Abstract
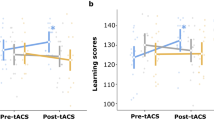
Understanding normal brain aging and developing methods to maintain or improve cognition in older adults are major goals of fundamental and translational neuroscience. Here we show a core feature of cognitive decline—working-memory deficits—emerges from disconnected local and long-range circuits instantiated by theta–gamma phase–amplitude coupling in temporal cortex and theta phase synchronization across frontotemporal cortex. We developed a noninvasive stimulation procedure for modulating long-range theta interactions in adults aged 60–76 years. After 25 min of stimulation, frequency-tuned to individual brain network dynamics, we observed a preferential increase in neural synchronization patterns and the return of sender–receiver relationships of information flow within and between frontotemporal regions. The end result was rapid improvement in working-memory performance that outlasted a 50 min post-stimulation period. The results provide insight into the physiological foundations of age-related cognitive impairment and contribute to groundwork for future non-pharmacological interventions targeting aspects of cognitive decline.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data and software code that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bishop, N. A., Lu, T. & Yankner, B. A. Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 (2010).
Hebert, L. E., Weuve, J., Scherr, P. A. & Evans, D. A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783 (2013).
Hayutin, A. M. Global demographic shifts create challenges and opportunities. PREA Q. 46–53 (2007).
Park, D. C. & Reuter-Lorenz, P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196 (2009).
Grady, C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 13, 491–505 (2012).
Hedden, T. & Gabrieli, J. D. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96 (2004).
Tomasi, D. & Volkow, N. D. Aging and functional brain networks. Mol. Psychiatry 17, 549–558 (2012).
Andrews-Hanna, J. R. et al. Disruption of large-scale brain systems in advanced aging. Neuron 56, 924–935 (2007).
Davis, S. W. et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46, 530–541 (2009).
O’Sullivan, M. et al. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 57, 632–638 (2001).
Siegel, M., Donner, T. H. & Engel, A. K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 13, 121–134 (2012).
Roux, F. & Uhlhaas, P. J. Working memory and neural oscillations: α–γ versus θ–γ codes for distinct WM information? Trends Cogn. Sci. 18, 16–25 (2014).
Helfrich, R. F. & Knight, R. T. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn. Sci. 20, 916–930 (2016).
Fell, J. & Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118 (2011).
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L. & von Stein, A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl Acad. Sci. USA 95, 7092–7096 (1998).
Axmacher, N. et al. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl Acad. Sci. USA 107, 3228–3233 (2010).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015).
Daume, J., Gruber, T., Engel, A. K. & Friese, U. Phase–amplitude coupling and long-range phase synchronization reveal frontotemporal interactions during visual working memory. J. Neurosci. 37, 313–322 (2017).
Sreenivasan, K. K., Curtis, C. E. & D’Esposito, M. Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 18, 82–89 (2014).
Lara, A. H. & Wallis, J. D. Executive control processes underlying multi-item working memory. Nat. Neurosci. 17, 876–883 (2014).
Reinhart, R. M. G. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc. Natl Acad. Sci. USA 114, 11542–11547 (2017).
Helfrich, R. F. et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 12, e1002031 (2014).
Polanía, R., Nitsche, M. A., Korman, C., Batsikadze, G. & Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 22, 1314–1318 (2012).
Wolinski, N., Cooper, N. R., Sauseng, P. & Romei, V. The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biol. 16, e2005348 (2018).
Alekseichuk, I., Turi, Z., Amador de Lara, G., Antal, A. & Paulus, W. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol. 26, 1513–1521 (2016).
Salthouse, T. A. The aging of working memory. Neuropsychology 8, 535–543 (1994).
Voytek, B. et al. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 35, 13257–13265 (2015).
Cole, S. R. & Voytek, B. Brain oscillations and the importance of waveform shape. Trends Cogn. Sci. 21, 137–149 (2017).
Scheffer-Teixeira, R. & Tort, A. B. On cross-frequency phase–phase coupling between theta and gamma oscillations in the hippocampus. Elife 5, 423–435 (2016).
Daume, J., Graetz, S., Gruber, T., Engel, A. K. & Friese, U. Cognitive control during audiovisual working memory engages frontotemporal theta-band interactions. Sci. Rep. 7, 12585 (2017).
Ranganath, C. & D’Esposito, M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31, 865–873 (2001).
