Abstract
Pioneer transcription factors are vital for cell fate changes. PU.1 and C/EBPα work together to regulate hematopoietic stem cell differentiation. However, how they recognize in vivo nucleosomal DNA targets remains elusive. Here we report the structures of the nucleosome containing the mouse genomic CX3CR1 enhancer DNA and its complexes with PU.1 alone and with both PU.1 and the C/EBPα DNA binding domain. Our structures reveal that PU.1 binds the DNA motif at the exit linker, shifting 17 bp of DNA into the core region through interactions with H2A, unwrapping ~20 bp of nucleosomal DNA. C/EBPα binding, aided by PU.1’s repositioning, unwraps ~25 bp of entry DNA. The PU.1 Q218H mutation, linked to acute myeloid leukemia, disrupts PU.1-H2A interactions. PU.1 and C/EBPα jointly displace linker histone H1 and open the H1-condensed nucleosome array. Our study unveils how two pioneer factors can work cooperatively to open closed chromatin by altering DNA positioning in the nucleosome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM reconstructions and atomic models of the CX3CR1 nucleosome and the nucleosome–pioneer factors complexes have been deposited in the Electron Microscopy Data Bank and the Protein Data Bank under the following accession codes: EMD-40889 and PDB 8SYP for the 162-bp CX3CR1 DNA nucleosome; EMD-28629 and PDB 8EVH for the wild-type nucleosome–PU.1 complex; EMD-28630 and PDB 8EVI for the nucleosome containing T77CH2A bound G220CPU.1; EMD-28631 and PDB 8EVJ for the nucleosome (T77CH2A) bound G220CPU.1 and MBP-DBDRC/EBPα. Structures used for model building can be found in the PDB, including PDB 7K61 for nucleosome bound to scFv, PDB 1PUE for PU.1 bound to DNA and PDB 1NWQ for C/EBPα bound to DNA. Source data are provided with this paper.
References
Larson, E. D., Marsh, A. J. & Harrison, M. M. Pioneering the developmental frontier. Mol. Cell 81, 1640–1650 (2021).
Balsalobre, A. & Drouin, J. Pioneer factors as master regulators of the epigenome and cell fate. Nat. Rev. Mol. Cell Biol. 23, 449–464 (2022).
Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997).
Zhou, B. R. et al. Distinct structures and dynamics of chromatosomes with different human linker histone isoforms. Mol. Cell 81, 166–182 e166 (2021).
Zaret, K. S. Pioneer transcription factors initiating gene network changes. Annu. Rev. Genet. 54, 367–385 (2020).
Iwafuchi-Doi, M. & Zaret, K. S. Pioneer transcription factors in cell reprogramming. Genes Dev. 28, 2679–2692 (2014).
Zhu, F. et al. The interaction landscape between transcription factors and the nucleosome. Nature 562, 76–81 (2018).
Donovan, B. T., Chen, H., Jipa, C., Bai, L. & Poirier, M. G. Dissociation rate compensation mechanism for budding yeast pioneer transcription factors. eLife https://doi.org/10.7554/eLife.43008 (2019).
Michael, A. K. et al. Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Science 368, 1460–1465 (2020).
Dodonova, S. O., Zhu, F., Dienemann, C., Taipale, J. & Cramer, P. Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature 580, 669–672 (2020).
Tanaka, H. et al. Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat. Commun. 11, 4136 (2020).
Guan, R. et al. Structural and dynamic mechanisms of CBF3-guided centromeric nucleosome formation. Nat. Commun. 12, 1763 (2021).
Echigoya, K. et al. Nucleosome binding by the pioneer transcription factor OCT4. Sci. Rep. 10, 11832 (2020).
Roberts, G. A. et al. Dissecting OCT4 defines the role of nucleosome binding in pluripotency. Nat. Cell Biol. 23, 834–845 (2021).
Takizawa, Y. et al. Cryo-EM structure of the nucleosome containing the the ALB1 enhancer DNA sequence. Open Biol. https://doi.org/10.1098/rsob.170255 (2018).
