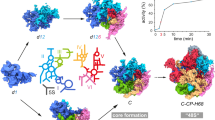
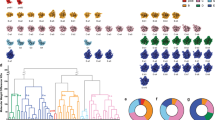
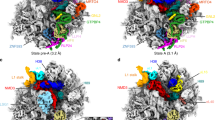
Abstract
Ribosome assembly is orchestrated by many assembly factors, including ribosomal RNA methyltransferases, whose precise role is poorly understood. Here, we leverage the power of cryo-EM and machine learning to discover that the E. coli methyltransferase KsgA performs a ‘proofreading’ function in the assembly of the small ribosomal subunit by recognizing and partially disassembling particles that have matured but are not competent for translation. We propose that this activity allows inactive particles an opportunity to reassemble into an active state, thereby increasing overall assembly fidelity. Detailed structural quantifications in our datasets additionally enabled the expansion of the Nomura assembly map to highlight rRNA helix and r-protein interdependencies, detailing how the binding and docking of these elements are tightly coupled. These results have wide-ranging implications for our understanding of the quality-control mechanisms governing ribosome biogenesis and showcase the power of heterogeneity analysis in cryo-EM to unveil functionally relevant information in biological systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The density map and the model for the KsgA-bound 30SΔksgA and untreated 30SΔksgA structures were deposited in the Electron Microscopy Data Bank under codes EMD-28720 and EMD-28692, respectively, and in the Protein Data Bank using codes 8EYT and 8EYQ, respectively. EMDB and PDB codes are also indicated in Table 1. Unfiltered particle stacks were deposited at EMPIAR with the following IDs: untreated dataset (EMPIAR-11529), small KsgA-treated dataset used for cryoDRGN and4MAVEn (EMPIAR-11526), and large KsgA-treated dataset used for high-resolution reconstruction of the KsgA-bound structure (EMPIAR-11528). Trained cryoDRGN models have been deposited at Zenodo at https://doi.org/10.5281/zenodo.7884215. Source data are provided with this paper.
Code availability
The MAVEn software, including scripts for on-the-fly reconstruction and analysis and voxel PCA, is available at: https://github.com/lkinman/MAVEn.
References
Shajani, Z., Sykes, M. T. & Williamson, J. R. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 80, 501–526 (2011).
Duss, O., Stepanyuk, G. A., Puglisi, J. D. & Williamson, J. R. Transient protein-RNA interactions guide nascent ribosomal RNA folding. Cell 179, 1357–1369(2019).
Rodgers, M. L. & Woodson, S. A. Transcription increases the cooperativity of ribonucleoprotein assembly. Cell 179, 1370–1381(2019).
Machnicka, M. A. et al. MODOMICS: a database of RNA modification pathways—2013 update. Nucleic Acids Res. 41, D262–D267 (2013).
Popova, A. M. & Williamson, J. R. Quantitative analysis of rRNA modifications using stable isotope labeling and mass spectrometry. J. Am. Chem. Soc. 136, 2058–2069 (2014).
Pletnev, P. et al. Comprehensive functional analysis of Escherichia coli ribosomal RNA methyltransferases. Front. Genet. 11, 97 (2020).
Mangat, C. S. & Brown, E. D. Ribosome biogenesis; the KsgA protein throws a methyl-mediated switch in ribosome assembly. Mol. Microbiol. 70, 1051–1053 (2008).
Van Knippenberg, P. H., Van Kimmenade, J. M. & Heus, H. A. Phylogeny of the conserved 3′ terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 12, 2595–2604 (1984).
Poldermans, B., Roza, L. & Van Knippenberg, P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3′ end of 16 S ribosomal RNA of Escherichia coli. III. Purification and properties of the methylating enzyme and methylase-30 S interactions. J. Biol. Chem. 254, 9094–9100 (1979).
Poldermans, B., Van Buul, C. P. & Van Knippenberg, P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3′ end of 16 S ribosomal RNA of Escherichia coli. II. The effect of the absence of the methyl groups on initiation of protein biosynthesis. J. Biol. Chem. 254, 9090–9093 (1979).
Poldermans, B., Goosen, N. & Van Knippenberg, P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3′ end of 16 S ribosomal RNA of Escherichia coli. I. The effect of kasugamycin on initiation of protein synthesis. J. Biol. Chem. 254, 9085–9089 (1979).
Lafontaine, D. L., Preiss, T. & Tollervey, D. Yeast 18S rRNA dimethylase Dim1p: a quality control mechanism in ribosome synthesis? Mol. Cell. Biol. 18, 2360–2370 (1998).
