Abstract
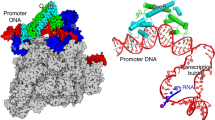
Transcription factors respond to multilevel stimuli and co-occupy promoter regions of target genes to activate RNA polymerase (RNAP) in a cooperative manner. To decipher the molecular mechanism, here we report two cryo-electron microscopy structures of Anabaena transcription activation complexes (TACs): NtcA-TAC composed of RNAP holoenzyme, promoter and a global activator NtcA, and NtcA–NtcB-TAC comprising an extra context-specific regulator, NtcB. Structural analysis showed that NtcA binding makes the promoter DNA bend by ∼50°, which facilitates RNAP to contact NtcB at the distal upstream NtcB box. The sequential binding of NtcA and NtcB induces looping back of promoter DNA towards RNAP, enabling the assembly of a fully activated TAC bound with two activators. Together with biochemical assays, we propose a ‘DNA looping’ mechanism of cooperative transcription activation in bacteria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The crystal structure of NtcB-EBD has been deposited at Protein Data Bank (PDB) under accession code 8H3Z. The cryo-EM maps and coordinates of NtcA-TAC and NtcA–NtcB-TAC have been deposited at the Electron Microscopy Data Bank (EMDB) (EMD-34476 for NtcA-TAC and EMD-34475 for NtcA–NtcB-TAC) and PDB (8H40 for NtcA-TAC and 8H3V for NtcA–NtcB-TAC). The supplementary map of NtcA–NtcB-TAC focusing on NtcA and NtcB has been deposited at EMDB under accession code of EMD-34477. Source data are provided with this paper.
References
Browning, D. F. & Busby, S. J. Local and global regulation of transcription initiation in bacteria. Nat. Rev. Microbiol. 14, 638–650 (2016).
Cramer, P. Organization and regulation of gene transcription. Nature 573, 45–54 (2019).
Browning, D. F. & Busby, S. J. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2, 57–65 (2004).
Lee, D. J., Minchin, S. D. & Busby, S. J. Activating transcription in bacteria. Annu. Rev. Microbiol. 66, 125–152 (2012).
Busby, S. J. W. Transcription activation in bacteria: ancient and modern. Microbiology 165, 386–395 (2019).
Feng, Y., Zhang, Y. & Ebright, R. H. Structural basis of transcription activation. Science 352, 1330–1333 (2016).
Liu, B., Hong, C., Huang, R. K., Yu, Z. & Steitz, T. A. Structural basis of bacterial transcription activation. Science 358, 947–951 (2017).
Shi, W., Jiang, Y., Deng, Y., Dong, Z. & Liu, B. Visualization of two architectures in class-II CAP-dependent transcription activation. PLoS Biol. 18, e3000706 (2020).
Forchhammer, K. & Selim, K. A. Carbon/nitrogen homeostasis control in cyanobacteria. FEMS Microbiol. Rev. 44, 33–53 (2020).
Ohashi, Y. et al. Regulation of nitrate assimilation in cyanobacteria. J. Exp. Bot. 62, 1411–1424 (2011).
Flores, E., Frias, J. E., Rubio, L. M. & Herrero, A. Photosynthetic nitrate assimilation in cyanobacteria. Photosynth. Res. 83, 117–133 (2005).
Flores, E. & Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 33, 164–167 (2005).
Zhao, M. X. et al. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc. Natl Acad. Sci. USA 107, 12487–12492 (2010).
Llacer, J. L. et al. Structural basis for the regulation of NtcA-dependent transcription by proteins PipX and PII. Proc. Natl Acad. Sci. USA 107, 15397–15402 (2010).
Picossi, S., Flores, E. & Herrero, A. ChIP analysis unravels an exceptionally wide distribution of DNA binding sites for the NtcA transcription factor in a heterocyst-forming cyanobacterium. BMC Genomics 15, 22 (2014).
Mitschke, J. et al. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc. Natl Acad. Sci. USA 108, 20130–20135 (2011).
Dominguez-Martin, M. A. et al. Differential NtcA responsiveness to 2-oxoglutarate underlies the diversity of C/N balance regulation in Prochlorococcus. Front. Microbiol. 8, 2641 (2017).
