Abstract
The recycling of membrane proteins from endosomes to the cell surface is vital for cell signaling and survival. Retriever, a trimeric complex of vacuolar protein-sorting-associated protein (VPS)35L, VPS26C and VPS29, together with the CCC complex comprising coiled-coil domain-containing (CCDC)22, CCDC93 and copper metabolism domain-containing (COMMD) proteins, plays a crucial role in this process. The precise mechanisms underlying retriever assembly and its interaction with CCC have remained elusive. Here, we present a high-resolution structure of retriever in humans determined using cryogenic electron microscopy. The structure reveals a unique assembly mechanism, distinguishing it from its remotely related paralog retromer. By combining AlphaFold predictions and biochemical, cellular and proteomic analyses, we further elucidate the structural organization of the entire retriever–CCC complex across evolution and uncover how cancer-associated mutations in humans disrupt complex formation and impair membrane protein homeostasis. These findings provide a fundamental framework for understanding the biological and pathological implications associated with retriever–CCC-mediated endosomal recycling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Cryo-EM maps and models have been deposited in the EMDB (accession numbers EMD-40884, EMD-40885 and EMD-40886) and the PDB (accession numbers PDB 8SYM, PDB 8SYN and PDB 8SYO). AlphaFold-Multimer-derived models are available in ModelArchive (https://modelarchive.org) under the accession codes ma-cfy9y (human retriever), ma-h9nwf (human retriever–CCDC22–CCDC93), ma-o592z (human CCDC22–CCDC93–DENND10), ma-itenz (human COMMD1–COMMD10 ring–CCDC22–CCDC93), ma-icsco (Danio rerio COMMD1–COMMD10 ring–CCDC22–CCDC93), ma-45mmt (Dictyostelium discoideum COMMD1–COMMD10 ring–CCDC22–CCDC93) and ma-2g80v (human retriever–CCC complex). MS data have been deposited at the MassIVE repository (accession numbers MSV000092100, MSV000092102, MSV000092103, MSV000092104). To our knowledge, all information required to reanalyze the data reported here is publicly available. Any additional data that we inadvertently missed will be shared upon reasonable request. This paper does not report original code. Source data are provided with this paper.
References
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Uhlen, M. et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
Seaman, M. N., McCaffery, J. M. & Emr, S. D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142, 665–681 (1998).
Haft, C. R. et al. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell 11, 4105–4116 (2000).
Edgar, A. J. & Polak, J. M. Human homologues of yeast vacuolar protein sorting 29 and 35. Biochem. Biophys. Res. Commun. 277, 622–630 (2000).
Lucas, M. et al. Structural mechanism for cargo recognition by the retromer complex. Cell 167, 1623–1635 (2016).
Steinberg, F. et al. A global analysis of SNX27–retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15, 461–471 (2013).
Harrison, M. S. et al. A mechanism for retromer endosomal coat complex assembly with cargo. Proc. Natl Acad. Sci. USA 111, 267–272 (2014).
Yong, X. et al. SNX27–FERM–SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27. Proc. Natl Acad. Sci. USA 118, e2105510118 (2021).
Simonetti, B. et al. SNX27–retromer directly binds ESCPE-1 to transfer cargo proteins during endosomal recycling. PLoS Biol. 20, e3001601 (2022).
Fjorback, A. W. et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J. Neurosci. 32, 1467–1480 (2012).
Gomez, T. S. & Billadeau, D. D. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev. Cell 17, 699–711 (2009).
Gomez, T. S., Gorman, J. A., de Narvajas, A. A., Koenig, A. O. & Billadeau, D. D. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Mol. Biol. Cell 23, 3215–3228 (2012).
Jia, D., Gomez, T. S., Billadeau, D. D. & Rosen, M. K. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol. Biol. Cell 23, 2352–2361 (2012).
Derivery, E. et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell 17, 712–723 (2009).
Phillips-Krawczak, C. A. et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol. Biol. Cell 26, 91–103 (2015).
Burstein, E. et al. COMMD proteins: a novel family of structural and functional homologs of MURR1. J. Biol. Chem. 280, 22222–22232 (2005).
Schou, K. B., Andersen, J. S. & Pedersen, L. B. A divergent calponin homology (NN-CH) domain defines a novel family: implications for evolution of ciliary IFT complex B proteins. Bioinformatics 30, 899–902 (2014).
Singla, A. et al. Endosomal PI3P regulation by the COMMD/CCDC22/CCDC93 (CCC) complex controls membrane protein recycling. Nat. Commun. 10, 4271 (2019).
Singla, A. et al. Regulation of murine copper homeostasis by members of the COMMD protein family. Dis. Model. Mech. 14, dmm045963 (2021).
McNally, K. E. et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 19, 1214–1225 (2017).
