Abstract
Parkinson’s disease is a synucleinopathy that is characterized by motor dysfunction, death of midbrain dopaminergic neurons and accumulation of α-synuclein (α-Syn) aggregates. Evidence suggests that α-Syn aggregation can originate in peripheral tissues and progress to the brain via autonomic fibers. We tested this by inoculating the duodenal wall of mice with α-Syn preformed fibrils. Following inoculation, we observed gastrointestinal deficits and physiological changes to the enteric nervous system. Using the AAV-PHP.S capsid to target the lysosomal enzyme glucocerebrosidase for peripheral gene transfer, we found that α-Syn pathology is reduced due to the increased expression of this protein. Lastly, inoculation of α-Syn fibrils in aged mice, but not younger mice, resulted in progression of α-Syn histopathology to the midbrain and subsequent motor defects. Our results characterize peripheral synucleinopathy in prodromal Parkinson’s disease and explore cellular mechanisms for the gut-to-brain progression of α-Syn pathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study. The data that support the findings of this study are available from the corresponding author upon request.
References
McCann, H., Stevens, C. H., Cartwright, H. & Halliday, G. M. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 20, S62–S67 (2014).
Lees, A. J., Hardy, J. & Revesz, T. Parkinson’s disease. Lancet 373, 2055–2066 (2009).
Hawkes, C. H., Del Tredici, K. & Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 16, 79–84 (2010).
Sánchez-Ferro, Á. et al. In vivo gastric detection of α-synuclein inclusions in Parkinson’s disease. Mov. Disord. 30, 517–524 (2015).
Shannon, K. M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 27, 709–715 (2012).
Yoo, B. B. & Mazmanian, S. K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46, 910–926 (2017).
Braak, H., Rüb, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014).
Uemura, N. et al. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 13, 21 (2018).
Kim, S. et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron https://doi.org/10.1016/j.neuron.2019.05.035 (2019).
Devos, D. et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 50, 42–48 (2013).
Codolo, G. et al. Triggering of inflammasome by aggregated α–synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8, e55375 (2013).
Amor, S., Puentes, F., Baker, D. & Valk, P. V. D. Inflammation in neurodegenerative diseases. Immunology 129, 154–169 (2010).
Chu, Y., Dodiya, H., Aebischer, P., Olanow, C. W. & Kordower, J. H. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol. Dis. 35, 385–398 (2009).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132, 1783–1794 (2009).
Fishbein, I., Kuo, Y.-M., Giasson, B. I. & Nussbaum, R. L. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a gaucher mutation. Brain 137, 3235–3247 (2014).
Mazzulli, J. R. et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52 (2011).
Sardi, S. P. et al. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl Acad. Sci. USA 108, 12101–12106 (2011).
O’Sullivan, S. S. et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov. Disord. 23, 101–106 (2008).
Volpicelli-Daley, L. A., Luk, K. C. & Lee, V. M.-Y. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014).
Luk, K. C. et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Volpicelli-Daley, L. A. et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Hallett, P. J., McLean, J. R., Kartunen, A., Langston, J. W. & Isacson, O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol. Dis. 47, 258–267 (2012).
Chesselet, M.-F. et al. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9, 297–314 (2012).
Schafer, K.-H., Mestres, P., Marz, P. & Rose-John, S. The IL-6/sIL-6R fusion protein hyper-IL-6 promotes neurite outgrowth and neuron survival in cultured enteric neurons. J. Interferon Cytokine Res. 19, 527–532 (1999).
De Schepper, S. et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415.e13 (2018).
Sehgal, A. et al. The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat. Commun. 9, 1272 (2018).
Barrenschee, M. et al. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun. 5, 1 (2017).
Fujiwara, H. et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 (2002).
Grassi, D. et al. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl Acad. Sci. USA 115, E2634–E2643 (2018).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Morabito, G. et al. AAV-PHP.B-mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol. Ther. https://doi.org/10.1016/j.ymthe.2017.08.004 (2017).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Challis, R. C. et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. https://doi.org/10.1038/s41596-018-0097-3 (2019).
