complex have been deposited in the Electron Microscopy Data Bank under accession code EMD-40603. The molecular dynamics simulation trajectories have been deposited in the Zenodo database under https://doi.org/10.5281/zenodo.7887650. Publicly available PDB entries used in this study are 6LI3, 4LDO, 3SN6, 7Y89 and 8HMV. Protein sequence data for sequence alignments are available from the National Center for Biotechnology Information’s RefSeq. Sequences used in the alignment in Extended Data Fig. 7 are NP_001254539, NP_001074595, XP_004938289, XP_041427552, NP_001007200, XP_019638841, XM_002731669 and XP_782439. Mass spectrometry data and are available online. Source data are provided with this paper.
References
Civelli, O., Saito, Y., Wang, Z., Nothacker, H.-P. & Reinscheid, R. K. Orphan GPCRs and their ligands. Pharmacol. Ther. 110, 525–532 (2006).
Mukhopadhyay, S. et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic Hedgehog pathway via cAMP signaling. Cell 152, 210–223 (2013).
Hwang, S.-H., Somatilaka, B. N., White, K. & Mukhopadhyay, S. Ciliary and extraciliary Gpr161 pools repress hedgehog signaling in a tissue-specific manner. eLife 10, e67121 (2021).
Shimada, I. S. et al. Basal suppression of the Sonic Hedgehog pathway by the G-protein-coupled receptor Gpr161 restricts medulloblastoma pathogenesis. Cell Rep. 22, 1169–1184 (2018).
Hwang, S.-H. et al. The G protein-coupled receptor Gpr161 regulates forelimb formation, limb patterning and skeletal morphogenesis in a primary cilium-dependent manner. Development 145, dev154054 (2018).
Kim, S.-E. et al. Wnt1 lineage specific deletion of Gpr161 results in embryonic midbrain malformation and failure of craniofacial skeletal development. Front. Genet. 12, 761418 (2021).
Shimada, I. S. et al. Derepression of sonic hedgehog signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities. Dev. Biol. 450, 47–62 (2019).
Kim, S.-E. et al. Dominant negative GPR161 rare variants are risk factors of human spina bifida. Hum. Mol. Genet. 28, 200–208 (2019).
Li, B. I. et al. The orphan GPCR, Gpr161, regulates the retinoic acid and canonical Wnt pathways during neurulation. Dev. Biol. 402, 17–31 (2015).
Matteson, P. G. et al. The orphan G protein-coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Proc. Natl Acad. Sci. USA 105, 2088–2093 (2008).
Karaca, E. et al. Whole-exome sequencing identifies homozygous GPR161 mutation in a family with pituitary stalk interruption syndrome. J. Clin. Endocrinol. Metab. 100, E140–7 (2015).
Begemann, M. et al. Germline GPR161 mutations predispose to pediatric medulloblastoma. J. Clin. Oncol. 38, 43–50 (2020).
Feigin, M. E., Xue, B., Hammell, M. C. & Muthuswamy, S. K. G-protein–coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc. Natl Acad. Sci. USA 111, 4191–4196 (2014).
Patel, K. & Smith, N. J. Primary cilia, A-kinase anchoring proteins and constitutive activity at the orphan G protein-coupled receptor GPR161: a tale about a tail. Br. J. Pharmacol. 10.1111/bph.16053 (2023).
McMahon, A. P., Ingham, P. W. & Tabin, C. J. in Current Topics in Developmental Biology Vol. 53 (eds Iomini, C. & Sun. Y.) 1–114 (Academic Press, 2003).
Kopinke, D., Norris, A. M. & Mukhopadhyay, S. Developmental and regenerative paradigms of cilia regulated Hedgehog signaling. Semin. Cell Dev. Biol. 110, 89–103 (2021).
Truong, M. E. et al. Vertebrate cells differentially interpret ciliary and extraciliary cAMP. Cell 184, 2911–2926.e18 (2021).
Hilgendorf, K. I., Johnson, C. T. & Jackson, P. K. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr. Opin. Cell Biol. 39, 84–92 (2016).
Mukhopadhyay, S. & Rohatgi, R. G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin. Cell Dev. Biol. 33, 63–72 (2014).