Giner-Lamia, J. et al. Identification of the direct regulon of NtcA during early acclimation to nitrogen starvation in the cyanobacterium Synechocystis sp. PCC 6803. Nucleic Acids Res. 45, 11800–11820 (2017).
Imamura, S. et al. Nitrate assimilatory genes and their transcriptional regulation in a unicellular red alga Cyanidioschyzon merolae: genetic evidence for nitrite reduction by a sulfite reductase-like enzyme. Plant Cell Physiol. 51, 707–717 (2010).
Lopatovskaia, K. V., Seliverstov, A. V. & Liubetskii, V. A. NtcA- and NtcB-regulons in cyanobacteria and Rhodophyta chloroplasts. Mol. Biol. 45, 570–574 (2011).
Frias, J. E., Flores, E. & Herrero, A. Activation of the Anabaena nir operon promoter requires both NtcA (CAP family) and NtcB (LysR family) transcription factors. Mol. Microbiol. 38, 613–625 (2000).
Aichi, M. & Omata, T. Involvement of NtcB, a LysR family transcription factor, in nitrite activation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 179, 4671–4675 (1997).
Aichi, M., Takatani, N. & Omata, T. Role of NtcB in activation of nitrate assimilation genes in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183, 5840–5847 (2001).
Maddocks, S. E. & Oyston, P. C. F. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154, 3609–3623 (2008).
Shen, L. Q. et al. An SI3-σ arch stabilizes cyanobacteria transcription initiation complex. Proc. Natl Acad. Sci. USA 120, e2219290120 (2023).
Lawson, C. L. et al. Catabolite activator protein: DNA binding and transcription activation. Curr. Opin. Struct. Biol. 14, 10–20 (2004).
Busby, S. & Ebright, R. H. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293, 199–213 (1999).
Blanco, A. G., Canals, A., Bernues, J., Sola, M. & Coll, M. The structure of a transcription activation subcomplex reveals how σ70 is recruited to PhoB promoters. EMBO J. 30, 3776–3785 (2011).
Panigrahi, A. & O’Malley, B. W. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 108 (2021).
Haberle, V. & Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637 (2018).
Sikorski, T. W. & Buratowski, S. The basal initiation machinery: beyond the general transcription factors. Curr. Opin. Cell Biol. 21, 344–351 (2009).
Giannopoulou, E. A. et al. Crystal structure of the full-length LysR-type transcription regulator CbnR in complex with promoter DNA. FEBS J. 288, 4560–4575 (2021).
Alanazi, A. M., Neidle, E. L. & Momany, C. The DNA-binding domain of BenM reveals the structural basis for the recognition of a T-N11-A sequence motif by LysR-type transcriptional regulators. Acta Crystallogr. D Biol. Crystallogr. 69, 1995–2007 (2013).
Jiang, Y. L. et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl Acad. Sci. USA 115, 403–408 (2018).
Mazumder, A. & Kapanidis, A. N. Recent advances in understanding σ70-dependent transcription initiation mechanisms. J. Mol. Biol. 431, 3947–3959 (2019).
Hao, M. et al. Structures of class I and class II transcription complexes reveal the molecular basis of RamA-dependent transcription activation. Adv. Sci. 9, e2103669 (2022).
Holst, B. et al. The cAMP-CRP/CytR nucleoprotein complex in Escherichia coli: two pairs of closely linked binding sites for the cAMP-CRP activator complex are involved in combinatorial regulation of the cdd promoter. EMBO J. 11, 3635–3643 (1992).
Rossiter, A. E. et al. Expression of different bacterial cytotoxins is controlled by two global transcription factors, CRP and Fis, that co-operate in a shared-recruitment mechanism. Biochem. J. 466, 323–335 (2015).
Danson, A. E., Jovanovic, M., Buck, M. & Zhang, X. Mechanisms of σ54-dependent transcription initiation and regulation. J. Mol. Biol. 431, 3960–3974 (2019).
Weintraub, A. S. et al. YY1 is a structural regulator of enhancer-promoter loops. Cell 171, 1573–1588.e28 (2017).
Chen, X. et al. Structures of the human Mediator and Mediator-bound preinitiation complex. Science 372, eabg0635 (2021).