Froula, J. M. et al. α-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons. Acta Neuropathol. Commun. 6, 35 (2018).
Boesmans, W., Hao, M. M. & Berghe, P. V. Optical tools to investigate cellular activity in the intestinal wall. J. Neurogastroenterol. Motil. 21, 337–351 (2015).
Treweek, J. B. et al. Whole-body tissue stabilization and selective extractions via tissue–hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 10, 1860–1896 (2015).
Reeve, A., Simcox, E. & Turnbull, D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 14, 19–30 (2014).
Kordower, J. H. et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain J. Neurol 136, 2419–2431 (2013).
Salvatore, M. F., Pruett, B. S., Dempsey, C. & Fields, V. Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area. J. Vis. Exp. https://doi.org/10.3791/4171 (2012).
Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993 (2014).
Tanner, C. M. et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872 (2011).
Rosenbloom, B. et al. The incidence of parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol. Dis. 46, 95–102 (2011).
Sidransky, E. & Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 11, 986–998 (2012).
Rocha, E. M. et al. Glucocerebrosidase gene therapy prevents α-synucleinopathy of midbrain dopamine neurons. Neurobiol. Dis. 82, 495–503 (2015).
Whitton, P. S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 150, 963–976 (2009).
Neunlist, M. et al. Enteric glial cells: recent developments and future directions. Gastroenterology 147, 1230–1237 (2014).
Chandra, R., Hiniker, A., Kuo, Y.-M., Nussbaum, R. L. & Liddle, R. A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2, e92295 (2017).
Douglas, P. M. & Dillin, A. Protein homeostasis and aging in neurodegeneration. J. Cell Biol. 190, 719–729 (2010).
Fox, E. A., Phillips, R. J., Martinson, F. A., Baronowsky, E. A. & Powley, T. L. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J. Comp. Neurol. 428, 558–576 (2000).
Rockenstein, E. et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 68, 568–578 (2002).
Li, Z. et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009 (2011).
Fleming, S. M., Ekhator, O. R. & Ghisays, V. Assessment of sensorimotor function in mouse mods of Parkinson’s disease. J. Vis. Exp. https://doi.org/10.3791/50303 (2013).
Deacon, R. M. J. Measuring the strength of mice. J. Vis. Exp. https://doi.org/10.3791/2610 (2013).
Bannon, A. W. & Malmberg, A. B. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 41, 8.9.1–8.9.16 (2007).
Park, J. et al. Pain perception in acute model mice of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol. Pain 11, 28 (2015).
Acknowledgements
We thank C. Bedbrook for help with calcium imaging and K. Beadle and E. Mackey for additional technical support. This work was supported by the following grants to V.G.: NIH Director’s New Innovator IDP20D017782-01 and PECASE; NIH/NIA R01AG047664-01; NIH BRAIN 1U01NS090577; the Heritage Medical Research Institute; the Pew Charitable Trust; the Rogers Fellowship for Parkinson’s Research and the CZI Neurodegeneration Challenge Network. C.C. was supported by NIH/NIA F32AG054101. S.K.M. was supported by NIH/NINDS R01NS085910. V.G. and S.K.M. were supported by Department of Defense grant W81XWH-17-1-0588. L.A.V.-D. was supported by NINDS R01NS102257 and Morris K. Udall Centers of Excellence for Parkinson’s Disease Research P50NS108675. T.R.S was supported by the Larry L. Hillblom Foundation. B.B.Y. was supported by NIH/NIGMS 5T32GM007616 and the Caltech Center for Environmental Microbial Interactions (CEMI).