Kyuma, T., Kizaki, H., Ryuno, H., Sekimizu, K. & Kaito, C. 16S rRNA methyltransferase KsgA contributes to oxidative stress resistance and virulence in Staphylococcus aureus. Biochimie 119, 166–174 (2015).
Chiok, K. L., Addwebi, T., Guard, J. & Shah, D. H. Dimethyl adenosine transferase (KsgA) deficiency in Salmonella enterica Serovar Enteritidis confers susceptibility to high osmolarity and virulence attenuation in chickens. Appl. Environ. Microbiol. 79, 7857–7866 (2013).
Cunningham, P. R. et al. Site-specific mutation of the conserved m62A m62A residues of E. coli 16S ribosomal RNA. Effects on ribosome function and activity of the ksgA methyltransferase. Biochim. Biophys. Acta 1050, 18–26 (1990).
Connolly, K., Rife, J. P. & Culver, G. Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA. Mol. Microbiol. 70, 1062–1075 (2008).
Himeno, H. et al. A novel GTPase activated by the small subunit of ribosome. Nucleic Acids Res. 32, 5303–5309 (2004).
Leong, V., Kent, M., Jomaa, A. & Ortega, J. Escherichia coli rimM and yjeQ null strains accumulate immature 30S subunits of similar structure and protein complement. RNA 19, 789–802 (2013).
Thurlow, B. et al. Binding properties of YjeQ (RsgA), RbfA, RimM and Era to assembly intermediates of the 30S subunit. Nucleic Acids Res. 44, 9918–9932 (2016).
Zhong, E. D., Bepler, T., Berger, B. & Davis, J. H. CryoDRGN: reconstruction of heterogeneous cryo-EM structures using neural networks. Nat. Methods 18, 176–185 (2021).
Held, W. A., Ballou, B., Mizushima, S. & Nomura, M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J. Biol. Chem. 249, 3103–3111 (1974).
Mizushima, S. & Nomura, M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature 226, 1214 (1970).
Stern, S., Powers, T., Changchien, L. M. & Noller, H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science 244, 783–790 (1989).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006). [pii].
Qin, D., Liu, Q., Devaraj, A. & Fredrick, K. Role of helix 44 of 16S rRNA in the fidelity of translation initiation. RNA 18, 485–495 (2012).
Schuwirth, B. S. et al. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310, 827–834 (2005).
Scheres, S. H. Processing of structurally heterogeneous cryo-EM Data in RELION. Methods Enzymol. 579, 125–157 (2016).
Kinman, L. F., Powell, B. M., Zhong, E. D., Berger, B. & Davis, J. H. Uncovering structural ensembles from single-particle cryo-EM data using cryoDRGN. Nat. Protoc. 18, 319–339 (2023).
Davis, J. H. & Williamson, J. R. Structure and dynamics of bacterial ribosome biogenesis. Philos. Trans. R. Soc. Lond. Ser. B 372, 20160181 (2017).
Davis, J. H. et al. Modular assembly of the bacterial large ribosomal subunit. Cell 167, 1610–1622 (2016).
Mulder, A. M. et al. Visualizing ribosome biogenesis: parallel assembly pathways for the 30S subunit. Science 330, 673–677 (2010).
Sashital, D. G. et al. A combined quantitative mass spectrometry and electron microscopy analysis of ribosomal 30S subunit assembly in E. coli. eLife 3, e04491 (2014).
Nomura, M. Biosynthesis of bacterial ribosomes. Symp. Soc. Dev. Biol. 30, 195–199 (1974).
Schedlbauer, A. et al. A conserved rRNA switch is central to decoding site maturation on the small ribosomal subunit. Sci. Adv. 7, eabf7547 (2021).
Stephan, N. C., Ries, A. B., Boehringer, D. & Ban, N. Structural basis of successive adenosine modifications by the conserved ribosomal methyltransferase KsgA. Nucleic Acids Res. 49, 6389–6398 (2021).
O’Farrell, H. C., Scarsdale, J. N. & Rife, J. P. Crystal structure of KsgA, a universally conserved rRNA adenine dimethyltransferase in Escherichia coli. J. Mol. Biol. 339, 337–353 (2004).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Nakane, T., Kimanius, D., Lindahl, E. & Scheres, S. H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife 7, e36861 (2018).
O’Farrell, H. C., Musayev, F. N., Scarsdale, J. N. & Rife, J. P. Control of substrate specificity by a single active site residue of the KsgA methyltransferase. Biochemistry 51, 466–474 (2012).
