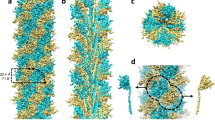
Abstract
Self-assembly of proteins into filaments, such as actin and tubulin filaments, underlies essential cellular processes in all three domains of life. The early emergence of filaments in evolutionary history suggests that filament genesis might be a robust process. Here we describe the fortuitous construction of GFP fusion proteins that self-assemble as fluorescent polar filaments in Escherichia coli. Filament formation is achieved by appending as few as 12 residues to GFP. Crystal structures reveal that each protomer donates an appendage to fill a groove between the two following protomers along the filament. This exchange of appendages resembles runaway domain swapping but is distinguished by higher efficiency because monomers cannot competitively bind their own appendages. Ample evidence for this ‘runaway domain coupling’ mechanism in nature suggests it could facilitate the evolutionary pathway from globular protein to polar filament, requiring a minimal extension of protein sequence and no substantial refolding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shih, Y.-L. & Rothfield, L. The bacterial cytoskeleton. Microbiol. Mol. Biol. Rev. 70, 729–754 (2006).
Jockusch, B.M. & Graumann, P.L. The long journey: actin on the road to pro- and eukaryotic cells. in Reviews of Physiology, Biochemistry and Pharmacology vol. 161 (eds. Amara, S. G. et al.) 67–85 https://doi.org/10.1007/112_2011_1 (Springer, Berlin, 2011).
Bernander, R., Lind, A. E. & Ettema, T. J. G. An archaeal origin for the actin cytoskeleton: implications for eukaryogenesis. Commun. Integr. Biol. 4, 664–667 (2011).
Wickstead, B. & Gull, K. The evolution of the cytoskeleton. J. Cell Biol. 194, 513–525 (2011).
O’Connell, J. D., Zhao, A., Ellington, A. D. & Marcotte, E. M. Dynamic reorganization of metabolic enzymes into intracellular bodies. Annu. Rev. Cell Dev. Biol. 28, 89–111 (2012).
Petrovska, I. et al. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. Elife https://doi.org/10.7554/eLife.02409 (2014).
Aughey, G. N. & Liu, J.-L. Metabolic regulation via enzyme filamentation. Crit. Rev. Biochem. Mol. Biol. 51, 282–293 (2015).
Barry, R. M. et al. Large-scale filament formation inhibits the activity of CTP synthetase. Elife 3, e03638 (2014).
Liu, Y. & Eisenberg, D. 3D domain swapping: as domains continue to swap. Protein Sci. 11, 1285–1299 (2002).
Bennett, M. J., Sawaya, M. R. & Eisenberg, D. Deposition diseases and 3D domain swapping. Structure 14, 811–824 (2006).
Ogihara, N. L. et al. Design of three-dimensional domain-swapped dimers and fibrous oligomers. Proc. Natl Acad. Sci. USA 98, 1404–1409 (2001).
Huntington, J. A. et al. A 2.6 Å structure of a serpin polymer and implications for conformational disease. J. Mol. Biol. 293, 449–455 (1999).
Strop, P., Smith, K. S., Iverson, T. M., Ferry, J. G. & Rees, D. C. Crystal structure of the “cab”-type β class carbonic anhydrase from the archaeon Methanobacterium thermoautotrophicum. J. Biol. Chem. 276, 10299–10305 (2001).
Guo, Z., & Eisenberg, D. Runaway domain swapping in amyloid-like fibrils of T7 endonuclease I. Proc. Natl Acad. Sci. USA 103, 8042–8047 (2006).
Wahlbom, M. et al. Fibrillogenic oligomers of human cystatin C are formed by propagated domain swapping. J. Biol. Chem. 282, 18318–18326 (2007).
Jaskólski, M. 3D domain swapping, protein oligomerization, and amyloid formation. Acta Biochim. Pol. 48, 807–827 (2001).
Sambashivan, S., Liu, Y., Sawaya, M. R., Gingery, M. & Eisenberg, D. Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature 437, 266–269 (2005).