Nichols, E. A., Kao, Y. C., Verfaellie, M. & Gabrieli, J. D. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus 16, 604–616 (2006).
Axmacher, N. et al. Sustained neural activity patterns during working memory in the human medial temporal lobe. J. Neurosci. 27, 7807–7816 (2007).
Axmacher, N., Schmitz, D. P., Wagner, T., Elger, C. E. & Fell, J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J. Neurosci. 28, 7304–7312 (2008).
Lueschow, A., Miller, E. K. & Desimone, R. Inferior temporal mechanisms for invariant object recognition. Cereb. Cortex 4, 523–531 (1994).
Woloszyn, L. & Sheinberg, D. L. Neural dynamics in inferior temporal cortex during a visual working memory task. J. Neurosci. 29, 5494–5507 (2009).
Powell, H. W. et al. Material-specific lateralization of memory encoding in the medial temporal lobe: blocked versus event-related design. Neuroimage 27, 231–239 (2005).
Henson, R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q. J. Exp. Psychol. B 58, 340–360 (2005).
Ali, M. M., Sellers, K. K. & Fröhlich, F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 33, 11262–11275 (2013).
Herrmann, C. S., Rach, S., Neuling, T. & Strüber, D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 7, 279 (2013).
Vossen, A., Gross, J. & Thut, G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 8, 499–508 (2015).
Zaehle, T., Rach, S. & Herrmann, C. S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One 5, e13766 (2010).
Bergmann, T. O. & Born, J. Phase-amplitude coupling: a general mechanism for memory processing and synaptic plasticity? Neuron 97, 10–13 (2018).
Gregoriou, G. G., Gotts, S. J., Zhou, H. & Desimone, R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210 (2009).
Wang, X. J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 90, 1195–1268 (2010).
Violante, I. R. et al. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife 6, e22001 (2017).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Beck, A. T., Steer, R. A. & Brown, G. K. Manual for the Beck Depression Inventory (The Psychological Association, 1996).
Sabia, S. et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch. Gen. Psychiatry 69, 627–635 (2012).
Grundey, J., Amu, R., Batsikadze, G., Paulus, W. & Nitsche, M. A. Diverging effects of nicotine on motor learning performance: improvement in deprived smokers and attenuation in non-smokers. Addict. Behav. 74, 90–97 (2017).
Grundey, J. et al. Double dissociation of working memory and attentional processes in smokers and non-smokers with and without nicotine. Psychopharmacology (Berl.) 232, 2491–2501 (2015).
Noury, N., Hipp, J. F. & Siegel, M. Physiological processes non-linearly affect electrophysiological recordings during transcranial electric stimulation. Neuroimage 140, 99–109 (2016).
Noury, N. & Siegel, M. Analyzing EEG and MEG signals recorded during tES, a reply. Neuroimage 167, 53–61 (2018).
Konkle, T., Brady, T. F., Alvarez, G. A. & Oliva, A. Conceptual distinctiveness supports detailed visual long-term memory for real-world objects. J. Exp. Psychol. Gen. 139, 558–578 (2010).
Brady, T. F., Konkle, T., Alvarez, G. A. & Oliva, A. Visual long-term memory has a massive storage capacity for object details. Proc. Natl Acad. Sci. USA 105, 14325–14329 (2008).
Park, J. Y., Jhung, K., Lee, J. & An, S. K. Theta–gamma coupling during a working memory task as compared to a simple vigilance task. Neurosci. Lett. 532, 39–43 (2013).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J. M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Nguyen, J., Deng, Y. & Reinhart, R. M. G. Brain-state determines learning improvements after transcranial alternating-current stimulation to frontal cortex. Brain Stimul. 11, 723–726 (2018).
Kanai, R., Chaieb, L., Antal, A., Walsh, V. & Paulus, W. Frequency-dependent electrical stimulation of the visual cortex. Curr. Biol. 18, 1839–1843 (2008).
Schutter, D. J. & Hortensius, R. Retinal origin of phosphenes to transcranial alternating current stimulation. Clin. Neurophysiol. 121, 1080–1084 (2010).
Paulus, W. On the difficulties of separating retinal from cortical origins of phosphenes when using transcranial alternating current stimulation (tACS). Clin. Neurophysiol. 121, 987–991 (2010).
Lachaux, J. P., Rodriguez, E., Martinerie, J. & Varela, F. J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999).
Reinhart, R. M. G., Zhu, J., Park, S. & Woodman, G. F. Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction-error signaling. J. Neurosci. 35, 12232–12240 (2015).