Tschaikner, P. M. et al. Feedback control of the Gpr161-Gαs-PKA axis contributes to basal Hedgehog repression in zebrafish. Development 148, dev192443 (2021).
Pal, K. et al. Smoothened determines β-arrestin–mediated removal of the G protein–coupled receptor Gpr161 from the primary cilium. J. Cell Biol. 212, 861–875 (2016).
Kroeze, W. K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–369 (2015).
Pusapati, G. V. et al. G protein–coupled receptors control the sensitivity of cells to the morphogen Sonic Hedgehog. Sci. Signal. 11, eaao5749 (2018).
Foster, S. R. et al. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell 179, 895–908.e21 (2019).
Bachmann, V. A. et al. Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling. Proc. Natl Acad. Sci. USA 113, 7786–7791 (2016).
Nehmé, R. et al. Mini-G proteins: novel tools for studying GPCRs in their active conformation. PLoS ONE 12, e0175642 (2017).
Carpenter, B. & Tate, C. G. Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation. Protein Eng. Des. Sel. 29, 583–594 (2016).
Rasmussen, S. G. F. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011).
Zhou, Q. et al. Common activation mechanism of class A GPCRs. eLife 8, e50279 (2019).
Lin, X. et al. Structural basis of ligand recognition and self-activation of orphan GPR52. Nature 579, 152–157 (2020).
Ye, F. et al. Cryo-EM structure of G-protein-coupled receptor GPR17 in complex with inhibitory G protein. MedComm. 3, e159 (2022).
Wong, T.-S. et al. Cryo-EM structure of orphan G protein-coupled receptor GPR21. MedComm. 4, e205 (2023).
Wan, Q. et al. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 293, 7466–7473 (2018).
Dixon, A. S. et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 (2016).
Copp, A. J. et al. Spina bifida. Nat. Rev. Dis. Primers 1, 15007 (2015).
Eaton, S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat. Rev. Mol. Cell Biol. 9, 437–445 (2008).
Luchetti, G. et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. eLife 5, e20304 (2016).
Cooper, M. K. et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 33, 508–513 (2003).
Budelier, M. M. et al. Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J. Biol. Chem. 292, 9294–9304 (2017).
Krishnan, K. et al. Validation of trifluoromethylphenyl diazirine cholesterol analogues as cholesterol mimetics and photolabeling reagents. ACS Chem. Biol. 16, 1493–1507 (2021).
Castellano, B. M. et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355, 1306–1311 (2017).
Shin, H. R. et al. Lysosomal GPCR-like protein LYCHOS signals cholesterol sufficiency to mTORC1. Science 377, 1290–1298 (2022).
Chen, M.-H. et al. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 23, 1910–1928 (2009).
Haycraft, C. J. et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, e53 (2005).
Kim, J., Kato, M. & Beachy, P. A. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl Acad. Sci. USA 106, 21666–21671 (2009).
Chen, Q. et al. Structures of rhodopsin in complex with G-protein-coupled receptor kinase 1. Nature 595, 600–605 (2021).
Duan, J. et al. GPCR activation and GRK2 assembly by a biased intracellular agonist. Nature 620, 676–681 (2023).
Huang, W. et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308 (2020).
Staus, D. P. et al. Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579, 297–302 (2020).
Nie, Y. et al. Specific binding of GPR174 by endogenous lysophosphatidylserine leads to high constitutive Gs signaling. Nat. Commun. 14, 5901 (2023).
Yang, X. et al. Molecular mechanism of allosteric modulation for the cannabinoid receptor CB1. Nat. Chem. Biol. 18, 831–840 (2022).
Draper-Joyce, C. J. et al. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597, 571–576 (2021).
Song, G. et al. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature 546, 312–315 (2017).
Liu, X. et al. An allosteric modulator binds to a conformational hub in the β2 adrenergic receptor. Nat. Chem. Biol. 16, 749–755 (2020).
Robertson, N. et al. Structure of the complement C5a receptor bound to the extra-helical antagonist NDT9513727. Nature 553, 111–114 (2018).
Dessauer, C. W. Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol. Pharmacol. 76, 935–941 (2009).
Moore, B. S. et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc. Natl Acad. Sci. USA 113, 13069–13074 (2016).