Jomaa, A. et al. Functional domains of the 50S subunit mature late in the assembly process. Nucleic Acids Res. 42, 3419–3435 (2014).
Razi, A. et al. Role of Era in assembly and homeostasis of the ribosomal small subunit. Nucleic Acids Res. 47, 8301–8317 (2019).
Ni, X. et al. YphC and YsxC GTPases assist the maturation of the central protuberance, GTPase associated region and functional core of the 50S ribosomal subunit. Nucleic Acids Res. 44, 8442–8455 (2016).
Melero, R. et al. Continuous flexibility analysis of SARS-CoV-2 spike prefusion structures. IUCrJ 7, 1059–1069 (2020).
Tagare, H. D., Kucukelbir, A., Sigworth, F. J., Wang, H. & Rao, M. Directly reconstructing principal components of heterogeneous particles from cryo-EM images. J. Struct. Biol. 191, 245–262 (2015).
Haselbach, D. et al. Structure and conformational dynamics of the human spliceosomal Bact complex. Cell 172, 454–464 (2018).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Hopfield, J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl Acad. Sci. USA 71, 4135–4139 (1974).
Jomaa, A. et al. Understanding ribosome assembly: the structure of in vivo assembled immature 30S subunits revealed by cryo-electron microscopy. RNA 17, 697–709 (2011).
Jahagirdar, D. et al. Alternative conformations and motions adopted by 30S ribosomal subunits visualized by cryo-electron microscopy. RNA 26, 2017–2030 (2020).
Zamir, A., Miskin, R. & Elson, D. Interconversions between inactive and active forms of ribosomal subunits. FEBS Lett. 3, 85–88 (1969).
Sparling, P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science 167, 56–58 (1970).
Cunningham, P. R., Richard, R. B., Weitzmann, C. J., Nurse, K. & Ofengand, J. The absence of modified nucleotides affects both in vitro assembly and in vitro function of the 30S ribosomal subunit of Escherichia coli. Biochimie 73, 789–796 (1991).
Igarashi, K. et al. Relationship between methylation of adenine near the 3′ end of 16-S ribosomal RNA and the activity of 30-S ribosomal subunits. Eur. J. Biochem. 113, 587–593 (1981).
Inoue, K., Basu, S. & Inouye, M. Dissection of 16S rRNA methyltransferase (KsgA) function in Escherichia coli. J. Bacteriol. 189, 8510–8518 (2007).
Seffouh, A. et al. RbgA ensures the correct timing in the maturation of the 50S subunits functional sites. Nucleic Acids Res. 50, 10801–10816 (2022).
Dutca, L. M. & Culver, G. M. Assembly of the 5′ and 3′ minor domains of 16S ribosomal RNA as monitored by tethered probing from ribosomal protein S20. J. Mol. Biol. 376, 92–S108 (2008).
Jagannathan, I. & Culver, G. M. Assembly of the central domain of the 30S ribosomal subunit: roles for the primary binding ribosomal proteins S15 and S8. J. Mol. Biol. 330, 373–383 (2003).
Woodson, S. A. RNA folding pathways and the self-assembly of ribosomes. Acc. Chem. Res. 44, 1312–1319 (2011).
Rodgers, M. L. & Woodson, S. A. A roadmap for rRNA folding and assembly during transcription. Trends Biochem. Sci. 46, 889–901 (2021).
Rabuck-Gibbons, J. N., Lyumkis, D. & Williamson, J. R. Quantitative mining of compositional heterogeneity in cryo-EM datasets of ribosome assembly intermediates. Structure 30, 498–509(2022).
Chen, M. & Ludtke, S. J. Deep learning-based mixed-dimensional Gaussian mixture model for characterizing variability in cryo-EM. Nat. Methods 18, 930–936 (2021).
Sykes, M. T. & Williamson, J. R. A complex assembly landscape for the 30S ribosomal subunit. Annu. Rev. Biophys. 38, 197–215 (2009).
Eng, J. K., Jahan, T. A. & Hoopmann, M. R. Comet: an open-source MS/MS sequence database search tool. Proteomics 13, 22–24 (2013).
Shteynberg, D. et al. iProphet: multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol. Cell Proteom. 10, M111 007690 (2011).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Schorb, M., Haberbosch, I., Hagen, W. J. H., Schwab, Y. & Mastronarde, D. N. Software tools for automated transmission electron microscopy. Nat. Methods 16, 471–477 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Gomez-Blanco, J., Kaur, S., Ortega, J. & Vargas, J. A robust approach to ab initio cryo-electron microscopy initial volume determination. J. Struct. Biol. 208, 107397 (2019).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Henderson, R. et al. Outcome of the first electron microscopy validation task force meeting. Structure 20, 205–214 (2012).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018).