Author information
Authors and Affiliations
Contributions
C.C. and V.G. conceptualized the study and developed the research plan. C.C., V.G. and L.A.V.-D. designed the study. L.A.V.-D. generated the α-Syn PFFs and α-Syn monomers. C.C. performed the animal surgeries, tissue clearing, histology, calcium imaging and retro-orbital viral injections. C.C., A.H. and T.R.S. performed the behavior experiments. C.C., B.B.Y. and R.C.C. performed virus production, purification and verification. C.C. and T.R.S. performed protein analyses. C.C., B.B.Y. and R.C.C. performed confocal imaging. A.M.H. and T.R.S. performed RNA extraction and quantitative PCR analyses. C.C. performed data analyses. S.K.M. provided key reagents and methods. C.C. and V.G. wrote the manuscript. All authors contributed to discussion. V.G. supervised all the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effect of αSyn pathology on GI health and cytokine expression.
a-d, GI function in WT, ASO, and aged mice is characterized by fecal pellet production (a; WT vs. ASO **p = 0.022, ASO vs. Aged **p = 0.0075), fecal pellet weight (b; WT vs. ASO ****p < 0.0001, WT vs. Aged ***p = 0.0009, ASO vs. Aged ****p < 0.0001), proportion fecal water weight (c; WT vs. ASO ****p < 0.0001, ASO vs. Aged ****p < 0.0001), and whole gut fecal transit time (d; WT vs. ASO ****p < 0.0001, ASO vs. Aged **p = 0.0038). e, Animal weight (WT vs. Aged ****p < 0.0001, ASO vs. Aged ****p < 0.0001). f, qPCR analysis of GBA1 expression in duodenum. Dots represent average of technical triplicates from a single animal. g, Densitometry analysis of cytokine panel from duodenal lysates after inoculation with α-Syn PFF or α-Syn monomer 7 dpi (Fractalkine **p = 0.0030, IL-1a **p = 0.0070, IL-6 *p = 0.0415, IL-7 * p = 0.0400, MCP-1 *p = 0.0416, MCSF *p = 0.0467, MIG *p = 0.0412, TECK *p = 0.0365, TIMP-2 *p = 0.0400). h, Representative cytokine panels detected by chemiluminescence. i, Representative Western blot immunostained for IL-6 and densitometry analysis normalized to WT integrated densities (WT vs. PFF 60 dpi *p = 0.0327, 60 dpi PFF vs. monomer *p = 0.0480, WT vs. ASO *p = 0.0304). Data depicted are mean ± s.e.m. P values were determined by one-way ANOVA (a-f,i). The following n values represents number of independent animals used for statistical evaluation: e1a-c, WT = 42, ASO = 20, Aged = 19; e1d, WT = 13, ASO = 9, Aged = 12; e1e, WT = 42, ASO = 20, Aged = 19; e1f, WT = 3, ASO = 4, Aged = 4; e1g, monomer and PFF = 4 each; e1i, all conditions = 4 each.
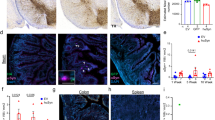
Extended Data Fig. 2 Visualization of the duodenal ENS and cell proliferation.
a, Maximum intensity projection confocal image of duodenum whole-mount stained for PGP9.5 (neurons) and GFAP (EGCs). Higher magnification of dashed box shown in Fig. 1g. Scale bar, 200 µm. b, Virtual cross-section of the duodenal wall after optical clearing distinguishes myenteric and submucosal plexuses. Intestinal crypt layer is marked in blue. Scale bar, 100 µm. c, DAPI nuclear labeling visualizes the intestinal crypt layer used for histological analysis of the ENS (top). High-magnification of dashed box shows individual crypts (bottom). Scale bars, 100 µm. Experimental images for (a-c) were obtained from 8 independent mice, with similar results obtained. d, Quantification of number of myenteric neurons per crypt (top) or myenteric EGCs per crypt (bottom; WT vs. PFF 60 dpi *p = 0.0329, WT vs. PFF 120 dpi *p = 0.0232), e, Immunohistochemical labeling of EdU detects recently proliferated extraganglionic (open arrows) or myenteric ganglion cells (closed arrows). EGCs are co-labeled with GFAP. Scale bar, 20 µm. f, Quantification of total EdU+ cells per crypt (Monomer vs. PFF *p = 0.0071). Data depicted is mean ± s.e.m. P values were determined by One-Way ANOVA (d) or one-tailed Student’s t-test (f). The following n values represents number of independent animals used for statistical evaluation: e2d enteric neurons per crypt, WT = 6, PFF 0 dpi = 6, PFF 7 dpi = 6, PFF 21 dpi = 6, PFF 60 dpi = 6, PFF 120 dpi = 5, monomer conditions = 5 each; e2d EGCs per crypt, WT = 4, all PFF = 5 each, monomer 7 dpi = 4, monomer 60 dpi = 5; e2f, monomer = 4, PFF = 5.