Somatilaka, B. N. et al. Ankmy2 prevents smoothened-independent hyperactivation of the Hedgehog pathway via cilia-regulated adenylyl cyclase signaling. Dev. Cell 54, 710–726.e8 (2020).
Jiang, J. Y., Falcone, J. L., Curci, S. & Hofer, A. M. Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc. Natl Acad. Sci. USA 116, 12066–12071 (2019).
Smith, F. D. et al. Local protein kinase A action proceeds through intact holoenzymes. Science 356, 1288–1293 (2017).
May, E. A. et al. Time-resolved proteomics profiling of the ciliary Hedgehog response. J. Cell Biol. 220, e202007207 (2021).
Calebiro, D. et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7, e1000172 (2009).
Crilly, S. E. & Puthenveedu, M. A. Compartmentalized GPCR signaling from intracellular membranes. J. Membr. Biol. 254, 259–271 (2021).
Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 (2013).
Vilardaga, J.-P., Jean-Alphonse, F. G. & Gardella, T. J. Endosomal generation of cAMP in GPCR signaling. Nat. Chem. Biol. 10, 700–706 (2014).
Kinnebrew, M. et al. Cholesterol accessibility at the ciliary membrane controls Hedgehog signaling. eLife 8, e50051 (2019).
Mesmin, B. & Maxfield, F. R. Intracellular sterol dynamics. Biochim. Biophys. Acta 1791, 636–645 (2009).
Ogden, S. K. et al. G protein Gαi functions immediately downstream of Smoothened in Hedgehog signalling. Nature 456, 967–970 (2008).
Ayers, K. L. & Thérond, P. P. Evaluating Smoothened as a G-protein-coupled receptor for Hedgehog signalling. Trends Cell Biol. 20, 287–298 (2010).
Happ, J. T. et al. A PKA inhibitor motif within SMOOTHENED controls Hedgehog signal transduction. Nat. Struct. Mol. Biol. 29, 990–999 (2022).
Stubbs, T., Bingman, J. I., Besse, J. & Mykytyn, K. Ciliary signaling proteins are mislocalized in the brains of Bardet-Biedl syndrome 1-null mice. Front. Cell Dev. Biol. 10, 109216 (2023).
Badgandi, H. B., Hwang, S. H., Shimada, I. S., Loriot, E. & Mukhopadhyay, S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 216, 743–760 (2017).
Sheu, S. H. et al. A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell 185, 3390–3407.e18 (2022).
Chou, C.-H. et al. Bisdemethoxycurcumin promotes apoptosis and inhibits the epithelial-mesenchymal transition through the inhibition of the G-protein-coupled receptor 161/mammalian target of rapamycin signaling pathway in triple negative breast cancer cells. J. Agric. Food Chem. 69, 14557–14567 (2021).
Bock, A. et al. Optical mapping of cAMP signaling at the nanometer scale. Cell 182, 1519–1530.e17 (2020).
Zhang, J. Z. et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell 182, 1531–1544.e15 (2020).
Ring, A. M. et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579 (2013).
Staus, D. P. et al. Sortase ligation enables homogeneous GPCR phosphorylation to reveal diversity in β-arrestin coupling. Proc. Natl Acad. Sci. USA 115, 3834–3839 (2018).
Faust, B. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Asarnow, D., Palovcak, E. & Cheng, Y. asarnow/pyem: UCSF pyem v.0.5. Zenodo https://doi.org/10.5281/zenodo.3576630 (2019).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. eLife 7, e42166 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Schüttelkopf, A. W. & van Aalten, D. M. F. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 (2004).
Darbandi-Tonkabon, R. et al. Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. J. Biol. Chem. 278, 13196–13206 (2003).
Zhang, L. & Hermans, J. Hydrophilicity of cavities in proteins. Proteins 24, 433–438 (1996).
Qu, C. et al. Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E2 receptor EP2 subtype. Sci. Adv. 7, eabf1268 (2021).
Lomize, M. A., Lomize, A. L., Pogozheva, I. D. & Mosberg, H. I. OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625 (2006).
Betz, R. Dabble. Zenodo https://doi.org/10.5281/zenodo.836914 (2017).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Case, D. A. et al. Amber21 (Univ. California San Francisco, 2022).
