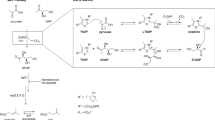
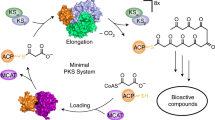
Abstract
The mycolic acid layer of the Mycobacterium tuberculosis cell wall is essential for viability and virulence, and the enzymes responsible for its synthesis are targets for antimycobacterial drug development. Polyketide synthase 13 (Pks13) is a module encoding several enzymatic and transport functions that carries out the condensation of two different long-chain fatty acids to produce mycolic acids. We determined structures by cryogenic-electron microscopy of dimeric multi-enzyme Pks13 purified from mycobacteria under normal growth conditions, captured with native substrates. Structures define the ketosynthase (KS), linker and acyl transferase (AT) domains at 1.8 Å resolution and two alternative locations of the N-terminal acyl carrier protein. These structures suggest intermediate states on the pathway for substrate delivery to the KS domain. Other domains, visible at lower resolution, are flexible relative to the KS-AT core. The chemical structures of three bound endogenous long-chain fatty acid substrates were determined by electrospray ionization mass spectrometry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The atomic coordinates for five structures of Pks13 have been deposited in the PDB with the accession codes 7UK4, 8CUY, 8CV1, 8CUZ and 8CV0. The corresponding maps have been deposited in the Electron Microscopy Data Bank with the accession codes EMD-26574, EMD-27002, EMD-27005, EMD-27003 and EMD-27004. Proteomics data have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD033471. Files containing the input data, scripts and output results for integrative modeling are freely available at https://github.com/integrativemodeling/Pks13. The details about the approach are given in Supplementary Table 4. Source data are provided with this paper.
References
Global Tuberculosis Report 2021 (World Health Organization, 2021).
Zuber, B. et al. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190, 5672–5680 (2008).
Hoffmann, C., Leis, A., Niederweis, M., Plitzko, J. M. & Engelhardt, H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl Acad. Sci. USA 105, 3963–3967 (2008).
Sani, M. et al. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog. 6, e1000794 (2010).
Grzegorzewicz, A. E. et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 8, 334–341 (2012).
Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 8, 19 (2020).
Ioerger, T. R. et al. Identification of new drug targets and resistance mechanisms in Mycobacterium tuberculosis. PLoS ONE 8, e75245 (2013).
Sacchettini, J. C., Rubin, E. J. & Freundlich, J. S. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat. Rev. Microbiol. 6, 41–52 (2008).
Portevin, D. et al. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc. Natl Acad. Sci. USA 101, 314–319 (2004).
Gavalda, S. et al. The Pks13/FadD32 crosstalk for the biosynthesis of mycolic acids in Mycobacterium tuberculosis. J. Biol. Chem. 284, 19255–19264 (2009).
Trivedi, O. A. et al. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428, 441–445 (2004).
Léger, M. et al. The dual function of the Mycobacterium tuberculosis FadD32 required for mycolic acid biosynthesis. Chem. Biol. 16, 510–519 (2009).
Marrakchi, H., Lanéelle, M.-A. & Daffé, M. Mycolic acids: structures, biosynthesis, and beyond. Chem. Biol. 21, 67–85 (2014).
Herbst, D. A. et al. The structural organization of substrate loading in iterative polyketide synthases. Nat. Chem. Biol. 14, 474–479 (2018).
Meniche, X. et al. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc. Natl Acad. Sci. USA 111, E3243–E3251 (2014).
Khosla, C. From active sites to machines: a challenge for enzyme chemists. Isr. J. Chem. 59, 37–40 (2019).
Aggarwal, A. et al. Development of a novel lead that targets M. tuberculosis polyketide synthase 13. Cell 170, 249–259.e25 (2017).
Pan, H. et al. Crystal structure of the priming beta-ketosynthase from the R1128 polyketide biosynthetic pathway. Structure 10, 1559–1568 (2002).
Tang, Y., Kim, C.-Y., Mathews, I. I., Cane, D. E. & Khosla, C. The 2.7-Angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc. Natl Acad. Sci. USA 103, 11124–11129 (2006).
Barry, C. E. et al. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 37, 143–179 (1998).