Extended Data Fig. 3 Inoculation of duodenum with αSyn PFF promotes formation of αSyn filaments.
a, Representative dot blot images of duodenal homogenates immunostained with an aggregation-specific α-Syn antibody (α-Syn filament) show an increase after α-Syn PFF inoculation and in ASO mice. b, Densitometry analysis of α-Syn filament dot blots of duodenal homogenates normalized to pre-injection WT mice integrated densities (int. den.) (WT vs. ASO **p = 0.0092). c, Representative dot blot image of controls immunostained with α-Syn filament antibody: 50 ng BSA, 50 ng and 100 ng α-Syn monomer, 50 ng and 100 ng α-Syn PFF. d, Densitometry analysis of control blots normalized to 50 ng BSA integrated densities (BSA vs. PFF 50ng **p = 0.0080, BSA vs. PFF 100ng ***p = 0.0009, monomer 50ng vs. PFF 50ng *p = 0.0178, monomer 100ng vs. PFF 100ng **p = 0.0078). Data depicted is mean ± s.e.m. P values were determined by one-way ANOVA (a, d). The following n values represents number of independent animals used for statistical evaluation: e3b, WT = 8, all PFF = 5 each, all monomer = 4 each, ASO = 8, Aged = 5; e3d, BSA = 4, all other groups = 3 each.
Extended Data Fig. 4 GBA1 gene transfer increases GCase function and improves GI health.
a-d, Characterization of GI function before and after peripheral GBA1 gene transfer by quantifying fecal pellet production (b), fecal pellet weight (c; ASO 0 vs. 60 dpvi *p = 0.0265), proportion fecal water weight (d), and whole gut fecal transit time (e). e-h, Characterization of GI function before and after peripheral EGFP gene transfer by quantifying fecal pellet production (e), fecal pellet weight (f), proportion fecal water weight (g), and whole gut fecal transit time (h; 0 dpi WT vs. ASO **p = 0.0085, 60 dpi WT vs. ASO *p = 0.0293). Data depicted are mean ± s.e.m. P values were determined by two-way ANOVA (a-h). The following n values represents number of independent animals used for statistical evaluation: e4a-d, for 0 / 7 / 21 / 60 dpi: WT = 17 / 12 / 11 / 8, ASO = 13 / 12 / 11 / 10; e4e-h, all conditions = 4 each.
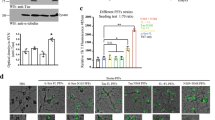
Extended Data Fig. 5 ENS neurotransmission is disrupted by αSyn pathology and restored by GBA1 gene transfer.
a, Quantification of average peak percent change in fluorescence and area under the curve after photostimulation pulse for jRGECO1a+-only or jRGECO1a+/ChR2+ duodenal neurons before and after inoculation (all ****p < 0.0001). b, Quantification of average peak percent change in fluorescence and area under the curve after photostimulation pulse for jRGECO1a+-only or jRGECO1a+/ChR2+ duodenal neurons before and after systemic delivery of AAV-PHP.S::ihSyn:GBA1 (Peak ΔF/F jRGECO1a+ 0 dpvi WT vs. ASO ****p < 0.0001, 7 dpvi WT vs. ASO ****p < 0.0001; Peak ΔF/F jRGECO1a+/ChR2+ 0 dpvi WT vs. ASO ****p < 0.0001, 7 dpvi WT vs. ASO ***p = 0.0006; AUC jRGECO1a+ 0 dpvi WT vs. ASO ****p < 0.0001, 7 dpvi WT vs. ASO **p = 0.0031; AUC jRGECO1a+/ChR2+ 0 dpi WT vs. ASO *p = 0.0398). Data depicted are mean ± s.e.m. P values were determined by two-way ANOVA. The following n values represents number of independent animals used for statistical evaluation: e5a, 0 dpi = 3, PFF 7 dpi = 4, PFF 60 dpi = 3, monomer 7 dpi = 3, monomer 60 dpi = 3; e5b, all conditions = 3.