Schoenfelder, S. & Fraser, P. Long-range enhancer-promoter contacts in gene expression control. Nat. Rev. Genet. 20, 437–455 (2019).
Revyakin, A., Liu, C., Ebright, R. H. & Strick, T. R. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science 314, 1139–1143 (2006).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Fang, C. L. et al. CueR activates transcription through a DNA distortion mechanism. Nat. Chem. Biol. 17, 57–64 (2021).
Mekler, V., Pavlova, O. & Severinov, K. Interaction of Escherichia coli RNA polymerase σ70 subunit with promoter elements in the context of free σ70, RNA polymerase holoenzyme, and the β′-σ70 complex. J. Biol. Chem. 286, 270–279 (2011).
Mekler, V. & Severinov, K. RNA polymerase molecular beacon as tool for studies of RNA polymerase–promoter interactions. Methods 86, 19–26 (2015).
Stark, H. GraFix: stabilization of fragile macromolecular complexes for single particle cryo-EM. Methods Enzymol. 481, 109–126 (2010).
Chen, J., Noble, A. J., Kang, J. Y. & Darst, S. A. Eliminating effects of particle adsorption to the air/water interface in single-particle cryo-electron microscopy: bacterial RNA polymerase and CHAPSO. J. Struct. Biol. X 1, 100005 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank the staff at the Shanghai Synchrotron Radiation Facility (SSRF) for X-ray diffraction data collection of NtcB-EBD; P.-P. Tang and Y.-X. Gao at the Cryo-EM Center at University of Science and Technology of China for cryo-EM data acquisition. This work is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (http://www.cas.cn; grant nos. XDB37020301; C.-Z.Z., and XDA24020302; Y.-L.J.), the National Natural Science Foundation of China (http://www.nsfc.gov.cn; grant no. 32171198; Y.-L.J.), National Key Research and Development Program of China (grant no. 2018YFA0900700; Y.Z.) and Anhui Provincial Natural Science Foundation (http://kjt.ah.gov.cn; grant no. 2108085J14; Y.-L.J.). Y.-L.J. thanks the Youth Innovation Promotion Association of Chinese Academy of Sciences for their support (membership no. 2020452).
Author information
Authors and Affiliations
Contributions
C.-Z.Z., Y.Z., Y.-L.J. and Y.C. conceived, designed and supervised the project. C.-Z.Z., Y.Z., Y.-L.J., Y.C. and S.-J.H. analyzed data and wrote the manuscript. S.-J.H., L.-Q.S., H.S., H.-C.M., K.Z. and N.C. performed the molecular cloning, protein expression and purification. S.-J.H., L.-L.Y. and W.-W.K. conducted the cryo-EM sample preparation and data acquisition. S.-J.H., Y.-L.J. and F.Y. performed cryo-EM data processing and model building. S.-J.H. and X.W. performed the biochemical assays. S.-J.H., Y.-L.J. and Z.-P.C. carried out the protein crystallization, X-ray data collection and structure determination. All authors discussed the data and read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Sara Osman in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The coordinated regulation of NtcA and NtcB on a PnirA derivative.
a-b, The transcriptional activity of Anabaena sp. PCC 7120 RNAP in the presence of NtcA (a) or NtcB (b) at increasing concentrations (0, 25, 50, 100, 200, 400, and 800 nM). c, The transcriptional activity of RNAP preincubated with 200 nM NtcA, followed by adding NtcB at increasing concentrations (0, 50, 100, 200, 400, 800, and 1600 nM). Data are presented as mean ± S.E.M., n = 3 biologically independent experiments. Two-tailed unpaired t-tests. No p value adjustments for multiple comparisons. The lower panel shows the representative gel image. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 indicate significant difference compared to lane 1 (a) and lane 2 (c). RNA transcripts, 102 nt. d, The multiple-sequence alignment of the upstream promoter region of nitrate assimilation genes in β-cyanobacteria. The NtcA and NtcB boxes are colored green and cyan, respectively. The numbers on the top indicate the positions of NtcA and NtcB relative to transcription start site from Anabaena sp. PCC 7120. Sequence logos derived from the multiple-sequence alignment. e, The DNA templates used for DNA scaffold of Cryo-EM, in vitro transcription assays and the molecular beacon assays. NtcA and NtcB boxes, −35 element and −10 element are colored as shown in the color key. T, template; NT, nontemplate.