Byers, D. M. & Gong, H. Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem. Cell Biol. 85, 649–662 (2007).
Robbins, T., Kapilivsky, J., Cane, D. E. & Khosla, C. Roles of conserved active site residues in the ketosynthase domain of an assembly line polyketide synthase. Biochemistry 55, 4476–4484 (2016).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Klaus, M. et al. Protein-protein interactions, not substrate recognition, dominate the turnover of chimeric assembly line polyketide synthases. J. Biol. Chem. 291, 16404–16415 (2016).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Vernon, R. M. et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife 7, e31486 (2018).
Lee, D., Lee, J. & Seok, C. What stabilizes close arginine pairing in proteins? Phys. Chem. Chem. Phys. 15, 5844–5853 (2013).
Bergeret, F. et al. Biochemical and structural study of the atypical acyltransferase domain from the mycobacterial polyketide synthase Pks13. J. Biol. Chem. 287, 33675–33690 (2012).
Gande, R. et al. Acyl-CoA carboxylases (accD2 and accD3), together with a unique polyketide synthase (Cg-pks), are key to mycolic acid biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 279, 44847–44857 (2004).
Lucas, X., Bauzá, A., Frontera, A. & Quiñonero, D. A thorough anion-π interaction study in biomolecules: on the importance of cooperativity effects. Chem. Sci. 7, 1038–1050 (2016).
Bon, C. et al. Solution structure of the type I polyketide synthase Pks13 from Mycobacterium tuberculosis. BMC Biol. 20, 147 (2022).
George, K. M., Yuan, Y., Sherman, D. R. & Barry, C. E. The biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Identification and functional analysis of CMAS-2. J. Biol. Chem. 270, 27292–27298 (1995).
Yuan, Y. & Barry, C. E. A common mechanism for the biosynthesis of methoxy and cyclopropyl mycolic acids in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 93, 12828–12833 (1996).
Piersimoni, L. & Sinz, A. Cross-linking/mass spectrometry at the crossroads. Anal. Bioanal. Chem. 412, 5981–5987 (2020).
Kaake, R. M. et al. A new in vivo cross-linking mass spectrometry platform to define protein-protein interactions in living cells. Mol. Cell Proteom. 13, 3533–3543 (2014).
Merkley, E. D. et al. Distance restraints from crosslinking mass spectrometry: mining a molecular dynamics simulation database to evaluate lysine-lysine distances. Protein Sci. 23, 747–759 (2014).
Kaake, R. M. et al. Characterization of an A3G-VifHIV-1-CRL5-CBFβ structure using a cross-linking mass spectrometry pipeline for integrative modeling of host-pathogen complexes. Mol. Cell Proteom. 20, 100132 (2021).
Sali, A. From integrative structural biology to cell biology. J. Biol. Chem. 296, 100743 (2021).
Saltzberg, D. J. et al. Using Integrative Modeling Platform to compute, validate, and archive a model of a protein complex structure. Protein Sci. 30, 250–261 (2021).
Saltzberg, D. et al. Modeling biological complexes using integrative modeling platform. Methods Mol. Biol. 2022, 353–377 (2019).
Kim, S. J. et al. Integrative structure and functional anatomy of a nuclear pore complex. Nature 555, 475–482 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Russel, D. et al. Putting the pieces together: integrative modeling platform software for structure determination of macromolecular assemblies. PLoS Biol. 10, e1001244 (2012).
Tsai, S. C. et al. Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: versatility from a unique substrate channel. Proc. Natl Acad. Sci. USA 98, 14808–14813 (2001).
Gokulan, K., Aggarwal, A., Shipman, L., Besra, G. S. & Sacchettini, J. C. Mycobacterium tuberculosis acyl carrier protein synthase adopts two different pH-dependent structural conformations. Acta Crystallogr. D 67, 657–669 (2011).
Keatinge-Clay, A. T. et al. Catalysis, specificity, and ACP docking site of Streptomyces coelicolor malonyl-CoA:ACP transacylase. Structure 11, 147–154 (2003).
Tsai, S.-C., Lu, H., Cane, D. E., Khosla, C. & Stroud, R. M. Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases. Biochemistry 41, 12598–12606 (2002).
