Abstract
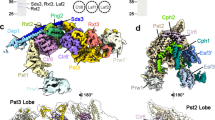
SIN3–HDAC (histone deacetylases) complexes have important roles in facilitating local histone deacetylation to regulate chromatin accessibility and gene expression. Here, we present the cryo-EM structure of the budding yeast SIN3–HDAC complex Rpd3L at an average resolution of 2.6 Å. The structure reveals that two distinct arms (ARM1 and ARM2) hang on a T-shaped scaffold formed by two coiled-coil domains. In each arm, Sin3 interacts with different subunits to create a different environment for the histone deacetylase Rpd3. ARM1 is in the inhibited state with the active site of Rpd3 blocked, whereas ARM2 is in an open conformation with the active site of Rpd3 exposed to the exterior space. The observed asymmetric architecture of Rpd3L is different from those of available structures of other class I HDAC complexes. Our study reveals the organization mechanism of the SIN3–HDAC complex and provides insights into the interaction pattern by which it targets histone deacetylase to chromatin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vidal, M. & Gaber, R. F. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 6317–6327 (1991).
Taunton, J., Hassig, C. A. & Schreiber, S. L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 (1996).
Verdin, E. & Ott, M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 (2015).
Micelli, C. & Rastelli, G. Histone deacetylases: structural determinants of inhibitor selectivity. Drug Discov. Today 20, 718–735 (2015).
Yang, X. J. & Seto, E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9, 206–218 (2008).
Moser, M. A., Hagelkruys, A. & Seiser, C. Transcription and beyond: the role of mammalian class I lysine deacetylases. Chromosoma 123, 67–78 (2014).
Millard, C. J., Watson, P. J., Fairall, L. & Schwabe, J. W. R. Targeting class I histone deacetylases in a “complex” environment. Trends Pharmacol. Sci. 38, 363–377 (2017).
Wang, Z. A. et al. Diverse nucleosome site-selectivity among histone deacetylase complexes. eLife 9, e57663 (2020).
Laugesen, A. & Helin, K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735–751 (2014).
Adams, G. E., Chandru, A. & Cowley, S. M. Co-repressor, co-activator and general transcription factor: the many faces of the Sin3 histone deacetylase (HDAC) complex. Biochem. J. 475, 3921–3932 (2018).
Nascimento, E. M. et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat. Cell Biol. 13, 1395–1405 (2011).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Kwon, Y. J. et al. Selective inhibition of SIN3 corepressor with avermectins as a novel therapeutic strategy in triple-negative breast cancer. Mol. Cancer Ther. 14, 1824–1836 (2015).
Rielland, M. et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J. Clin. Invest. 124, 2125–2135 (2014).
Falkenberg, K. J. & Johnstone, R. W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 13, 673–691 (2014).
Adams, M. K. et al. Differential complex formation via paralogs in the human Sin3 protein interaction network. Mol. Cell Proteomics 19, 1468–1484 (2020).
Witteveen, J. S. et al. Haploinsufficiency of MeCP2-interacting transcriptional co-repressor SIN3A causes mild intellectual disability by affecting the development of cortical integrity. Nat. Genet. 48, 877–887 (2016).
Latypova, X. et al. Haploinsufficiency of the Sin3/HDAC corepressor complex member SIN3B causes a syndromic intellectual disability/autism spectrum disorder. Am. J. Hum. Genet 108, 929–941 (2021).
Kasten, M. M., Dorland, S. & Stillman, D. J. A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol. 17, 4852–4858 (1997).
Keogh, M. C. et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123, 593–605 (2005).
Carrozza, M. J. et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123, 581–592 (2005).
van Oevelen, C. et al. A role for mammalian Sin3 in permanent gene silencing. Mol. Cell 32, 359–370 (2008).
Kadosh, D. & Struhl, K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89, 365–371 (1997).
Bing Li et al. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 316, 1050–1054 (2007).