Dunkle, J. A. et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984 (2011).
Boehringer, D., O’Farrell, H. C., Rife, J. P. & Ban, N. Structural insights into methyltransferase KsgA function in 30S ribosomal subunit biogenesis. J. Biol. Chem. 287, 10453–10459 (2012).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Noeske, J. et al. High-resolution structure of the Escherichia coli ribosome. Nat. Struct. Mol. Biol. 22, 336–341 (2015).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr. 60, 2126–2132 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D. Biol. Crystallogr. 66, 486–501 (2010).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Acknowledgements
We thank K. Sears, M. Strauss, K. Basu and other staff members at the Facility for Electron Microscopy Research (FEMR) at McGill University for help with microscope operation and data collection; the MIT-Satori administrative team for providing computational resources and support; and B. Powell and E. Zhong, and other members of the Davis and Ortega labs, for constructive feedback on this work. This work was funded by the Hugh Hampton Young Fellowship to L.F.K.; National Science Foundation CAREER grant 2046778 and National Institutes of Health grant R01-GM144542 to J.H.D.; and the Canadian Institutes of Health Research grant CIHR PJT-180305 to J.O. FEMR is supported by the Canadian Foundation for Innovation, Quebec Government and McGill University. Research in the Davis lab is supported by the Alfred P. Sloan Foundation, the James H. Ferry Fund, the MIT J-Clinic, and the Whitehead Family.
Author information
Authors and Affiliations
Contributions
Investigation: J.S., L.F.K., D.J.; Software: L.F.K.; Writing – Original draft: J.S., L.F.K., J.O., J.H.D.; Writing – Review & editing: J.S., L.F.K., J.O., J.H.D.; Visualization: J.S., L.F.K., J.H.D.; Supervision, Project administration, and Funding acquisition: J.O., J.H.D. Peer reviewer reports are available.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Sara Osman and Dimitris Typas were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–17, Supplementary Video Legends 1 and 2, and Uncropped Gel
Supplementary Video 1
Supplementary Video 1: Rotation of the head density in KsgA-treated volumes. For each of the KsgA-treated and untreated datasets, 500 volumes were resampled from the subset of latent space corresponding to particles exhibiting strong head density (see Methods). The full set of 1,000 volumes were aligned and amplitude-scaled, and a mask corresponding to the native H32 and H33 density in PDB model 4V9D was applied to the volumes. Principal component analysis was performed on the resulting voxel array (see Methods and Supplementary Figure 12). Volumes shown here are sampled from low to high values of the first principal component. Only the head portion of each volume is shown (orange); the mature 30S is overlaid for reference (gray).
Supplementary Video 2
Supplementary Video 2: H44 flexibility in the absence of KsgA. For each of the treated and untreated datasets, 500 volumes were resampled from the subset of latent space corresponding to particles exhibiting strong H44 density (see Methods). The full set of 1,000 volumes were aligned and amplitude-scaled, and a mask corresponding to the native H44 density in PDB model 4V9D was applied to the volumes. Principal component analysis (vPCA) was performed on the resulting voxel array (see Methods and Supplementary Figure 12). Volumes shown here were sampled from low to high value of the first principal component. Only the H44-masked portion of each volume is shown (orange); a volume lacking H44 is overlaid for reference (grey).
Source data
Source Data Fig. 2
Normalized MAVEn occupancies table for the KsgA-treated sample.
Source Data Fig. 5
Normalized MAVEn occupancies table for the untreated sample.
Source Data Extended Data Fig. 1
R-protein occupancy as measured by mass spectrometry. Data are plotted in Supplementary Figure 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Kinman, L.F., Jahagirdar, D. et al. KsgA facilitates ribosomal small subunit maturation by proofreading a key structural lesion. Nat Struct Mol Biol 30, 1468–1480 (2023). https://doi.org/10.1038/s41594-023-01078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01078-5
This article is cited by
-
Learning structural heterogeneity from cryo-electron sub-tomograms with tomoDRGN
Nature Methods (2024)
-
A closed translocation channel in the substrate-free AAA+ ClpXP protease diminishes rogue degradation
Nature Communications (2023)
-
Assembly landscape for the bacterial large ribosomal subunit
Nature Communications (2023)