Oikawa, T. et al. The role of Ets family transcription factor PU.1 in hematopoietic cell differentiation, proliferation and apoptosis. Cell Death Differ. 6, 599–608 (1999).
Mueller, B. U. et al. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood 100, 998–1007 (2002).
Le Coz, C. et al. Constrained chromatin accessibility in PU.1-mutated agammaglobulinemia patients. J. Exp. Med. https://doi.org/10.1084/jem.20201750 (2021).
Rosenbauer, F. et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat. Genet. 36, 624–630 (2004).
Friedman, A. D. Transcriptional control of granulocyte and monocyte development. Oncogene 26, 6816–6828 (2007).
Pham, T. H. et al. Mechanisms of in vivo binding site selection of the hematopoietic master transcription factor PU.1. Nucleic Acids Res. 41, 6391–6402 (2013).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
van Oevelen, C. et al. C/EBPα activates pre-existing and de novo macrophage enhancers during induced pre-B cell transdifferentiation and myelopoiesis. Stem Cell Rep. 5, 232–247 (2015).
Barozzi, I. et al. Coregulation of transcription factor binding and nucleosome occupancy through DNA features of mammalian enhancers. Mol. Cell 54, 844–857 (2014).
Fernandez Garcia, M. et al. Structural features of transcription factors associating with nucleosome binding. Mol. Cell 75, 921–932 (2019).
Nerlov, C. C/EBPα mutations in acute myeloid leukaemias. Nat. Rev. Cancer 4, 394–400 (2004).
van Riel, B. & Rosenbauer, F. Epigenetic control of hematopoiesis: the PU.1 chromatin connection. Biol. Chem. 395, 1265–1274 (2014).
Yamamoto, H., Kihara-Negishi, F., Yamada, T., Hashimoto, Y. & Oikawa, T. Physical and functional interactions between the transcription factor PU.1 and the coactivator CBP. Oncogene 18, 1495–1501 (1999).
Nerlov, C. & Ziff, E. B. CCAAT/enhancer binding protein-α amino acid motifs with dual TBP and TFIIB binding ability co-operate to activate transcription in both yeast and mammalian cells. EMBO J. 14, 4318–4328 (1995).
Minderjahn, J. et al. Mechanisms governing the pioneering and redistribution capabilities of the non-classical pioneer PU.1. Nat. Commun. 11, 402 (2020).
Kovács, K. A., Steinmann, M., Magistretti, P. J., Halfon, O. & Cardinaux, J. R. CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation. J. Biol. Chem. 278, 36959–36965 (2003).
Pedersen, T. A., Kowenz-Leutz, E., Leutz, A. & Nerlov, C. Cooperation between C/EBPα TBP/TFIIB and SWI/SNF recruiting domains is required for adipocyte differentiation. Genes Dev. 15, 3208–3216 (2001).
Zhou, B. R. et al. Atomic resolution cryo-EM structure of a native-like CENP-A nucleosome aided by an antibody fragment. Nat. Commun. 10, 2301 (2019).
Solomon, L. A., Li, S. K., Piskorz, J., Xu, L. S. & DeKoter, R. P. Genome-wide comparison of PU.1 and Spi-B binding sites in a mouse B lymphoma cell line. BMC Genomics 16, 76 (2015).
Polach, K. J. & Widom, J. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol. 254, 130–149 (1995).
Soufi, A. et al. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161, 555–568 (2015).
Donovan, B. T. et al. Basic helix-loop-helix pioneer factors interact with the histone octamer to invade nucleosomes and generate nucleosome-depleted regions. Mol. Cell 83, 1251–1263 (2023).
Frederick, M. A. et al. A pioneer factor locally opens compacted chromatin to enable targeted ATP-dependent nucleosome remodeling. Nat. Struct. Mol. Biol. 30, 31–37 (2023).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855 (2018).
Hall, M. A. et al. High-resolution dynamic mapping of histone-DNA interactions in a nucleosome. Nat. Struct. Mol. Biol. 16, 124–129 (2009).