Silverstein, R. A. & Ekwall, K. Sin3: a flexible regulator of global gene expression and genome stability. Curr. Genet. 47, 1–17 (2005).
Lechner, T. et al. Sds3 (suppressor of defective silencing 3) is an integral component of the yeast Sin3·Rpd3 histone deacetylase complex and is required for histone deacetylase activity. J. Biol. Chem. 275, 40961–40966 (2000).
Lamping, E., Lückl, J., Paltauf, F., Henry, S. A. & Kohlwein, S. D. Isolation and characterization of a mutant of Saccharomyces cerevisiae with pleiotropic deficiencies in transcriptional activation and repression. Genetics 137, 55–65 (1994).
Colina, A. R. & Young, D. Raf60, a novel component of the Rpd3 histone deacetylase complex required for Rpd3 activity in Saccharomyces cerevisiae. J. Biol. Chem. 280, 42552–42556 (2005).
Loewith, R. et al. Pho23 is associated with the Rpd3 histone deacetylase and is required for its normal function in regulation of gene expression and silencing in Saccharomyces cerevisiae. J. Biol. Chem. 276, 24068–24074 (2001).
Xie, T. et al. Structure of the 30-kDa Sin3-associated protein (SAP30) in complex with the mammalian Sin3A corepressor and its role in nucleic acid binding. J. Biol. Chem. 286, 27814–27824 (2011).
Clark, M. D. et al. Structural insights into the assembly of the histone deacetylase-associated Sin3L/Rpd3L corepressor complex. Proc. Natl Acad. Sci. USA 112, E3669–E3678 (2015).
Brubaker, K. et al. Solution structure of the interacting domains of the Mad-Sin3 complex: implications for recruitment of a chromatin-modifying complex. Cell 103, 655–665 (2000).
Nomura, M., Uda-Tochio, H., Murai, K., Mori, N. & Nishimura, Y. The neural repressor NRSF/REST binds the PAH1 domain of the Sin3 corepressor by using its distinct short hydrophobic helix. J. Mol. Biol. 354, 903–915 (2005).
Pena, P. V. et al. Histone H3K4me3 binding is required for the DNA repair and apoptotic activities of ING1 tumor suppressor. J. Mol. Biol. 380, 303–312 (2008).
Narumi-Kishimoto, Y. et al. Novel SIN3A mutation identified in a Japanese patient with Witteveen-Kolk syndrome. Eur. J. Med. Genet. 62, 103547 (2019).
Bansal, N., David, G., Farias, E. & Waxman, S. Emerging roles of epigenetic regulator Sin3 in cancer. Adv. Cancer Res. 130, 113–135 (2016).
Watanabe, K. et al. A novel somatic mutation of SIN3A detected in breast cancer by whole-exome sequencing enhances cell proliferation through ERα expression. Sci. Rep. 8, 16000 (2018).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005).
Laherty, C. D. et al. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell 89, 349–356 (1997).
Finnin, M. S. et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401, 188–193 (1999).
Watson, P. J., Fairall, L., Santos, G. M. & Schwabe, J. W. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 481, 335–340 (2012).
Millard, C. J., Fairall, L., Ragan, T. J., Savva, C. G. & Schwabe, J. W. R. The topology of chromatin-binding domains in the NuRD deacetylase complex. Nucleic Acids Res. https://doi.org/10.1093/nar/gkaa1121 (2020).
Turnbull, R. E. et al. The MiDAC histone deacetylase complex is essential for embryonic development and has a unique multivalent structure. Nat. Commun. 11, 3252 (2020).
Pratap, J. et al. The histone deacetylase inhibitor, vorinostat, reduces tumor growth at the metastatic bone site and associated osteolysis, but promotes normal bone loss. Mol. Cancer Ther. 9, 3210–3220 (2010).
Subramanian, S., Bates, S. E., Wright, J. J., Espinoza-Delgado, I. & Piekarz, R. L. Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals 3, 2751–2767 (2010).
