Abstract
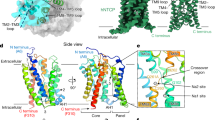
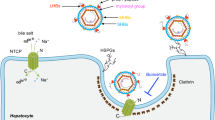
Hepatitis B virus (HBV), a leading cause of developing hepatocellular carcinoma affecting more than 290 million people worldwide, is an enveloped DNA virus specifically infecting hepatocytes. Myristoylated preS1 domain of the HBV large surface protein binds to the host receptor sodium-taurocholate cotransporting polypeptide (NTCP), a hepatocellular bile acid transporter, to initiate viral entry. Here, we report the cryogenic-electron microscopy structure of the myristoylated preS1 (residues 2–48) peptide bound to human NTCP. The unexpectedly folded N-terminal half of the peptide embeds deeply into the outward-facing tunnel of NTCP, whereas the C-terminal half formed extensive contacts on the extracellular surface. Our findings reveal an unprecedented induced-fit mechanism for establishing high-affinity virus–host attachment and provide a blueprint for the rational design of anti-HBV drugs targeting virus entry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps and related structure coordinates of human NTCP–myr-preS1–YN9048Fab and NTCP–myr-preS1–YN9016Fab have been deposited in the EMDB and PDB under accession codes EMD-34981 (PDB 8HRX) and EMD-34982 (PDB 8HRY), respectively. Source data are provided with this paper.
References
Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403 (2018).
World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections (WHO, 2021).
Gripon, P., Cannie, I. & Urban, S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 79, 1613–1622 (2005).
Glebe, D. et al. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 129, 234–245 (2005).
Bruss, V., Hagelsten, J., Gerhardt, E. & Galle, P. R. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 218, 396–399 (1996).
Gripon, P., Leseyec, J., Rumin, S. & Guguenguillouzo, C. Myristylation of the hepatitis-B virus large surface protein is essential for viral infectivity. Virology 213, 292–299 (1995).
Urban, S., Bartenschlager, R., Kubitz, R. & Zoulim, F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology 147, 48–64 (2014).
Ni, Y. et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146, 1070–1083 (2014).
Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife https://doi.org/10.7554/eLife.00049 (2012).
Schulze, A., Schieck, A., Ni, Y., Mier, W. & Urban, S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 84, 1989–2000 (2010).
Hollnberger, J. et al. No virologic resistance to bulevirtide monotherapy detected in patients through 24 weeks treatment in phase II and III clinical trials for chronic hepatitis delta. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.04.027 (2023).
Anwer, M. S. & Stieger, B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflug. Arch. 466, 77–89 (2014).
Appelman, M. D., Wettengel, J. M., Protzer, U., Oude Elferink, R. P. J. & van de Graaf, S. F. J. Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids 1866, 158960 (2021).
Park, J. H. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027 (2022).
Liu, H. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 32, 773–776 (2022).
Goutam, K., Ielasi, F. S., Pardon, E., Steyaert, J. & Reyes, N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015 (2022).
Asami, J. et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022).
Fu, L., Hu, H., Liu, Y., Jing, Z. & Li, W. Woodchuck sodium taurocholate cotransporting polypeptide supports low-level hepatitis B and D virus entry. Virology 505, 1–11 (2017).
Jacquet, S. et al. Evolution of hepatitis B virus receptor NTCP reveals differential pathogenicities and species specificities of hepadnaviruses in primates, rodents, and bats. J. Virol. https://doi.org/10.1128/JVI.01738-18 (2019).
Muller, S. F., Konig, A., Doring, B., Glebe, D. & Geyer, J. Characterisation of the hepatitis B virus cross-species transmission pattern via Na+/taurocholate co-transporting polypeptides from 11 New World and Old World primate species. PLoS ONE 13, e0199200 (2018).
Saito, S. et al. Catalog of 238 variations among six human genes encoding solute carriers (hSLCs) in the Japanese population. J. Hum. Genet. 47, 576–584 (2002).