Teng, P. K. & Eisenberg, D. Short protein segments can drive a non-fibrillizing protein into the amyloid state. Protein Eng. Des. Sel. 22, 531–536 (2009).
Goldschmidt, L., Teng, P. K., Riek, R., & Eisenberg, D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl Acad. Sci. USA 107, 3487–3492 (2010).
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993).
Yang, F., Moss, L. G. & Phillips, G. N. Jr. The molecular structure of green fluorescent protein. Nat. Biotechnol. 14, 1246–1251 (1996).
Ponstingl, H., Henrick, K. & Thornton, J. M. Discriminating between homodimeric and monomeric proteins in the crystalline state. Proteins 41, 47–57 (2000).
Wellems, T. E. & Josephs, R. Crystallization of deoxyhemoglobin S by fiber alignment and fusion. J. Mol. Biol. 135, 651–674 (1979).
Harrington, D. J., Adachi, K. & Royer, W. E. Jr. The high resolution crystal structure of deoxyhemoglobin S. J. Mol. Biol. 272, 398–407 (1997).
Liu, Y., Gotte, G., Libonati, M. & Eisenberg, D. Structures of the two 3D domain-swapped RNase A trimers. Protein Sci. 11, 371–380 (2002).
Wang, Z., Kishchenko, G., Chen, Y. & Josephs, R. Polymerization of deoxy-sickle cell hemoglobin in high-phosphate buffer. J. Struct. Biol. 131, 197–209 (2000).
Lomas, D. A., Evans, D. L., Finch, J. T. & Carrell, R. W. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature 357, 605–607 (1992).
Bennett, M. J., Choe, S., & Eisenberg, D. Domain swapping: entangling alliances between proteins. Proc. Natl Acad. Sci. USA 91, 3127–3131 (1994).
Raymond, D. D., Piper, M. E., Gerrard, S. R., Skiniotis, G., & Smith, J. L. Phleboviruses encapsidate their genomes by sequestering RNA bases. Proc. Natl Acad. Sci. USA 109, 19208–19213 (2012).
Hospenthal, M. K. et al. The cryoelectron microscopy structure of the type 1 chaperone-usher pilus rod. Structure 25, 1829–1838.e4 (2017).
Kuhlman, B., O’Neill, J. W., Kim, D. E., Zhang, K. Y., & Baker, D. Conversion of monomeric protein L to an obligate dimer by computational protein design. Proc. Natl Acad. Sci. USA 98, 10687–10691 (2001).
Kelley, B. S., Chang, L. C. & Bewley, C. A. Engineering an obligate domain-swapped dimer of cyanovirin-N with enhanced anti-HIV activity. J. Am. Chem. Soc. 124, 3210–3211 (2002).
Ruigrok, R. W. H. & DiCapua, E. On the polymerization state of recA in the absence of DNA. Biochimie 73, 191–198 (1991).
von der Ecken, J. et al. Structure of the F-actin-tropomyosin complex. Nature 519, 114–117 (2015).
Greenberg, M. J., Wang, C.-L. A., Lehman, W. & Moore, J. R. Modulation of actin mechanics by caldesmon and tropomyosin. Cell Motil. Cytoskeleton 65, 156–164 (2008).
Jung, J. & Lee, B. Circularly permuted proteins in the protein structure database. Protein Sci. 10, 1881–1886 (2001).
Sawaya, M. R., Guo, S., Tabor, S., Richardson, C. C. & Ellenberger, T. Crystal structure of the helicase domain from the replicative helicase-primase of bacteriophage T7. Cell 99, 167–177 (1999).
Lynch, E. M. et al. Human CTP synthase filament structure reveals the active enzyme conformation. Nat. Struct. Mol. Biol. 24, 507–514 (2017).
Barry, R. M. & Gitai, Z. Self-assembling enzymes and the origins of the cytoskeleton. Curr. Opin. Microbiol. 14, 704–711 (2011).
Shen, Q.-J. et al. Filamentation of metabolic enzymes in Saccharomyces cerevisiae. J. Genet. Genomics 43, 393–404 (2016).