Maxon, M. E. & Herskowitz, I. Ash1p is a site-specific DNA-binding protein that actively represses transcription. Proc. Natl Acad. Sci. USA 98, 1495–1500 (2001).
Le Guezennec, X., Vermeulen, M. & Stunnenberg, H. G. Molecular characterization of Sin3 PAH-domain interactor specificity and identification of PAH partners. Nucleic Acids Res. 34, 3929–3937 (2006).
Strich, R. et al. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 8, 796–810 (1994).
Lee, B. B. et al. Rpd3L HDAC links H3K4me3 to transcriptional repression memory. Nucleic Acids Res. 46, 8261–8274 (2018).
Zhu, C. et al. High-resolution DNA-binding specificity analysis of yeast transcription factors. Genome Res. 19, 556–566 (2009).
Crisp, R. J., Adkins, E. M., Kimmel, E. & Kaplan, J. Recruitment of Tup1p and Cti6p regulates heme-deficient expression of Aft1p target genes. EMBO J. 25, 512–521 (2006).
DeLano, W. L. The PyMOL Molecular Graphics System. http://www.pymol.org (2002).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Gietz, R. D. & Schiestl, R. H. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 35–37 (2007).
Yang, B. et al. Identification of cross-linked peptides from complex samples. Nat. Methods 9, 904–906 (2012).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e18722 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zhan, X., Yan, C., Zhang, X., Lei, J. & Shi, Y. Structure of a human catalytic step I spliceosome. Science 359, 537–545 (2018).
Chen, S. X. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Swint-Kruse, L. & Brown, C. S. Resmap: automated representation of macromolecular interfaces as two-dimensional networks. Bioinformatics 21, 3327–3328 (2005).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Acknowledgements
We thank Y. Shi for support and suggestions, and the cryo-EM facility and supercomputer center of Westlake University for providing cryo-EM and computation support, respectively. This work was supported by funds from the National Natural Science Foundation of China (32100978 to C.W.).
Author information
Authors and Affiliations
Contributions
C.W. and Z.G. conceived the project and designed the experiments. C.W., Z.G. and C.C. performed the majority of the experiments. X. Zhang contributed to EM sample preparation. X. Zhan and Y.L. carried out the structure determination. Y.X. and M.W. contributed to the preparation of nucleosomes. S.G. and C.C.L.W. performed MS proteomics experiments. C.W., Z.G. and X. Zhan wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Tiago Faial and Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification of the Rpd3L complex from S. cerevisiae.
a and b, Gel filtration analysis of the Rpd3L complex purified by 3xFlag-tag on Sin3 (a) or Rxt1 (b). The peak fractions from gel filtration were visualized on SDS-PAGE by Coomassie blue staining or silver staining. Peak Fractions were collected for cryo-EM sample preparation. However, these cryo-EM sample were in bad behavior. c, The peak fractions from glycerol density gradient centrifugation were visualized on SDS-PAGE by silver staining. Fractions 8–12 were collected for cryo-EM sample preparation. We finally used these samples to collect cryo-EM data. These purification experiments have been repeated at least three times.
Extended Data Fig. 2 Characterization of the Rpd3L complex.
a, Protein components of the purified Rpd3L complex were confirmed by mass spectrometry analysis. b, The cross-linking mass spectrometry (XL-MS) analysis of the Rpd3L complex The purified complex was crosslinked by BS3. This analysis identified 24 pairs of inter-molecular interaction among the Rpd3L proteins. This data facilitates structural identification of the Rpd3L components in the EM density. c, Schematic representation of the intermolecular cross-links within the Rpd3L complex.
Extended Data Fig. 3 Micrographs, 2D classes and a flow chart of cryo-EM data (Sin3-Flag) processing for the Rpd3L complex.
a, Representative electron micrograph of the Sin3-Flag sample. The dimeter of the green circle is 25 nm. b, 2D classes of the Sin3-Flag sample. c, All processing steps were carried out in RELION 3.0 and cryoSPARC. Please refer to Methods for details.