Takeuchi, J. S. et al. A single adaptive mutation in sodium taurocholate cotransporting polypeptide induced by hepadnaviruses determines virus species specificity. J. Virol. https://doi.org/10.1128/JVI.01432-18 (2019).
Wang, P. et al. Genetic variations of NTCP are associated with susceptibility to HBV infection and related hepatocellular carcinoma. Oncotarget 8, 105407–105424 (2017).
Yan, H. et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 87, 7977–7991 (2013).
Chi, S. W., Kim, D. H., Lee, S. H., Chang, I. & Han, K. H. Pre-structured motifs in the natively unstructured preS1 surface antigen of hepatitis B virus. Protein Sci. 16, 2108–2117 (2007).
Zakrzewicz, D. et al. Tyrosine 146 of the human Na(+)/taurocholate cotransporting polypeptide (NTCP) is essential for its hepatitis B virus (HBV) receptor function and HBV entry into hepatocytes. Viruses https://doi.org/10.3390/v14061259 (2022).
Yan, H. et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J. Virol. 88, 3273–3284 (2014).
Petersen, J. et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat. Biotechnol. 26, 335–341 (2008).
Manel, N. et al. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115, 449–459 (2003).
Kim, J. W., Closs, E. I., Albritton, L. M. & Cunningham, J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352, 725–728 (1991).
Sugase, K., Dyson, H. J. & Wright, P. E. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447, 1021–1025 (2007).
Dunker, A. K. et al. Intrinsically disordered protein. J. Mol. Graph Model 19, 26–59 (2001).
Yan, R. et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367, 1444–1448 (2020).
Hwang, S. S. et al. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science 367, 1255–1260 (2020).
Gong, X. et al. Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell 165, 1467–1478 (2016).
Kirstgen, M. et al. Selective hepatitis B and D virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci. Rep. 10, 21772 (2020).
Hu, H. H. et al. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut 65, 1514–1521 (2016).
Peng, L. et al. The p.Ser267Phe variant in SLC10A1 is associated with resistance to chronic hepatitis B. Hepatology 61, 1251–1260 (2015).
Ho, R. H., Leake, B. F., Roberts, R. L., Lee, W. & Kim, R. B. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J. Biol. Chem. 279, 7213–7222 (2004).
Winer, B. Y. & Ploss, A. Determinants of hepatitis B and delta virus host tropism. Curr. Opin. Virol. 13, 109–116 (2015).
Watashi, K. & Wakita, T. Hepatitis B virus and hepatitis D virus entry, species specificity, and tissue tropism. Cold Spring Harb. Perspect. Med. 5, a021378 (2015).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D.-Struct. Biol. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Iwamoto, M. et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem. Biophys. Res. Commun. 443, 808–813 (2014).
Shibuya, K. et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J. Exp. Med. 198, 1829–1839 (2003).
Kobayashi, C. et al. Fungal secondary metabolite exophillic acid selectively inhibits the entry of Hepatitis B and D viruses. Viruses https://doi.org/10.3390/v14040764 (2022).
Acknowledgements
We thank Y. Sakamaki and M. Kikkawa (Cryo-EM facility in the University of Tokyo, Tokyo, Japan) for their help in cryo-EM data collection. This work was supported by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology under grant numbers 19H00976 (T.S.), 20H03499 (K.W.), JP19H05779 (S.-Y.P.), 21H02449 (S.-Y.P.), 18K05334 (N.N.), 19H00923 (S.I. and N.N.), 22H02556 (U.O.) and 23H02724 (K.W. and N.N.). This work was supported by Japan Agency for Medical Research and Development, AMED under grant numbers JP22fk0310517 (S.-Y.P. and M.M.) and JP23fk0310504 (K.W.). This work is partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research) from AMED under grant numbers JP19am0101115 (support nos. 1570, 1846 and 1848) and JP22ama121007j0001 (S.I., K.W. and N.N.), Joint Usage/Research Center Program of Institute for Life and Medical Sciences, Kyoto University (N.N.), Japan Foundation for Applied Enzymology (K.W.), Takeda Science Foundation (K.W. and N.N.), Mitsubishi Foundation (N.N.) and the Knut and Alice Wallenberg foundation (D.D.).