Bartuzi, P. et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat. Commun. 7, 10961 (2016).
Li, H. et al. Endosomal sorting of Notch receptors through COMMD9 dependent pathways modulates Notch signaling. J. Cell Biol. 211, 605–617 (2015).
Zhang, J. et al. DENN domain-containing protein FAM45A regulates the homeostasis of late/multivesicular endosomes. Biochim. Biophys. Acta Mol. Cell Res. 1866, 916–929 (2019).
Borchers, A. C., Langemeyer, L. & Ungermann, C. Who’s in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond. J. Cell Biol. 220, e202105120 (2021).
Wan, C. et al. Panorama of ancient metazoan macromolecular complexes. Nature 525, 339–344 (2015).
Mallam, A. L. & Marcotte, E. M. Systems-wide studies uncover commander, a multiprotein complex essential to human development. Cell Syst. 4, 483–494 (2017).
Healy, M. D. et al. Structure of the endosomal commander complex linked to Ritscher–Schinzel syndrome. Cell 186, 2219–2237 (2023).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Collins, B. M., Skinner, C. F., Watson, P. J., Seaman, M. N. & Owen, D. J. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat. Struct. Mol. Biol. 12, 594–602 (2005).
Cleary, S. P. et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology 58, 1693–1702 (2013).
Hecht, M., Bromberg, Y. & Rost, B. Better prediction of functional effects for sequence variants. BMC Genomics 16, S1 (2015).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Wu, X. et al. Insights regarding guanine nucleotide exchange from the structure of a DENN-domain protein complexed with its Rab GTPase substrate. Proc. Natl Acad. Sci. USA 108, 18672–18677 (2011).
Healy, M. D. et al. Structural insights into the architecture and membrane interactions of the conserved COMMD proteins. eLife 7, e35898 (2018).
Voineagu, I. et al. CCDC22: a novel candidate gene for syndromic X-linked intellectual disability. Mol. Psychiatry 17, 4–7 (2012).
Kolanczyk, M. et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher–Schinzel/3C syndrome. Eur. J. Hum. Genet. 23, 633–638 (2014).
Kato, K. et al. Biallelic VPS35L pathogenic variants cause 3C/Ritscher–Schinzel-like syndrome through dysfunction of retriever complex. J. Med. Genet. 57, 245–253 (2020).
Otsuji, S. et al. Clinical diversity and molecular mechanism of VPS35L-associated Ritscher–Schinzel syndrome. J. Med. Genet. 60, 359–367 (2022).
van de Sluis, B. et al. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol. Cell. Biol. 27, 4142–4156 (2007).
van de Sluis, B., Peter, A. T. & Wijmenga, C. Indirect molecular diagnosis of copper toxicosis in Bedlington terriers is complicated by haplotype diversity. J. Hered. 94, 256–259 (2003).
Vonk, W. I. et al. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS ONE 6, e29183 (2011).
van De Sluis, B., Rothuizen, J., Pearson, P. L., van Oost, B. A. & Wijmenga, C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 11, 165–173 (2002).
Vos, D. Y. et al. Cargo-specific role for retriever subunit VPS26C in hepatocyte lipoprotein receptor recycling to control postprandial triglyceride-rich lipoproteins. Arterioscler. Thromb. Vasc. Biol. 43, e29–e45 (2022).
Fedoseienko, A. et al. The COMMD family regulates plasma LDL levels and attenuates atherosclerosis through stabilizing the CCC complex in endosomal LDLR trafficking. Circ. Res. 122, 1648–1660 (2018).
Maine, G. N., Mao, X., Komarck, C. M. & Burstein, E. COMMD1 promotes the ubiquitination of NF-κB subunits through a cullin-containing ubiquitin ligase. EMBO J. 26, 436–447 (2007).
Geng, H., Wittwer, T., Dittrich-Breiholz, O., Kracht, M. & Schmitz, M. L. Phosphorylation of NF-κB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 10, 381–386 (2009).
Starokadomskyy, P. et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-κB signaling. J. Clin. Invest. 123, 2244–2256 (2013).
Murata, K. et al. Hypoxia-sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity 47, 66–79 (2017).
Li, H. et al. Copper metabolism domain-containing 1 represses genes that promote inflammation and protects mice from colitis and colitis-associated cancer. Gastroenterology 147, 184–195 (2014).
Nakai, A. et al. The COMMD3/8 complex determines GRK6 specificity for chemoattractant receptors. J. Exp. Med. 216, 1630–1647 (2019).
Shirai, T. et al. Celastrol suppresses humoral immune responses and autoimmunity by targeting the COMMD3/8 complex. Sci. Immunol. 8, eadc9324 (2023).
Jia, D. et al. Structural and mechanistic insights into regulation of the retromer coat by TBC1d5. Nat. Commun. 7, 13305 (2016).