Zhao, D. et al. Quantitative modeling of nucleosome unwrapping from both ends. Biophys. J. 117, 2204–2216 (2019).
Morgunova, E. & Taipale, J. Structural perspective of cooperative transcription factor binding. Curr. Opin. Struct. Biol. 47, 1–8 (2017).
Mirny, L. A. Nucleosome-mediated cooperativity between transcription factors. Proc. Natl Acad. Sci. USA 107, 22534–22539 (2010).
Zhou, B. R. & Bai, Y. Preparation of scFv stabilized chromatosomes for single-particle cryo-EM structure determination. STAR Protoc. 2, 100396 (2021).
Dorigo, B. et al. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306, 1571–1573 (2004).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife https://doi.org/10.7554/eLife.42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Shimko, J. C., North, J. A., Bruns, A. N., Poirier, M. G. & Ottesen, J. J. Preparation of fully synthetic histone H3 reveals that acetyl-lysine 56 facilitates protein binding within nucleosomes. J. Mol. Biol. 408, 187–204 (2011).
Acknowledgements
We thank R. Huang and A. Zeher for assistance with cryo-EM data collection. The cryo-EM work utilized the NCI-NIH IRP Cryo-EM Consortium (NICE) microscopy resource and the NIH Biowulf high-performance computing system for data processing. This work was supported by the intramural research program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health (Y.B.).
Author information
Authors and Affiliations
Contributions
Y.B. conceived the project. T.L. and R.G. conducted the experiment. B.-R.Z. provided the scFv, H1 protein and W601 array DNA. Y.B., R.G. and T.L. analyzed the structures and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Hitoshi Kurumizaka, Paul Wade and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Sara Osman and Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
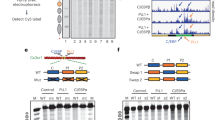
Extended Data Fig. 1 Characterization of PU.1 and C/EBPα binding to CX3CR1 nucleosome.
a, SDS-PAGE of the protein samples used in this study. b and c, EMSA of CX3CR1 nucleosome association to PU.1 without (b) and with scFv (c). d to f, EMSA of CX3CR1 nucleosome binding by DBDC/EBPα (d), MBP-DBDC/EBPα (e) and MBP-DBDC/EBPα with scFv (f). For the experiments, the nucleosome concentration is 0.5 μM. The ratios of PU.1 proteins over the nucleosomes are 0, 0.25, 0.5, 1.0, 1.5, 2.0 and 2.5, respectively. The ratios of the C/EBPα proteins over the nucleosomes are 0, 1.0, 2.0, 3.0, 4.0, 6.0 and 8.0, respectively. g, Quantification of the CX3CR1 nucleosome binding by PU.1 with and without scFv. h, Quantification of the CX3CR1 nucleosome binding by MBP-DBDC/EBPα with and without scFv. The Kdapp values were obtained by fitting the data to Hill equation. In g and h, data represent mean and s.d. based on at three parallel experiments.
Extended Data Fig. 2 Data collection and processing for the free 162 bp CX3CR1 nucleosome.
a, Raw image. The scale bar represents 50 nm. Particles are similar on all the other micrographs. b, 2D classification results. c, Typical particle analysis. Note that resolution of the blue class can reach 2.9 Å and it has the same dyad location as the gray class. The dyad location of the other classes could not be determined due to lower resolution. d, FSC curves. e, Particle orientation distribution. f, Local resolution. g, Model of the nucleosome with scFv shows that scFv does not interact with DNA.
Extended Data Fig. 3 Data collection and processing for the 162 bp CX3CR1 nucleosome bound to PU.1.
a, Raw image. The scale bar represents 50 nm. Particles are similar on all micrographs. b, 2D classification results. The black arrow indicates the PU.1 features. c, Particle analysis. d, FSC curves. e, Particle orientation distribution. f, Local resolution. DNA was built from scratch based on the Cryo-EM map. g, At the nucleosome DNA exit site, there is a ‘GGAA’ located at the position with the extra density, indicating the PU.1 binding on the canonical site. h, Fitting of PU.1 crystal structure (PDB: 1PUE) into the extra density on the unwrapped DNA. i, FRET results show that H2A-H2B is not dissociated from the nucleosome core after PU.1 binding.