Author information
Authors and Affiliations
Contributions
The experimental design was developed by J.A., K.W., S.-Y.P., N.N. and U.O. The preparation of recombinant proteins for antibody generation and structural analysis was carried out by J.A., J.-H., P., Y.N., N.I., Y.S., D.D. and N.N. Antibody was generated by Y.N., T.U., K.L., Y.S., S.I. and N.N. Cryo-EM analysis was carried out by J.A., Z.Z. and U.O. The transport assay, preS1-binding and HBV infection assays were done by C.K., J.M., M.M., T.W. and K.W. Visualization was done by J.A., Z.Z. and U.O. Validation, data curation and project administration were done by K.W., S.-Y.P., N.N. and U.O. The original draft was written by J.A. and U.O. Review and editing were carried out by J.A., D.D., K.W., S.-Y.P., N.N. and U.O. Supervision of the project was the responsibility of S.I., T.S., K.W., S.-Y.P., N.N. and U.O. Funding was acquired by M.M., D.D., S.I., T.S., K.W., S.-Y.P., N.N. and U.O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Edward Yu and Adam Zlotnick for their contribution to the peer review of this work. Peer reviewer reports are available. Sara Osman was the Primary Editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Topology of LHBs and NTCP.
a, Topology diagrams of LHBs (top) and NTCP (bottom). Orange, myristoyl moiety; red, preS1 (2–108). Helices of NTCP are colored from blue to yellow, from the N- to C-terminus. Core (TM2–4 and TM7–9) and panel (TM1, TM5 and TM6) domains are indicated. b, Amino acid sequence alignment of preS1(2–48) from HBV genotypes A-H. Consensus, consensus sequence of all HBV genotypes. The sequence of Myrcludex B (Myr-B) is derived from that of the HBV genotype C. Loop A (residues 7–19) is shaded orange. c, PreS1 (ribbon models, orange) in complex with human NTCP (surface representations, cyan) are shown parallel to the membrane (top) and from the extracellular side (bottom). Side chains of the residues different from the sequence of Myrcludex B (Myr-B) are displayed as stick representations.
Extended Data Fig. 2 Sequence alignment of mammalian NTCPs.
Sequence alignment of human, chimpanzee, hamadryas, squirrel monkey, horse, bovine, rat, and mouse NTCPs calculated using Clustal Omega. Colored bars above the sequence indicate the locations of transmembrane helices in human NTCP. Residues involved in preS1 binding are highlighted in yellow (site 1) and cyan (site 2). Residues 87, 158, and 267 are represented by black lines.
Extended Data Fig. 3 Cryo-EM analysis of NTCP/myr-preS1 complex.
a, Representative size-exclusion chromatography of the human NTCP/myr-preS1 complex. Absorbance profiles (top) and SDS-PAGE analysis after staining with Coomassie Brilliant Blue (CBB, bottom) are shown. The pooled fractions are indicated. Absorbances at 280 nm and 260 nm are shown as solid and dashed lines, respectively. Purifications were performed twice. b, c, Data processing workflow of cryo-EM analysis of human NTCP/myr-preS1 in complex with YN9048Fab (b) and YN9016Fab (c). Representative motion-corrected micrographs (out of 11,785 (b) and 5,711 (c) total micrographs), 2D class averages, gold-standard FSC curves of the final 3D reconstruction (resolution cut-off at FSC = 0.143), and the final 3D map (colored according to the local resolution) are shown. The 2D class averages were calculated using refined particles that were used for the final reconstruction.
Extended Data Fig. 4 Cryo-EM density maps of NTCP and preS1.
a, Cryo-EM density maps of the human NTCP/myr-preS1/YN9048Fab complex. Each TM helix of NTCP and preS1 is shown. b, PreS1 (density map and ribbon representation) binding to human NTCP (surface and ribbon representations), shown parallel to the membrane. Densities of possible myristoyl moieties at the N-terminus of preS1 (G2) are indicated. Cyan, NTCP; orange, myr-preS1.