Salomon-Ferrer, R., Götz, A. W., Poole, D., Le Grand, S. & Walker, R. C. Routine microsecond molecular dynamics simulations with aMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Hopkins, C. W., Le Grand, S., Walker, R. C. & Roitberg, A. E. Long-time-step molecular dynamics through hydrogen mass repartitioning. J. Chem. Theory Comput. 11, 1864–1874 (2015).
Roe, D. R. & Cheatham, T. E. 3rd PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Norman, R. X. et al. Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Hum. Mol. Genet. 18, 1740–1754 (2009).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant nos. R01GM108799 (A.S.E.), 1R35GM149287 (A.S.E.), Grant 1 P50 MH122379 (D.F.C.), R01AR054396 and R01HD089918 (J.F.R.), R35GM144136 (S.M.) and R01GM138992 (R.O.D. and A.M.). Additional support came from a National Science Foundation Graduate Research Fellowship (M.K.) and Human Frontier Science Program Long-Term Fellowship grant no. LT000916/2018-L (C.-M.S.). Cryo-EM equipment at UCSF is partially supported by NIH grant nos. S10OD020054 and S10OD021741. Some of this work was performed at the Stanford-SLAC Cryo-EM Center (S2C2), which is supported by the NIH Common Fund Transformative High-Resolution Cryo-Electron Microscopy program (grant no. U24 GM129541). We thank C. Hecksel at S2C2 personnel for invaluable support and assistance. We are indebted to J. Eggenschwiler for his generous gift of anti-Gli2 antibody. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. A.M. acknowledges support from the Edward Mallinckrodt, Jr. Foundation and the Vallee Foundation. A.M. is a Chan Zuckerberg Biohub Investigator.
Author information
Authors and Affiliations
Contributions
N.H., S.H. and I.D. cloned, expressed, and biochemically optimized the purification of GPR161 complex constructs for structural studies. N.H., S.H. and I.D. performed cryo-EM data collection, with help from the SLAC Cryo-EM Center and data processing. N.H., S.H., I.D. and A.M. built and refined models of GPR161. N.H. and S.H. generated receptor constructs and determined expression levels by flow cytometry and performed signaling studies, complementation assays and analyzed the data. M.K. and C.-M.S. performed and analyzed molecular dynamics simulations under the supervision of R.O.D. N.H. prepared samples for, performed and analyzed SPA data with A.M. Z.C. performed and analyzed mass spectrometry experiments using reagents provided by D.F.C. under the supervision of A.S.E. S.-H.H., V.R.P. and S.M. prepared constructs, performed and analyzed GPR161 localization and Hedgehog pathway repression experiments. S.P.B. performed phylogenetic analysis under the supervision of D.S.M. and additional phylogenetic analysis was provided by J.F.R. P.T., D.R. and E.S. analyzed GPR161 variants. All authors contributed to figures. N.H., S.H., A.M. and S.M. wrote the paper, with edits and approval from all authors. A.M. supervised the overall project.
Corresponding authors
Ethics declarations
Competing interests
A.M. is a founder of Epiodyne and Stipple Bio, consults for Abalone and serves on the scientific advisory board of Septerna. R.O.D. serves on the scientific advisory board of Septerna. The other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Jianhang Jia, Nicola Smith and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Biochemical preparation of GPR161-miniGs complex.
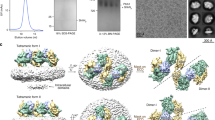
a) Cartoon depiction of GPR161 stabilization, solubilization, and purification. b) Size-exclusion chromatogram (left) and SDS-PAGE gel (right) of purified GPR161-Gs complex with Nb35. Purification and SDS-PAGE gel were done once and not repeated. Chromatogram and SDS-PAGE gel are shown for preparation used for cryo-EM analysis.
Extended Data Fig. 2 Cryogenic electron microscopy processing of GPR161.
a) A representative motion-corrected cryogenic electron microscopy (cryo-EM) micrograph obtained from a Titan Krios microscope (n = 8,294). b) A subset of highly populated, reference-free 2D-class averages. c) Schematic showing the cryo-EM data processing workflow. Initial processing was performed using UCSF MotionCor2 and cryoSPARC. Particles were transferred using the pyem script package to RELION for alignment-free 3D classification. Finally, particles were processed in cisTEM using the manual refinement job type with a 7TM mask followed by a full particle mask. Dashed boxes indicated selected classes. d) Gold-standard Fourier Shell Correlation (GSFSC) curve for final refined and sharpened map computed in cryoSPARC. e) Euler angle distribution of final refined map computed in cryoSPARC.