Extended Data Fig. 4 Data collection and processing for the 167 bp CX3CR1 nucleosome containing T77CH2A bound to G220CPU.1.
a, Raw image. The scale bar represents 50 nm. Particles are similar on all micrographs. b, 2D classification results. The black arrows indicate the densities of PU.1. c, Particle analysis. d, FSC curves. e, Particle orientation distribution. f, Local resolution. g, Cryo-EM map showing the disulfide bond between G220CPU.1 and T77CH2A. h, Non-reducing SDS-PAGE of nucleosome−PU.1 complex without and with the oxidant. The experiment was repeated three times with similar results. i, Structure of the nucleosome-PU.1 complex with scFv shows that scFv does not interact with PU.1.
Extended Data Fig. 5 Representative Cryo-EM densities and fitting of the structural models.
a, Histones and scFv in the H2A-T77Cnucleosome-G220CPU.1-scFv complex. b, Fitting of the H2A-T77Cnucleosome-G220CPU.1 model to the wild-type nucleosome-PU.1 density map, showing that the disulfide bond does not change the complex structure. c, Illustration of the density map at the interface of ETSPU.1 and DNA with highlights on the residues that interact with DNA. d, Fitting of H2A-T77Cnucleosome-G220CPU.1 structure into the density of the wild type nucleosome-PU.1-scFv complex.
Extended Data Fig. 6 Data collection and processing for the 167 bp CX3CR1 nucleosome containing T77CH2A bound to G220CPU.1 and MBP-DBDC/EBPα.
a, Raw image. The scale bar represents for 50 nm. Particles are similar on all micrographs. b, 2D classification results. c, Particle analysis. d, FSC curves. e, Particle orientation distribution. f, Local resolution. g, Alignment of three classes with different entry site DNA unwrapping degrees. h, Fitting of C/EBPα crystal structure (PDB: 1NWQ) into the extra density at unwrapped entry site DNA. i, Modeling of binding of C/EBPα binding to the fully wrapped nucleosome at the location corresponding to that in g.
Extended Data Fig. 7 Verification of C/EBPα binding location.
a, EMSA of Cmutnucleosome (the C/EBPα binding location ‘CAGCTGGTTG’ suggested by the cryo-EM result was mutated to’CAGCAACTTG’) binding by MBP-DBDC/EBPα. For the experiment, the nucleosome concentration is 0.5 μM. The ratios of the C/EBPα proteins over the nucleosomes are 0, 1.0, 2.0, 3.0, 4.0, 6.0 and 8.0, respectively. b, Quantification of the wild type CX3CR1 nucleosome (Extended Data Fig. 1h) and Cmutnucleosome binding by MBP-DBDC/EBPα. The apparent Kdapp values were obtained by fitting the Data to Hill equation. Data represent mean and s.d. based on three independent experiments. c and d, EMSA of the W601 nucleosome (c) and 601CE-site nucleosome (‘CAGCTGGTTG’ was inserted into W601 at the corresponding location in CX3CR1 nucleosome, illustrated by the diagram on the right) (d) binding by MBP-DBDC/EBPα. For the experiment, the nucleosome concentration is 0.5 μM. The ratios of the C/EBPα proteins over the nucleosomes are 0, 1.0, 2.0, 3.0, 4.0, respectively. e, EMSA of 20 bp DNA containing wild type C/EBPα binding site (5′-ATCTTCAGCTGGTTGCTGAG-3′) and Cmut site (5′-ATCTTCAGCAACTTGCTGAG-3′). For the experiment, the DNA concentration is 5 μM. The ratios of C/EBPα proteins over the DNA are 0, 0.5, 1.0, 1.5 and 2.0, respectively. f and g, EMSA of CX3CR1 DNA (f) and nucleosome (g) binding by MBP. For the experiment, the DNA and nucleosome concentrations are 0.5 μM. The ratios of the MBP protein over the DNA and nucleosome are 0, 1.0, 2.0, 3.0, 4.0, 6.0 and 8.0, respectively. For a, c-g, each experiment was repeated three times with similar results.