Extended Data Fig. 6 Inoculation of duodenum with αSyn PFF in adult mice does not progress αSyn pathology to the nodose ganglion.
a, Maximum intensity 3D projection confocal image of a whole nodose ganglion from an α-Syn PFF inoculated WT mouse 60 dpi. Ganglion was PACT cleared and immunolabeled for PGP9.5 (neurons), S129P, and DAPI (nuclei). Experimental images were obtained from 14 independent mice, with similar results obtained. Scale bars, 100 µm. Z-stack depth, 500 µm. b, Maximum intensity 3D projection confocal image of a nodose ganglion from a WT mouse. Experimental images were obtained from 5 independent mice, with similar results obtained. Scale bar, 100 µm. Z-stack depth, 450 µm. c, Quantification of S129P+ nodose ganglion neurons (WT vs. ASO **p = 0.0011, monomer 7 dpi vs. ASO **p = 0.0027, monomer 60 dpi vs. ASO *p = 0.0176, ASO vs. Aged *p = 0.0323). Data depicted is mean ± s.e.m. P values were determined by one-way ANOVA. The following n values represents number of independent animals used for statistical evaluation: e6c, WT = 5, ASO = 5, Aged = 5, for 7 / 60 / 120 dpi, PFF = 5 / 5 / 4, monomer = 4 / 3 / 0.
Extended Data Fig. 7 Brain αSyn pathology observed in ASO mice but not after inoculation of duodenum with αSyn PFF in adult mice.
a-b, Maximum intensity projection confocal images of the brainstem (approximately −7.76 mm from Bregma) from an α-Syn PFF-inoculated 8 week old WT mouse 60 dpi (a) and an ASO mouse (b), immunolabeled for cholinergic neurons (ChAT) and S129P. Scale bars, 500 μm. Experimental images for (a-b) were obtained from 3 independent mice, with similar results obtained. c-d, Maximum intensity projection confocal images of the midbrain (approximately −3.64 mm from Bregma) from an α-Syn PFF-inoculated WT mouse 60 dpi (c) and an ASO mouse (d), immunolabeled for dopaminergic neurons (TH), S129P, and nuclei (DAPI). Scale bars: 500 μm. Experimental images for (c-d) were obtained from 3 independent mice, with similar results obtained. e-f, Densitometry analysis of S129P signal in the DMV (e; WT vs. ASO ***p = 0.0003, PFF 60 dpi vs. ASO ***p = 0.0007) and SNc (f; WT vs. ASO **p = 0.0011, PFF 60 dpi vs. ASO **p = 0.0014) per unit area, normalized to WT. Data depicted is mean ± s.e.m. P values were determined by one-way ANOVA (a, d). The following n values represents number of independent animals used for statistical evaluation: e7e-f, all conditions = 3 each.