Pandya, M. J. et al. Sticky-end assembly of a designed peptide fiber provides insight into protein fibrillogenesis. Biochemistry 39, 8728–8734 (2000).
Potekhin, S. A. et al. De novo design of fibrils made of short alpha-helical coiled coil peptides. Chem. Biol. 8, 1025–1032 (2001).
Padilla, J. E., Colovos, C., & Yeates, T. O. Nanohedra: using symmetry to design self assembling protein cages, layers, crystals, and filaments. Proc. Natl Acad. Sci. USA 98, 2217–2221 (2001).
Yeates, T. O. & Padilla, J. E. Designing supramolecular protein assemblies. Curr. Opin. Struct. Biol. 12, 464–470 (2002).
Mou, Y., Yu, J.-Y., Wannier, T. M., Guo, C.-L. & Mayo, S. L. Computational design of co-assembling protein-DNA nanowires. Nature 525, 230–233 (2015).
Sayre, T. C., Lee, T. M., King, N. P. & Yeates, T. O. Protein stabilization in a highly knotted protein polymer. Protein Eng. Des. Sel. 24, 627–630 (2011).
Bharat, T. A. M., Murshudov, G. N., Sachse, C. & Löwe, J. Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles. Nature 523, 106–110 (2015).
Ormö, M. et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273, 1392–1395 (1996).
Garrity, S. J., Sivanathan, V., Dong, J., Lindquist, S., & Hochschild, A. Conversion of a yeast prion protein to an infectious form in bacteria. Proc. Natl Acad. Sci. USA 107, 10596–10601 (2010).
Khlebnikov, A., Datsenko, K. A., Skaug, T., Wanner, B. L. & Keasling, J. D. Homogeneous expression of the PBAD promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147, 3241–3247 (2001).
Doan, T., Marquis, K. A. & Rudner, D. Z. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol. Microbiol. 55, 1767–1781 (2005).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Auerbach, D. et al. Replacement of highly conserved E222 by the photostable non-photoconvertible histidine in GFP. ChemBioChem 15, 1404–1408 (2014).
Bricogne, G. et al. Buster version 1.10.0 (Global Phasing Ltd., Cambridge, 2016).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Delano, W. The PyMOL Molecular Graphics System (Schrödinger LLC, New York, 2017).
Chou, P. Y. & Fasman, G. D. Empirical predictions of protein conformation. Annu. Rev. Biochem. 47, 251–276 (1978).
Acknowledgements
We thank M. Collazo and D. Cascio of the UCLA-DOE X-ray Crystallization and Crystallography Core Facilities for their assistance with crystallization and data collection, J. Abraham for discussion, and S. Dove for critical reading of the manuscript. We thank the Howard Hughes Medical Institute and National Science Foundation (grant 1616265) for support to D.S.E. and National Institutes of Health (grants OD003806 and GM115941) for support to A.H. Diffraction data were collected at the Northeastern Collaborative Access Team beamlines 24-ID-E and C, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P41 GM103403). The Pilatus 6 M detector on 24-ID-C beam line is funded by an NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
L.M. designed the study, performed cell biological and mutagenesis experiments and contributed to writing the manuscript. D.M.H. designed the study, performed cell biological experiments and protein purifications, and contributed to writing the manuscript. D.S.E. discussed plans and results with A.H. and M.R.S. A.H. designed the study and contributed to writing the manuscript. M.R.S. determined and analyzed the crystal structures, performed in cellulo diffraction experiments and contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Amyloid-forming propensities for each of the eight indicated GFP-RNase fusion linkers, as predicted by ZipperDB.
Orange bars represent hexapeptides predicted to form the spines of amyloid fibrils (energies below –23 kcal/mol). Most notably, the glutamine variant, which was predicted to have the strongest amyloid-forming propensity, produced no filaments, suggesting that the filaments are not amyloid-like.
Supplementary Figure 2 Crystals of GFP-RNase(1–8) invariably display a needle morphology.