Extended Data Fig. 3 Cryo-EM local density.
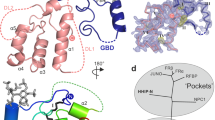
a) Orthogonal views of local resolution for the sharpened, final map of GPR161-Gs complex computed with local resolution in cryoSPARC. b) Close up of local resolution for sterol density. c) Isolated cryo-EM densities from the unsharpened, final map of GPR161 complex. Shown are the transmembrane (TM) helices, extracellular loops, and cholesterol-like density.
Extended Data Fig. 4 Comparison to additional GPCR structures.
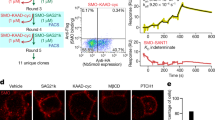
a) Structural comparison of GPR161 heterotrimer complex and β2AR heterotrimer complex (PDB ID: 3SN6) (ref. 28). GPR161 has the same hallmarks of GPCR activation as the prototypical receptor, β2AR. b) View of the GPR161 ECL2 inside the canonical Class A GPCR binding site. ECL2 makes multiple hydrophobic interactions deep within the pocket. The superficial part of the pocket harbors ionic interactions between ECL2 and the binding pocket. c) Structural comparison of GPR161 to other orphan GPCRs with self-activating ECL2, including GPR17 (PDB ID: 7Y89) and GPR21 (PDB ID: 8HMV)31,32. The cis-interaction of ECL2 with the canonical ligand-binding site is seen across self-activating orphan GPCRs but the precise loop conformation changes between receptors. d) Luminescence for β-arrestin recruitment in the PRESTO-Tango assay when compared across 314 GPCRs (data replotted from Kroeze WM et al.22, n = 4 for each target, shown as mean ± s.e.m. of technical replicates). GPR21 yields a signal slightly above the median. GPR52 yields a signal one order of magnitude above the median. GPR161 and GPR17 yield a signal about two orders of magnitude above the median.
Extended Data Fig. 5 GPR161 molecular dynamics simulation trajectories.
a) Time traces of ECL2 position in all six unrestrained simulations of GPR161 with miniGs removed. ECL2 position is represented by distance between W182 and T189. b) Time traces of distance between cholesterol and GPR161 residue W3277.56 in all six unrestrained simulations of GPR161 with miniGs removed. c) Time traces of distance between cholesterol and GPR161 residue W3277.56 in all six simulations where GPR161 residues that contact miniGs are restrained to their miniGs bound conformation. d) A comparison of the cryo-EM structure (green and magenta) to a representative snapshot from an unrestrained simulation of GPR161 with miniGs removed shows that, in the absence of miniGs, the intracellular ends of TM6 and TM7 move inwards, obstructing the Gs binding site.
Extended Data Fig. 6 Surface expression of GPR161 mutants.
a) Representative flow cytometry surface expression histograms for receptors and mutants used in cell-based assays. b) Surface expression of receptors and mutants quantified by anti-FLAG-A647 median fluorescence intensity ± sd from n = 3 (for L465PC-term) or n = 4 (for rest) biologically independent samples.
Extended Data Fig. 7 Phylogenetic analysis of GPR161.
a) BLAST search results for Human GPR161 (Uniprot: Q8N6U8). Sequences are plotted from highest confidence (E-Value) and highest sequence identity (% identity) to lowest. Representative organisms spanning the full range of homologous GPR161 sequences are listed. b) Full sequence alignment of eight GPR161 model organism sequences identified in BLAST search.
Extended Data Fig. 8 Photolabeling with LKM238 and mass spectrometry sequence coverage.
a) Product ion spectrum of LKM238-labeled GPR161-miniGs with peptides mapped to TM6. This peptide is modified with a mass consistent with LKM238 at position K2676.32. Red brackets and peaks indicate product ions that contain the LKM238 adduct. b) Mass spectrometric sequence coverage of GPR161-miniGs. Underlined segments indicate transmembrane spanning helices, red font indicates peptides identified by tandem MS analysis and gray font indicates glycosylation sites.