Yao, J. et al. Mechanism of inhibition of retromer transport by the bacterial effector RidL. Proc. Natl Acad. Sci. USA 115, E1446–E1454 (2018).
Kovtun, O. et al. Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature 561, 561–564 (2018).
Laulumaa, S., Kumpula, E.-P., Huiskonen, J. T. & Varjosalo, M. Structure and interactions of the endogenous human commander complex. Preprint at bioRxiv https://doi.org/10.1101/2023.04.03.535349 (2023).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Ismail, A. M., Padrick, S. B., Chen, B., Umetani, J. & Rosen, M. K. The WAVE regulatory complex is inhibited. Nat. Struct. Mol. Biol. 16, 561–563 (2009).
Giridharan, S. S. P. et al. Lipid kinases VPS34 and PIKfyve coordinate a phosphoinositide cascade to regulate retriever-mediated recycling on endosomes. eLife 11, e69709 (2022).
Mao, X. et al. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-κB/RelA. Genes Dev. 23, 849–861 (2009).
Chen, B., Padrick, S. B., Henry, L. & Rosen, M. K. Biochemical reconstitution of the WAVE regulatory complex. Methods Enzymol. 540, 55–72 (2014).
Chen, B. et al. Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. eLife 6, e29795 (2017).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Henderson, R. et al. Outcome of the first Electron Microscopy Validation Task Force meeting. Structure 20, 205–214 (2012).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Burley, S. K. et al. Protein Data Bank (PDB): the single global macromolecular structure archive. Methods Mol. Biol. 1607, 627–641 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
We thank the ResearchIT at Iowa State University for hardware resources, installation of AlphaFold and ongoing computational and diagnostic support. We also thank A. Lemoff and the Proteomics Core as well as A. Mobley and the Flow Cytometry Core at UT Southwestern. Electron microscopy data were collected in collaboration with the Structural Biology Laboratory with help from Y. Li, using the Cryo-Electron Microscopy Facility at the UT Southwestern Medical Center (partially supported by grant RP220582 from the Cancer Prevention and Research Institute of Texas for cryo-EM studies) and the Iowa State University Cryo-EM Facility (supported by the Roy J. Carver Structural Initiative). Research was supported by funding from the National Institutes of Health (R35 GM128786 to B.C., and R01 DK107733 to E.B. and D.D.B.), the National Science Foundation CAREER award (CDF 2047640 to B.C.) and Roy J. Carver Charitable Trust seed funds (to B.C.).
Author information
Authors and Affiliations
Contributions
E.B., B.C. and D.D.B. conceived the project. E.B. oversaw cell biological and proteomic experiments performed by A.S. with help from Q.L., K.S. and X.L. B.C. oversaw protein purification, biochemical experiments and AlphaFold predictions performed by D.J.B. with help from D.A.K. and X.Z. Z.C. and Y.H. oversaw cryo-EM grid preparation, data collection, single-particle reconstruction and atomic model building. P.J. supervised initial cryo-EM grid preparation and data collection performed by D.J.B. at Iowa State. M.J.M. and D.D.B. helped with cellular experiments and data interpretation. B.C., Z.C., D.J.B. and Y.H. analyzed structures. E.B., B.C. and Z.C. drafted the manuscript and prepared figures with assistance from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Structural & Molecular Biology thanks Oleksiy Kovtun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification and cryo-EM structural determination of Retriever.
(a) Representative gel filtration chromatography of the purified Retriever complex. Purification was repeated at least 5 times. (b) Representative cryo-EM micrograph from a total of 2,892 micrographs used for data processing. (c) Representative 2D class averages. (d) Euler angle distribution plots for Retriever (upper) and the locally refined VPS29 with the NT ‘belt’ peptide and the CT region of VPS35L (lower). (e) Local resolution map of Retriever (upper) and the locally refined VPS29 with the NT ‘belt’ peptide and the CT region of VPS35L (lower). (f) Fourier Shell Correlation (FSC) plot for Retriever (upper) and the locally refined VPS29 with the NT ‘belt’ peptide and the CT region of VPS35L (lower). (g) Schematic showing cryo-EM data processing steps for obtaining 3D reconstruction of Retriever. The three maps deposited to PDB/EMDB are labeled.
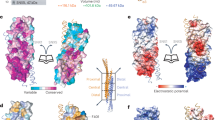
Extended Data Fig. 2 Structural comparison between Retriever and Retromer.