Extended Data Fig. 2 Cryo-EM data processing of NtcA-TAC.
a, A representative cryo-EM image of NtcA-TAC. Bar, 100 nm. The micrograph is a representative of 2,090 cryo-EM images. b, Representative 2D class averages of NtcA-TAC. c, Flowchart for cryo-EM data processing and map reconstruction for NtcA-TAC.
Extended Data Fig. 3 The interaction pattern of NtcA binding to the promoter DNA and RNAP in NtcA-TAC.
a, Superposition of promoter DNA between Anabaena NtcA-TAC and Synechocystis transcription initiation complex (synRPitc, PDB: 8GZG). The color schemes are as follows: NT (nontemplate DNA) of NtcA-TAC, light yellow; T (template DNA) of NtcA-TAC, light orange; σA of NtcA-TAC, green; NT, T and σA of synRPitc, blue; RNA of synRPitc, red. The σR2, σR3, and σR4 represent the region 2, region 3 and region 4 of σA, respectively. b, Cryo-EM map of NtcA and its binding boxes. c, Cryo-EM maps of NtcA-σR4. d, Superposition of σR4-DNA between NtcA-TAC and synRPitc. e, The interactions between NtcA and the NtcA box of promoter DNA. The key residues of NtcA involved in binding to DNA are shown as spheres. NT, nontemplate. f, The EMSA results showing binding of wild-type (NtcAWT) or mutant (R187A/V188A/R192A; NtcAM) NtcA with a DNA fragment containing the NtcA box. The EMSA analysis results is a representative (n = 1) of many times optimized experiments. Source data are provided in the Source Data file.
Extended Data Fig. 4 In vitro reconstitution of NtcA-NtcB-TAC.
a, Gel-shift result showing migration of protein-DNA complex reconstituted through indicated combinations of NtcA, NtcB, RNAP and the DNA in Fig. 1a. The Gel-shift result is a representative (n = 1) of many times optimized experiments. Source data are provided in the Source Data file. b, The size-exclusion chromatography of NtcA-NtcB-TAC. c, The SDS-PAGE analysis of the purified NtcA-NtcB-TAC complex. The protein components are labeled on the right of the gel. The chromatogram and SDS-PAGE are representatives of > 5 independent experiments that showed similar results. d, The native-PAGE analysis of NtcA-NtcB-TAC stained with SYBR Gold dye (left) or Coomassie Brilliant Blue (right). The native-PAGE analysis result is a representative of > 3 independent experiments that showed similar results. Source data are provided in the Source Data file.
Extended Data Fig. 5 Cryo-EM data processing of NtcA-NtcB-TAC.
a, A representative cryo-EM image of NtcA-NtcB-TAC. Bar, 100 nm. The micrograph is a representative of 5,047 cryo-EM images. b, Representative 2D class averages of NtcA-NtcB-TAC. c, Flowchart for cryo-EM data processing of NtcA-NtcB-TAC.
Extended Data Fig. 6 The crystal structure of NtcB-EBD.
a, A schematic presentation of domain organization of NtcB. b, Crystal structure of the NtcB-EBD dimer. The two subunits are colored yellow and green, respectively. The two subdomains RD I and RD II are labeled.
Extended Data Fig. 7 Structural comparison of NtcA-TAC and NtcA-NtcB-TAC.
a, Superposition of promoter DNA between NtcA-TAC and NtcA-NtcB-TAC. b, Superposition of the NtcA-σR4-DNA between NtcA-TAC and NtcA-NtcB-TAC.
Extended Data Fig. 8 NtcA and NtcB independently bind to their respective boxes.
a, The sequences of 5′-Cy5 labeled dsDNA that are used in the EMSA assays. PnirA, 76-bp DNA containing wild-type NtcA and NtcB boxes; PnirA/ntcAm, a PnirA derivative bearing mutations at the conserved palindromic nucleotides of NtcA box; PnirA/ntcBm, a PnirA derivative bearing mutations at the conserved palindromic nucleotides of NtcB box. The mutated nucleotides are colored red. b, NtcA and NtcB bind to PnirA independently. NtcAWT, wide-type NtcA protein; NtcBWT, wide-type NtcB protein. c, Mutating the binding boxes of NtcA abolished NtcA binding activity but did not affect NtcB binding. d, Mutating the binding boxes of NtcB abolished NtcB binding activity but did not affect NtcA binding. All EMSA results are representatives of > 5 independent experiments that showed similar results. Source data are provided in the Source Data file.