Extended Data Fig. 9 GPR161 localization and repression of GLI2 ciliary trafficking.
a) Representative images of GPR161 mutants on ciliary localization and GLI2 repression in ciliary tips in NIH 3T3 cells. NIH 3T3 Flp-In CRISPR based Gpr161−/− cells stably expressing untagged mouse wild-type or Gpr161 mutants were starved for 24 h upon confluence and were treated for further 24 h ± SAG (500 nM). After fixation, cells were immunostained with anti-GLI2 (red), anti-GPR161 (green), anti-acetylated, and centrosome (AcTub; PCNT purple) antibodies. Whole cell images with an arrow indicating imaged cilia. Scale bar, 5 µm. b) Quantification of GPR161 positive cilia indicating trafficking and egress of GPR161 from cilia in the pathway off and on state, respectively. ECL2 mutants do not traffic to cilia suggesting impaired biogenesis. GPR161-V129E3.54 does not egress from cilia following pathway activation and GPR161-L465PC-term has reduced egress compared to GPR161. (*P < 0.05; ns, not significant; two-way ANOVA followed by Šidák’s multiple comparison tests; Adjusted P values for DMSO vs. SAG: NIH3T3, Gpr161−/− + WT, +AAA7.52, 7.56, 8.51, +L465PC-term, + AAA7.52, 7.56, 8.51 L465PC-term = <0.0001, Gpr161−/−, Gpr161−/− + W182RECL2, +W182GECL2 = > 0.9999, Gpr161−/− + V129E3.54 = 0.997) c) Quantification of GLI2 positive cilia indicating Hedgehog pathway activation. ECL2 mutants and GPR161-V129E3.54 do not rescue, similar to Gpr161−/−. For b,c, data are shown from n = 3 independent experiments from images taken from 2-3 different regions/experiment and counting 15-30 cells/region. Data are mean ± s.d. (*P < 0.05; ns, not significant; two-way ANOVA followed by Šidák’s multiple comparison tests; Adjusted P values for DMSO vs. SAG: NIH3T3, Gpr161−/− + WT = < 0.0001, Gpr161−/− = >0.9999, Gpr161−/− + W182RECL2 = 0.9705, Gpr161−/− + W182GECL2 = 0.9724, Gpr161−/− + V129E3.54 = 0.9882, Gpr161−/− + AAA7.52, 7.56, 8.51 + L465PC-term = 0.9917). d) Transcript abundance of wild-type and mutant Gpr161 constructs stably expressed in Gpr161−/− NIH3T3 cells quantified by quantitative RT-PCR. e) GPR161-V129E3.54 has reduced recruitment of miniGs compared to WT. Nanoluc complementation assay for receptor recruitment of miniGs. Data are mean ± s.d., n = 2 (for V129E3.54) or n = 4 (for GPR161) biologically independent samples (*P < 0.05; ns, not significant; Unpaired two-tailed t test with Welch’s correction; Adjusted P value: GPR161 vs. V129E3.54 = 0.0008). f) GPR161-V129E3.54 has similar recruitment of PKA-RI compared to GPR161. Nanoluc complementation assay for receptor recruitment of PKA-RI. Data are mean ± s.d., n = 2 (for V129E3.54) or n = 5 (for GPR161) biologically independent samples (*P < 0.05; ns, not significant; Unpaired two-tailed t test with Welch’s correction; Adjusted P value: GPR161 vs. V129E3.54 = 0.9406).
Supplementary information
Source data
Source Data Figs. 2–5 and Extended Data Figs. 1, 6, 8 and 9.
Numerical data from graphs. Data from each figure appear on one Excel tab.
Source Data Extended Data Fig. 1
Unprocessed gel from Extended Data Fig.1.
Source Data Extended Data Fig. 6
Flow cytometry expression data and unprocessed gel from Extended Data Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoppe, N., Harrison, S., Hwang, SH. et al. GPR161 structure uncovers the redundant role of sterol-regulated ciliary cAMP signaling in the Hedgehog pathway. Nat Struct Mol Biol 31, 667–677 (2024). https://doi.org/10.1038/s41594-024-01223-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01223-8
This article is cited by
-
Shifting our perspective on orphan G protein-coupled receptors
Nature Structural & Molecular Biology (2024)