(a) Surface representation of electrostatic potentials of Retriever vs. Retromer (PDB: 7U6F). (b) Superimposition of individual subunits from Retriever (colored) vs. Retromer (gray). (c) Intermolecular interface between VPS35L and VPS29 vs. VPS35 and VPS29, shown as surface representations of electrostatic potentials. (d) Same as in (C), with VPS35L and VPS26C vs. VPS35 and VPS26A. (e) Detailed comparison of VPS29 binding surface on VPS35L in Retriever vs. VPS35 in Retromer. For clarity, the backbones of VPS29 are shown as loops. VPS35L and VPS35 are shown as cartoons and semi-transparent surface representations of electrostatic potentials.
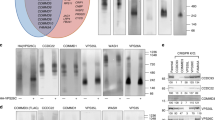
Extended Data Fig. 3 Cellular and proteomic analysis of VPS35L mutants.
(a) Huh-7 hepatocellular carcinoma cells carrying the indicated mutations in VPS35L (EV, empty vector). Immunoprecipitation of VPS35L followed by western blot for the indicated proteins is shown. (b) Immunoprecipitation of VPS35L followed by competitive elution of native complexes using HA peptide, and separation of the complexes in blue native gels. After transfer, the complexes were immunoblotted with the indicated antibodies. (c) Heatmap representation of protein-protein interaction results using proteomics. VPS35L was immunoprecipitated from the indicated Huh-7 stable cell lines (in triplicate samples) and the results are expressed as fold compared to Huh-7 control cells (darker blue depicts greater depletion compared to WT VPS35L). Statistical significance as determined by Student’s t-test is indicated in color scale (yellow indicating p<0.05, and grey indicating p>0.05). (d) Immunoprecipitation of VPS35L carrying indicated point mutations expressed in HEK293T cells and immunoblotting for the indicated proteins. Immunoblots are representative results of one iteration out of at least three independent experiments with the exception of VPS35L K940N and R766W mutants, which were tested only once.
Extended Data Fig. 4 VPS35L localization and PM proteome effects.
(a) Immunofluorescence staining for VPS35L (green channel, using HA antibody), FAM21 (red channel), and nuclei (DAPI, blue channel) in the indicated stable Huh-7 cell lines. Representative images of one out of two independent experiments are shown. (b) Immunofluorescence staining for VPS35L (green channel, using HA antibody), FAM21 (red channel), and nuclei (DAPI, blue channel) in the indicated HeLa knockout cell lines transfected with HA-VPS35L. Representative images of one experiment are shown. (c, d) Representative gating and acquisition parameters for Villin and CD14 staining by flow cytometry.
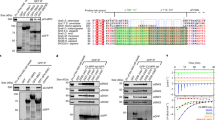
Extended Data Fig. 5 AlphaFold Multimer prediction of CCDC22-CCDC93 binding to Retriever.
(a & d) Overlay of all 25 AlphaFold Multimer models of Retriever alone (A) or CCDC22-CCDC93-Retriever (D) with the cryo-EM model of Retriever. AFM models of Retriever are grey. (b & e) Representative AFM models colored using pLDDT scores. High scores indicate high reliability in local structure prediction. (c & f) PAE score matrix of the AFM model shown in (B & E). Low scores (deep color) indicate high reliability in the relative position in the 3D space. Boundaries of protein sequences and important structure regions are indicated.
Extended Data Fig. 6 AlphaFold Multimer prediction of CCDC22-CCDC93 binding to DENND10.
(a) AlphaFold Multimer prediction of DENND10 binding to full-length (FL) CCDC22-CCDC93. (b) Representative AFM models colored using pLDDT scores. (c) PAE score matrix of the AFM model shown in (B). Boundaries of protein sequences and important structure regions are indicated. (d) Superimposition of the AFM model of DENND10 with the crystal structure of DENND1a (PDB: 6EKK). Rab35 binding surface of DENND1a and CCDC22-CCDC93 binding surface of DENN10 are colored to show the partial overlap of the two surfaces.
Extended Data Fig. 7 AlphaFold Multimer prediction of CCDC22-CCDC93 binding to COMMD.
(a–c) Overlays of AlphaFold Multimer models and schematic showing CCDC22-CCDC93 binding to COMMD decamer ring for proteins from Human (A), Zebrafish (B), and Amoeba (Dictyostelium) (C). (d–f) Representative AFM models colored using pLDDT scores. (g–i) PAE score matrices of the AFM models shown in (D-F). Boundaries of protein sequences and important structure regions are indicated.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Numerical data.
Source Data Fig. 3
Numerical data.
Source Data Fig. 4
Unprocessed western blots and Coomassie blue gels.
Source Data Fig. 5
Unprocessed western blots and Coomassie blue gels.
Source Data Fig. 5
Chromatography data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed Coomassie blue gels.
Source Data Extended Data Fig. 1
Chromatography data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boesch, D.J., Singla, A., Han, Y. et al. Structural organization of the retriever–CCC endosomal recycling complex. Nat Struct Mol Biol (2023). https://doi.org/10.1038/s41594-023-01184-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-023-01184-4