Extended Data Fig. 9 The interactions of NtcB to promoter DNA and RNAP in NtcA-NtcB-TAC.
a, Superposition of NtcB-DBD/DNA in NtcA-NtcB-TAC and the crystal structure of BenM-DBD/DNA complex (PDB:4IHS). The residues of NtcB (blue, yellow) and BenM (black) responsible for DNA motif recognition are labeled. NT, nontemplate. b, Alanine mutation of the DNA motif-recognition residues (R34A/H53A; NtcBM) of NtcB abolished the binding ability of NtcB to a DNA fragment containing NtcB boxes. The EMSA results are representatives of > 3 independent experiments that showed similar results. Source data are provided in the Source Data file. c, Mutations of the DNA motif-recognition or RNAP-contact residues of NtcB impairs the transcription activation activity of NtcB. Data are presented as mean ± S.E.M., n = 3 biologically independent experiments. Two-tailed unpaired t-tests. No p value adjustments for multiple comparisons. The lower panel shows the representative gel image. **P < 0.05 indicates significant difference compared to lane 3. RNA transcripts, 102 nt. 2-OG, 2-oxoglutarate. △151–160, deletion of the contact patch (residues Leu151-Gly160) of NtcB. d, Cryo-EM map showing the interface between RNAP and NtcB-αNTD.
Extended Data Fig. 10 Alteration of spacer between NtcA- and NtcB-boxes abolishes transcription activation activity of NtcB.
a, The sequences of three promoter DNAs that are used in in vitro transcription assays. PnirA/AB-WT, PnirA containing wild-type spacer length between NtcA and NtcB boxes; PnirA/AB + 5, a PnirA derivative containing insertion of the spacer between NtcA and NtcB boxes by 5 bp; PnirA/AB-5, a PnirA derivative containing deletion of the spacer between NtcA and NtcB boxes by 5 bp. The sequences of insertion or deletion of nucleotides are highlighted. b, c, Insertion (b) or deletion (c) of the spacer by 5 bp between NtcA and NtcB boxes impaired the transcription activation activity of NtcB. Data are presented as mean ± S.E.M., n = 3 biologically independent experiments. Two-tailed unpaired t-tests. No p value adjustments for multiple comparisons. The lower panel shows the representative gel image. RNA transcripts, 102 nt.
Supplementary information
Source data
Source Data Fig. 1
The raw data of five replicates for Fig. 1f.
Source Data Fig. 1
The statistical source data for Fig. 1f.
Source Data Fig. 3
The statistical source data for Fig. 3d.
Source Data Extended Data Fig. 1
The raw data of three replicates for Extended Data Fig. 1a–c.
Source Data Extended Data Fig. 1
The statistical source data for Extended Data Fig. 1a–c.
Source Data Extended Data Fig. 3
The unprocessed gels for Extended Data Fig. 3f.
Source Data Extended Data Fig. 4
The unprocessed gels for Extended Data Fig. 4a,c,d.
Source Data Extended Data Fig. 8
The unprocessed gels for Extended Data Fig. 8b–d.
Source Data Extended Data Fig. 9
The unprocessed gels for Extended Data Fig. 9b and the raw data of three replicates for Extended Data Fig. 9c.
Source Data Extended Data Fig. 9
The statistical source data for Extended Data Fig. 9c.
Source Data Extended Data Fig. 10
The raw data of three replicates for Extended Data Fig. 10b,c.
Source Data Extended Data Fig. 10
The statistical source data for Extended Data Fig. 10b,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, SJ., Jiang, YL., You, LL. et al. DNA looping mediates cooperative transcription activation. Nat Struct Mol Biol 31, 293–299 (2024). https://doi.org/10.1038/s41594-023-01149-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01149-7