Extended Data Fig. 8 Duodenal inoculation with αSyn PFF in adult mice produces only mild sensorimotor deficits.
a-f, Sensorimotor behaviors after inoculation were quantified using average time to turn and descend a pole (a; PFF 0 vs. 60 dpi **p = 0.0012, 90 dpi PFF vs. monomer *p = 0.0265), average time before falling during the inverted wire-hang paradigm (b; PFF 0 vs. 60 dpi *p = 0.0306, PFF 0 vs. 120 dpi *p = 0.0285), average time to remove adhesive from nasal bridge (c; PFF 0 vs. 90 dpi **p = 0.0014, PFF 0 vs. 120 dpi *p = 0.0285, 60 dpi PFF vs. monomer *p = 0.0193, 90 dpi PFF vs. monomer *p = 0.0342), average weightlifting paradigm score (d), average time to cross a narrowing beam (e), and total number of slips incurred during 3 trials of the narrowing beam paradigm (f; 60 dpi PFF vs. BSA **p = 0.0023). Boxplots represent median, interquartile range, and 1.5x the interquartile range. g, Hot plate test did not reveal any changes in nociception at 60 dpi. Data depicted is mean ± s.e.m. P values were determined by two-way ANOVA (a-f) or one-way ANOVA (g). The following n values represents number of independent animals used for statistical evaluation: e8a-c, for 0 / 7 / 21 / 60 / 90 / 120 dpi, PFF = 16 / 14 / 14 / 11 / 9 / 8, monomer = 9 / 9 / 9 / 8 / 8 / 8, BSA = 17 / 16 / 11 / 9 / 7 / 7; e8d, for 0 / 7 / 21 / 60 / 90 / 120 dpi, PFF = 8 / 8 / 12 / 8 / 8 / 8, monomer = 9 / 9 / 9 / 8 / 8 / 8, BSA = 17 / 16 / 11 / 9 / 7 / 7; e8e-f, for 0 / 7 / 21 / 60 / 90 / 120 dpi, PFF = 8 / 8 / 10 / 7 / 6 / 6, monomer = 9 / 9 / 9 / 8 / 8 / 8, BSA = 17 / 16 / 11 / 9 / 7 / 7; e8g, baseline = 10, monomer = 4, PFF = 6.
Extended Data Fig. 9 Duodenal αSyn PFF inoculation in aged mice does not result in SNc dopaminergic cell loss.
a-c, Representative images of the midbrain immunostained for dopaminergic neurons (TH), S129P, and nuclei (DAPI) from aged mice pre-inoculation (a), α-Syn PFF-inoculated aged mice 120 dpi, and α-Syn-monomer inoculated aged mice 120 dpi. Scale bars, 500 μm. Experimental images for (a-c) were obtained from 12 independent mice, with similar results obtained. d, Quantification of dopaminergic cell density within the boundary of the SNc as determined by TH+ signal (example shown in [a]). Data depicted is mean ± s.e.m. P values were determined by one-way ANOVA. The following n values represents number of independent animals used for statistical evaluation: e9d, all conditions = 4.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Video 1
Representative movie of optogenetic stimulation and calcium imaging of the duodenal enteric nervous system. Raw grayscale movie collecting jRGECO1a signal from duodenal enteric neurons in response to photostimulation of ChR2-EYFP by a 473-nm pulse (indicated in frames with the word “light” at the top right). For demonstrative purposes, the movie shows a stimulation train of 5 pulses, 10 s apart.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed dot blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Challis, C., Hori, A., Sampson, T.R. et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci 23, 327–336 (2020). https://doi.org/10.1038/s41593-020-0589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0589-7
This article is cited by
-
The involvement of α-synucleinopathy in the disruption of microglial homeostasis contributes to the pathogenesis of Parkinson’s disease
Cell Communication and Signaling (2024)
-
Addition of α-synuclein aggregates to the intestinal environment recapitulates Parkinsonian symptoms in model systems
Acta Pharmacologica Sinica (2024)
-
Phosphorylated α-synuclein deposited in Schwann cells interacting with TLR2 mediates cell damage and induces Parkinson’s disease autonomic dysfunction
Cell Death Discovery (2024)
-
Gut microbiota-induced CXCL1 elevation triggers early neuroinflammation in the substantia nigra of Parkinsonian mice
Acta Pharmacologica Sinica (2024)
-
Neuroimmune Connectomes in the Gut and Their Implications in Parkinson’s Disease
Molecular Neurobiology (2024)