Mindrebo, J. T. et al. Gating mechanism of elongating β-ketoacyl-ACP synthases. Nat. Commun. 11, 1727 (2020).
Portevin, D. et al. The acyl-AMP ligase FadD32 and AccD4-containing acyl-CoA carboxylase are required for the synthesis of mycolic acids and essential for mycobacterial growth: identification of the carboxylation product and determination of the acyl-CoA carboxylase components. J. Biol. Chem. 280, 8862–8874 (2005).
La Rosa, V. et al. MmpL3 is the cellular target of the antitubercular pyrrole derivative BM212. Antimicrob. Agents Chemother. 56, 324–331 (2012).
Poce, G. et al. Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection. PLoS ONE 8, e56980 (2013).
LaCava, J., Jiang, H. & Rout, M. P. Protein complex affinity capture from cryomilled mammalian cells. J. Vis. Exp. https://doi.org/10.3791/54518 (2016).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. eLife 7, e35383 (2018).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Terwilliger, T. C., Sobolev, O. V., Afonine, P. V. & Adams, P. D. Automated map sharpening by maximization of detail and connectivity. Acta Crystallogr. D 74, 545–559 (2018).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
van Zundert, G. C. P., Moriarty, N. W., Sobolev, O. V., Adams, P. D., & Borrelli, K. W. Macromolecular refinement of X-ray and cryoelectron microscopy structures with Phenix/OPLS3e for improved structure and ligand quality. Structure 29, 913–921.e4 (2021).
Pravda, L. et al. MOLEonline: a web-based tool for analyzing channels, tunnels and pores (2018 update). Nucleic Acids Res. 46, W368–W373 (2018).
Tan, B. K. et al. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl Acad. Sci. USA 109, 16504–16509 (2012).
Kao, A. et al. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol. Cell Proteom. 10, M110.002212 (2011).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Kessner, D., Chambers, M., Burke, R., Agus, D. & Mallick, P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24, 2534–2536 (2008).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Baker, P. R. & Chalkley, R. J. MS-viewer: a web-based spectral viewer for proteomics results. Mol. Cell Proteom. 13, 1392–1396 (2014).
Alber, F. et al. Determining the architectures of macromolecular assemblies. Nature 450, 683–694 (2007).
Viswanath, S., Chemmama, I. E., Cimermancic, P. & Sali, A. Assessing exhaustiveness of stochastic sampling for integrative modeling of macromolecular structures. Biophys. J. 113, 2344–2353 (2017).
Acknowledgements
We thank J. Sacchettini, Texas A&M University for the inhibitor TAM16 and C. Sassetti, University of Massachusetts Worcester for the Pks13-TEV-eGFP MSMEG strain and both for their insights, A. Keatinge-Clay and J. Sacchettini for their reading of the paper, counsel and advice, and G. Ashley, ProLynx, Inc. for Supplementary Fig. 1b. We acknowledge P. Thomas, M. Harrington, J. Baker-LePain and the Wynton HPC for computational support; and D. Bulkley, Z. Yu and G. Gilbert for their maintenance of the UCSF EM Core. We thank J. Wilkins and K. Li for performing initial mass spectrometry for lipid identification in the UCSF mass spectrometry core. Research was financially supported by grant nos. P01 AI095208 (J. Sacchettini), GM024485 (R.M.S.), AI48366 (Z.G.), GM083960 and GM109824 (A.S.), U19 A1135990 (N.J.K.) and R01 AI128214 (O.S.R.). M.S.D. acknowledges a National Science Federation graduate fellowship.