Extended Data Fig. 5 Interface between NTCP/myr-preS1 complex and Fab.
a, Overall structure of human NTCP/myr-preS1 in complex with YN9048Fab (left) and YN9016Fab (right). The cryo-EM maps (top) and ribbon models (bottom) are shown. NTCP, preS1, and the heavy and light chains of Fab are shown in cyan, orange, red, and pink, respectively. b, Fab-binding epitopes on the human NTCP/myr-preS1 complex. Surface and ribbon models of the human NTCP/myr-preS1 structure are shown at the top. The NTCP regions involved in Fab binding are indicated. Dashed line: regions involved in interaction with the heavy chain of Fab. Dotted line: regions involved in interaction with the light chain of Fab. c, List of residues involved in the interactions with Fab. d, Structural comparison of human NTCP/myr-preS1 in complex with YN9048Fab and YN9016Fab. A close-up view of preS1 on the extracellular surface, indicated by a black rectangle on the left, is shown (right). Side chains of the residues of preS1 are shown as stick representations. RMSD values are indicated. e, Structural comparison of human NTCP in the apo state (grey, PDB: 7VAD)17 and in complex with preS1 (cyan). Core (left) and panel (right) domains are shown. RMSD values are indicated.
Extended Data Fig. 6 Intramolecular interaction of preS1.
a, Cross-sectional views of human NTCP in complex with myr-preS1 (top). PreS1 is shown as a ribbon model. PreS1 is colored from blue to yellow, from the N- to C-terminus. Side chains of the residues involved in intramolecular interactions are displayed as stick representations. Red dashed line, salt bridge; black dashed line, hydrogen bond. Close-up views of the anti-parallel β-strand (residues 3–5 and 32–34) (bottom left) and two pairs of salt bridges (K24–D31 and D33–K38) (bottom right). b, List of residues involved in the intermolecular hydrogen bonds between preS1 and human NTCP.
Extended Data Fig. 7 Evaluation of preS1 binding.
a, Fluorescence images of the preS1 binding assay in HepG2 cells expressing the wild type NTCP treated with the myr-preS1 mutants at 250 nM. Scale bar, 100 μm. b, Fluorescence images of the preS1 binding assay in Huh-7 cells expressing the wild type NTCP or its mutants treated with the myr-2–48 at 40 nM (left). The expression levels of NTCP (upper) and actin as a loading control (lower) were monitored by western blotting (right). NTCP and actin were detected on different gels. Scale bar, 100 μm. The data shows the means of the results from three independent experiments.
Extended Data Fig. 8 Inhibition of substrate transport by preS1 binding and effect of mutations.
a, Superposition of human NTCP/myr-preS1 (cyan, this study) and human NTCP/GCDC (dark blue, PDB: 7ZYI ref. 15). Residues 9–15 of preS1 (red) occupy the same position as a part of the GCDC molecules. b, Structural comparison of the human NTCP/myr-preS1 complex (this study) and human NTCP in the inward-facing conformation (PDB: 7PQG ref. 16), shown at the top. Distance between A273 (TM8b) in the NTCP/myr-preS1 complex and inward-facing NTCP is indicated. Residues 10–16 of preS1 (red) occupy the same position as TM8b in the inward-facing NTCP. The inward facing NTCP alone is shown (right). c, Close-up views of S267 (top left, this study; bottom left, PDB: 7ZYI ref. 15), N87 (top right), and G158 (bottom right) with respect to preS1 or bile acid binding. Residues near S267, N87, and G158 are shown as stick and space-filling representations.
Supplementary information
Source data
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Uncropped gel image with molecular weight marker.
Source Data Extended Data Fig. 7
Uncropped and unprocessed images overlay with molecular weight marker.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asami, J., Park, JH., Nomura, Y. et al. Structural basis of hepatitis B virus receptor binding. Nat Struct Mol Biol 31, 447–454 (2024). https://doi.org/10.1038/s41594-023-01191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01191-5
This article is cited by
-
Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP
Nature Communications (2024)