Extended Data Fig. 4 Micrographs, 2D classes and a flow chart of cryo-EM data (Rxt1-Flag) processing for the Rpd3L complex.
a, Representative electron micrograph of the Rxt1-Flag sample. The dimeter of the green circle is 25 nm. b, 2D classes of the Rxt1-Flag sample. c, All processing steps were carried out in RELION 3.0 and cryoSPARC. Please refer to Methods for details.
Extended Data Fig. 5 Cryo-EM analysis of the yeast Rpd3L complex.
a, The final reconstruction for the Rpd3L complex displays an average resolution of 2.6 Å on the basis of the Fourier-shell correlation (FSC) value of 0.143. b, Two overall views of the EM density map. The local resolutions are color-coded for different regions of Rpd3L complex. c, Angular distribution of the particles used for the reconstruction. d, The FSC curves for cross-validation between the model and the cryo-EM maps of yeast Rpd3L complex. Shown here are the FSC curves between the final refined atomic model and the reconstruction from all particles (black), between the model refined in the reconstruction from only half of the particles and the reconstruction from that same half (red), and between that same model and the reconstruction from the other half of the particles (green).
Extended Data Fig. 6 The structure of the Ash1 (residue 188–221).
Depending on the XL-MS analysis and the AlphaFold model, we docked the region (residue 188–221) of Ash1 in the low-resolution map to interact with the coiled-coil domains of Sds3 and Dep1.
Extended Data Fig. 7 EM density maps for representative segments of the Rpd3L complex.
Close-up views of fragments of the Rpd3L subunits with cryo-EM densities shown as meshes. The bulk residues are shown as sticks representation. Most of the side chains fit into the cryo-EM map well, indicating the model was built correctly.
Extended Data Fig. 8 Structures of Sds3, Dep1, Rxt2 and Pho23.
a, Cartoon models of Sds3, Dep1, Rxt2 and Pho23. b, A close-up view on the interface between Pho23_α1 and the α10 of Sin3. c, A close-up view on the interface between Rxt2_α7 and the α5 of Sin3’. d, The α9-10 of Sin3_HID or Sin3’_HID interacts with Dep1_α5 or Sds3_α4, respectively, which is similar to the NMR structure of human SIN3A-SDS3 subcomplex (PDB code: 2N2H).
Extended Data Fig. 9 Sequence alignment and structural analysis of class I HDACs.
a, The structures of the Rpd3 and Rpd3’ are almost identical and similar to the structure of human HDAC1. b, The binding sites of the zinc metal in the two copies of Rpd3. c, A close-up view of the zinc binding sites.
Extended Data Fig. 10 Superposition of yeast Rpd3-Sin3HID and human HDAC1-MTA1SANT.
The N-terminus of Sin3_α6 is clashed with InsP6.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, sequence alignments of Sin3, Rpd3, Sds3, Pho23, Sap30, Ume1, Dep1, Rxt2 and Rxt3, and Table 1, summary of model building for the Rpd3L complex.
Source data
Source Data Fig. 1
Unprocessed gel for Fig. 1a.
Source Data Extended Data Fig. 1
Unprocessed gel for Extended Data Fig. 1a–c.
Source Data Extended Data Fig. 2
Source MS data for Extended Data Fig. 2a (Sin3-Flag sample) and Extended Data Fig. 2a (Rxt1-Flag sample), and source cross-linking MS data for Extended Data Fig. 2b,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Z., Chu, C., Lu, Y. et al. Structure of a SIN3–HDAC complex from budding yeast. Nat Struct Mol Biol 30, 753–760 (2023). https://doi.org/10.1038/s41594-023-00975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-00975-z
This article is cited by
-
Structural basis of nucleosome deacetylation and DNA linker tightening by Rpd3S histone deacetylase complex
Cell Research (2023)
-
Structure of the complete Saccharomyces cerevisiae Rpd3S-nucleosome complex
Nature Communications (2023)