Author information
Authors and Affiliations
Contributions
O.S.R., S.K.K. and R.M.S. conceived the project. S.K.K. and J.C. prepared the protein. M.S.D. prepared EM grids and performed EM imaging and image processing. M.S.D. interpreted density maps and determined the five structures reported. S.K.K., M.S.D. and J.F.-M. refined and interpreted the structures. Z.G. determined the lipid chains attached to PKS13 by mass spectrometry at ‘the lipidomics project’. E.H.P., R.M.K. and N.J.K. determined the DSSO cross-links formed by mass spectrometry. I.E. and A.S. performed integrative structure determination. R.M.S. and O.S.R. supervised the project. S.K.K., M.S.D., J.F.M., Z.G., O.S.R. and R.M.S. wrote the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The Krogan Laboratory has received research support from Vir Biotechnology, F. Hoffmann-La Roche and Rezo Therapeutics. N.J.K. has financially compensated consulting agreements with the Icahn School of Medicine at Mount Sinai, New York, Maze Therapeutics, Interline Therapeutics, Rezo Therapeutics, GEn1E Lifesciences, Inc. and Twist Bioscience Corp. He is on the Board of Directors of Rezo Therapeutics and is a shareholder in Tenaya Therapeutics, Maze Therapeutics, Rezo Therapeutics and Interline Therapeutics.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Florian Ullrich and Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification of native Pks13 from mycobacteria.
(a) Final Superose 6 size exclusion chromatography profile of Pks13. Two peaks containing Pks13 are labeled 1 and 2, in addition to the void peak and peak containing TEV protease. Pks13 peaks were run on non-reducing SDS-PAGE (Biorad #4561096) (b) and blue native PAGE (Invitrogen #BN1004BOX) (c), which were repeated twice with similar results. In SDS-PAGE (b) the protein runs at ~198 kDa, as expected for a protomer of Pks13. Blue native PAGE (c) indicates that majority of the protein is in a dimeric form, and higher oligomeric forms are present. Upon screening both peaks 1 and 2 on gold Quantifoil grids by cryoEM, peak 1 was selected for data collection since it produced more monodisperse particles that correspond to Pks13 dimers. We conclude that the Pks13 dimers associate loosely in solution, and that these readily dissociate to form monodisperse Pks13 dimers under the conditions in which the images were acquired. (d) 78.1% sequence coverage (highlighted red) by tryptic digested Pks13 fragments run on LC-MS-MS.
Extended Data Fig. 2 DE-rich linker.
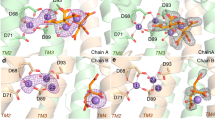
17 amino acid linking sequence (E76-92) between ACP1 and KS includes nine acidic amino acids (colored orange) and termed the ‘DE-rich linker’. First 11 amino acids (E76-E86) lie near positively charged surfaces of two different regions on the KS monomer surface (Extended Data Fig. 3). The last six amino acids (D87-R92) closely overlap in orientation between the ACP1a and ACP1b structures and interact electrostatically with the shared positively charged surface (Extended Data Fig. 3).
Extended Data Fig. 3 Electrostatic surface rendering of ACP1-KS-AT domains.
(a) The KS-AT surface is colored by electrostatic potential (red: acidic, blue: basic) of the residues. ACP1a and ACP1b and their ensuing linkers to the KS (yellow and lemon chiffon) are superimposed onto the KS-AT electrostatic potential surface, illustrating that the DE-rich linker lie near positively charged surfaces on the KS. (b) ACP1a, ACP1b, and the linkers to the KS are colored by electrostatic potential (red: acidic, blue: basic) of the residues. Model of KS-AT is superimposed onto the highly negative electrostatic potential surface of the DE-rich linker.
Extended Data Fig. 4 Interfacing residues between DE-rich linker and KS domain in ACP1a position.
Interfacing residues were determined by PISA and labeled. Hydrogen bond between E88(OE2):R384(NH2) is represented with a yellow dotted line.
Extended Data Fig. 5 Interfacing residues between DE-rich linker and KS domain in ACP1b position.
Interfacing residues were determined by PISA and labeled. Hydrogen bonds between Y84(OH):R395(NH1), E88(OE2):R384(NH1), and E88(OE2):K388(NZ) are labeled with yellow dotted lines.
Extended Data Fig. 6 ACP1a interaction with KS, KS-AT linker domain, and AT-ACP2 linker.
Interfacing residues between ACP1a and KS, KS-AT linker domain, and AT-ACP2 linker are shown as stick representation and labeled. Pi-pi stacking interaction is shown between R63 of ACP1a and R496 of KS. The Ppant arm is shown attached to S38 in helix II of ACP1a, and Ppant density is shown zoned around 2.3 Å around the model.