This needle morphology recapitulates on a larger scale the rod-like shape of GFP-RNase assemblies observed in cells. (a) A representative sampling of the many conditions producing GFP-RNase(1-8) crystals. Only a small percentage of these conditions were tested for diffraction. Each subpanel reports the crystallization kit and composition of the reservoir that produced the crystal. The scale bar at the top left applies to all subpanels. (b) The four crystals used for structure determination, each with distinct crystal packing and unit cell parameters. The scale bar at the left applies to all subpanels.
Supplementary Figure 3 Structural effects of point mutations on a major interface of the GFP-RNase protofilament assembly.
The donor helix (residues 233-247) is colored green and the acceptor barrel is colored brown. The top panel illustrates the crystal structure; the remaining panels are hypothetical models illustrating the compatibility or incompatibility of the designated mutation with the filament structure. The only variant of M240 that is structurally and physically compatible with the filament structure is L240. The A240 mutation creates a destabilizing gap. The E240 and Q240 mutations place polar atoms in a hydrophobic environment. Nitrogen, oxygen, and sulfur atoms are colored blue, red, and yellow, respectively. The yellow starbursts and red disks indicate steric clash.
Supplementary Figure 4 Protomer interfaces observed in the GFP-RNase(1–8) protofilament crystal structure: dimensions and organization.
Three distinct interface regions are marked with dashed lines. The amount of surface area buried in each interface is indicated. The filament axis is vertical in this view.
Supplementary Figure 5 Immunoblot showing that the GFP-V219E fusion protein variant and parent fusion protein are produced at comparable levels.
The blot was probed with anti-His antibody to detect the His6-tagged GFP fusion constructs, GFP-AMA-RNaseA(1-8)-His6 and GFP-V219E-AMA-RNaseA(1-8)His6, and. A separate blot prepared in tandem with identical samples was probed with an antibody to detect the RpoA protein of E. coli, which served as a loading control.
Supplementary Figure 6 Bragg spacings observed in the diffraction pattern of rod-containing E. coli match prominent molecular spacings in the GFP-RNase protofilament crystals in space group C2.
(a) The diffraction pattern from dried and aligned E. coli cells containing GFP-RNaseA(1-8) rods. The numbers in parentheses correspond to resolution (Bragg spacing) of the reflection in angstroms. The integers specify the Miller indices for the reflections based on correspondence with the C2 crystal form of GFP-RNase(1-8). Reflections related by symmetry share the same symbol. (b-g) Illustrations of the Bragg planes associated with six of the strongest reflections. The structure of GFP-RNase(1-8) filaments in the C2 crystal form is shown in cartoon ribbons. Individual barrels of GFP are highlighted by cylinders. A single reference protofilament is colored in light and dark green. Bragg planes are shown in blue. The Miller index is labeled above each panel. The unit cell is outlined in red. Unit cell directions (a, b and c) are labeled. (h) A clear outline of the filament architecture can be seen from the sum of the six Fourier waves illustrated in the previous panels.
Supplementary Figure 7 A mechanism for generating new protofilaments by circular permutation.
By this mechanism, components of preexisting intramolecular interfaces are split over distant surfaces, requiring protomers to assemble into a filament in order to reconstitute the interface. This mechanism of generating a protofilament-competent interface might occur faster than an alternative mechanism in which individual residues are mutated over generations to form a stable interface de novo.
Supplementary Figure 8 A plot of solvation free energy versus area buried in the interfaces of 28 surveyed filaments.
Values were calculated using the structures indicated. PDB accession codes are reported in parentheses. The plot indicates that GFP-RNase ranks lower in stability compared to most filaments surveyed.
Supplementary information
Supplementary Information
Supplementary Figures 1–8, Supplementary Notes 1 and 2 and Supplementary Table 1
Rights and permissions
About this article
Cite this article
McPartland, L., Heller, D.M., Eisenberg, D.S. et al. Atomic insights into the genesis of cellular filaments by globular proteins. Nat Struct Mol Biol 25, 705–714 (2018). https://doi.org/10.1038/s41594-018-0096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0096-7