Extended Data Fig. 8 Interactions between PU.1 and free CX3CR1 DNA.
EMSA of PU.1 and the 162 bp wild type CX3CR1 and mutated (GGAA in the two sites near the dyad to GGGG) DNA (Fig. 1a). The DNA concentration is at 0.5 μM. The ratios of the PU.1 proteins over the WT DNA are 0, 0.25, 0.5, 1.0, 1.5, 2.0 and 2.5, respectively. The ratios of the PU.1 proteins over the mutated DNA are 0, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0, respectively. The bottom panel shows the quantification of WT and mutated DNA binding by PU.1. The Kdapp values were obtained by fitting the data (fraction of the nucleosome bound to PU.1 versus PU.1 concentration) to Hill equation. Data represent mean and s.d. based on three independent experiments.
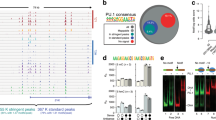
Extended Data Fig. 9 The FRET assay confirms DNA repositioning and PU.1-facilitated nucleosome binding by C/EBPα.
a, Design of the FRET assay. Cy3 and Cy5 are labeled on the H2A residue 116 and the end of the DNA at the entry site. b, FRET in buffer containing 140 mM KCl. c, FRET at 20 °C. d, FRET at 4 °C. e, FRET assay with Cy3 labeled on DNA entry site (nucleotide 19) and Cy5 on C/EBPα in the absence (left panel) and presence (right panel) of PU.1. f. FRET assay with Cy3 labeled on DNA exit site and Cy5 on PU.1 in the absence (left panel) and presence (right panel) of C/EBPα.
Extended Data Fig. 10 PU.1 and C/EBPα facilitate endonuclease digestion of the CX3CR1 chromatosomal DNA in the H1-condensed nucleosome array.
a, Sequence and the endonuclease sites in the CX3CR1 and neighboring Widom 601 (W601) DNA. b. The diagram shows the arrangement of the nucleosome array. c, Agarose gel showing purified 12 × 197 bp DNA. d, Agarose gel showing reconstituted nucleosome arrays digested by SmaI endonuclease. SmaI locates in all the linker DNA, so there are only mono-nucleosomes with 197 bp DNA after cutting. e, Agarose gel showing reconstituted nucleosome arrays digested by HaeIII enzyme with and without H1.4. HaeIII exists in the linker DNA of both W601 and CX3CR1 nucleosomes. f and g, Agarose gels showing the digestion of the H1.4-condensed nucleosome array by XhoI (f) and EcoRI (g) enzymes with different ratios of PU.1 and C/EBPα, respectively, over the W601 nucleosome array. h and i, Agarose gels showing the digestion of the H1.4-condensed PU.1 motif-mutated nucleosome array by XhoI (h) and the H1.4-condensed CEB/Pα motif-mutated nucleosome array by EcoRI (i) enzymes with different ratios of PU.1 and C/EBPα over the mutated nucleosome array, respectively. For c-i, each experiment was repeated three times with similar results.
Supplementary information
Supplementary Video 1
Cooperative binding of PU.1 and C/EBPα to the nucleosome.
Supplementary Table 1
A list of DNA sequences and primers.
Source data
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 4
Unprocessed gels.
Source Data Fig. 7
Unprocessed gels.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 7
Unprocessed gels.
Source Data Extended Data Fig. 8
Unprocessed gels.
Source Data Extended Data Fig. 10
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Lian, T., Guan, R., Zhou, BR. et al. Structural mechanism of synergistic targeting of the CX3CR1 nucleosome by PU.1 and C/EBPα. Nat Struct Mol Biol 31, 633–643 (2024). https://doi.org/10.1038/s41594-023-01189-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01189-z
This article is cited by
-
Nucleosome-bound NR5A2 structure reveals pioneer factor mechanism by DNA minor groove anchor competition
Nature Structural & Molecular Biology (2024)