Extended Data Fig. 7 ACP1b interaction with KS and AT’.
(a) ACP1b:AT’ interfacing residues’ side chains are shown and labeled, revealing a hydrophilic interface. (b) ACP1b:KS interfacing residues’ side chains are shown and labeled, revealing a hydrophobic interface.
Extended Data Fig. 8 The active center of the AT domain shows the bound substrate interactions.
(a) Crossed-eyes stereo figure showing the density for the active site of the AT domain with substrate bound. The α-carboxyl group on the bound substrate is stabilized by a π-anion interaction with the phenyl ring of F709. (b) Rotated ∼90° around the vertical axis and tilted forward from panel (a) shows interactions between the active center of the AT domain and the bound α-carboxy acyl substrate chain attached to S798. Distances between heavy atoms are tabulated in Å. The catalytic base H906 is ideally placed for the reaction at the γO of S798. The ester carbonyl on the substrate, and hence the oxyanion intermediate formed at that oxygen during the catalytic reaction may be stabilized by R823, H906, and S905. The H906 orientation is aligned by hydrogen bonds from the δN-H to the C = O of G959 and of H962.
Extended Data Fig. 9 Linkage map depicting all unique K-K linkages of DSSO cross-linked Pks13.
The 57 unique K-K inter-linked residues are summarized in this linkage map as black lines connecting two Pks13 lysine residues. Shown is a cartoon diagram of the primary sequence structure of Pks13 with domains labeled as in Fig. 1a. The sequence numbers of lysine residues indicated by linear position in this figure are included in Supplementary Tables 2, 3, 5, and 6.
Extended Data Fig. 10 Integrative structure modeling of the full-length Pks13 dimer.
(a) The localization probability density of the ensemble of structures is shown with representative (centroid) structure from the ensemble embedded within it. The three panels represent different solutions with their relative percentages listed above. The structured and unstructured regions are represented as beads, using the same color scheme as Fig. 1a. Regions with well-defined structures are modeled as rigid bodies consisting of beads representing each individual residue; the coordinates of a 1-residue bead were those of the corresponding Cα atom. Regions with no available structure or flexible regions are represented as flexible strings of beads, corresponding to up to 10 residues per bead. (b) Detail of cross-links mapped to the centroid structure of the integrative model of the Pks13 dimer. Satisfied and violated cross-links shown in blue and yellow, respectively. A cross-link is classified as satisfied if the Cα–Cα distance spanned by the cross-linked residues in any of the models of the cluster is less than 35 Å. (c) Histogram showing the distribution of the cross-linked Cα–Cα distances in the Pks13 dimer integrative structures. (d) Histogram showing the Cα–Cα distances between the active sites of the ACP1-KS, AT-ACP2, KS-ACP2, and ACP2-TE domains. The shortest distances (red lines) are 26, 13.7, 14.8, and 9.7 Å between the ACP1-KS, AT-ACP2, KS-ACP2, and ACP2-TE domains, respectively.
Supplementary information
Supplementary Information
Supplementary Text, Figs. 1–13, Table 1, captions for Tables 2–6, caption for Video 1 and References.
Supplementary Table 2
Summary of unique Pks13 interlinked residues excluding files from highly cross-linked samples.
Supplementary Table 3
Metadata of all DSSO cross-linked Pks13 XL–MS files.
Supplementary Table 5
Table with description and scores for overall DSSO cross-linked peptides for all processed files including interlinked, monolinked and single peptides.
Supplementary Table 6
Summary of all unique Pks13 interlinked residues from the full dataset.
Supplementary Video 1
Relative positions of the ACP1, KS, AT domains and substrates determined by cryo-EM.
Source data
Source Data Fig. 1
Chem draw data for Fig. 1 trehalose.
Source Data Fig. 1
Chem draw data for Fig. 1 mechanism.
Source Data Extended Data Fig. 1
Uncropped gel pictures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S.K., Dickinson, M.S., Finer-Moore, J. et al. Structure and dynamics of the essential endogenous mycobacterial polyketide synthase Pks13. Nat Struct Mol Biol 30, 296–308 (2023). https://doi.org/10.1038/s41594-022-00918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00918-0