Reinhart, R. M. G. & Woodman, G. F. Enhancing long-term memory with stimulation tunes visual attention in one trial. Proc. Natl Acad. Sci. USA 112, 625–630 (2015).
Reinhart, R. M. G. & Woodman, G. F. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J. Neurosci. 34, 4214–4227 (2014).
Reinhart, R. M. G., Zhu, J., Park, S. & Woodman, G. F. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc. Natl Acad. Sci. USA 112, 9448–9453 (2015).
Reinhart, R. M. G., Cosman, J. D., Fukuda, K. & Woodman, G. F. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten. Percept. Psychophys. 79, 3–23 (2017).
Reinhart, R. M. G., Xiao, W., McClenahan, L. J. & Woodman, G. F. Electrical stimulation of visual cortex can immediately improve spatial vision. Curr. Biol. 26, 1867–1872 (2016).
Poreisz, C., Boros, K., Antal, A. & Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 72, 208–214 (2007).
Gandiga, P. C., Hummel, F. C. & Cohen, L. G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 117, 845–850 (2006).
Jung, T. P. et al. Imaging brain dynamics using independent component analysis. Proc. IEEE Inst. Electr. Electron. Eng. 89, 1107–1122 (2001).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Berens, P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 21 (2009).
van Driel, J., Cox, R. & Cohen, M. X. Phase-clustering bias in phase-amplitude cross-frequency coupling and its removal. J. Neurosci. Methods 254, 60–72 (2015).
Van Veen, B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880 (1997).
Hipp, J. F., Engel, A. K. & Siegel, M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 69, 387–396 (2011).
Sekihara, K. & Nagarajan, S. S. Adaptive Spatial Filters for Electromagnetic Brain Imaging (Springer, 2008).
Darvas, F., Pantazis, D., Kucukaltun-Yildirim, E. & Leahy, R. M. Mapping human brain function with MEG and EEG: methods and validation. Neuroimage 23 (Suppl. 1), S289–S299 (2004).
Dannhauer, M., Lanfer, B., Wolters, C. H. & Knösche, T. R. Modeling of the human skull in EEG source analysis. Hum. Brain Mapp. 32, 1383–1399 (2011).
Wolters, C. H. et al. Influence of tissue conductivity anisotropy on EEG/MEG field and return current computation in a realistic head model: a simulation and visualization study using high-resolution finite element modeling. Neuroimage 30, 813–826 (2006).
Cho, J. H., Vorwerk, J., Wolters, C. H. & Knösche, T. R. Influence of the head model on EEG and MEG source connectivity analyses. Neuroimage 110, 60–77 (2015).
Buchner, H. et al. Inverse localization of electric dipole current sources in finite element models of the human head. Electroencephalogr. Clin. Neurophysiol. 102, 267–278 (1997).
Wolters, C. H., Grasedyck, L. & Hackbusch, W. Efficient computation of lead field bases and influence matrix for the FEM-based EEG and MEG inverse problem. Inverse Probl. 20, 1099–1116 (2004).
Lew, S., Wolters, C. H., Dierkes, T., Röer, C. & Macleod, R. S. Accuracy and run-time comparison for different potential approaches and iterative solvers in finite element method based EEG source analysis. Appl. Numer. Math. 59, 1970–1988 (2009).
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage 27, 842–851 (2005).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Lancaster, J. L. et al. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 10, 120–131 (2000).
Jiang, H., Bahramisharif, A., van Gerven, M. A. & Jensen, O. Measuring directionality between neuronal oscillations of different frequencies. Neuroimage 118, 359–367 (2015).
Gross, J. et al. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl Acad. Sci. USA 98, 694–699 (2001).
Nolte, G. et al. Robustly estimating the flow direction of information in complex physical systems. Phys. Rev. Lett. 100, 234101 (2008).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 29, 169–195 (1999).
Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617 (2012).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Nolte, G. et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 115, 2292–2307 (2004).
Storey, J. D. A direct approach to false discovery rates. J. R. Stat. Soc. B 64, 479–498 (2002).
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01 MH 114877) awarded to R.M.G.R.
Author information
Authors and Affiliations
Contributions
R.M.G.R. conceived the experiments. R.M.G.R. and J.A.N. conducted the experiments and analyzed the data. R.M.G.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Michael Nitsche and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Experiment 1: Age, but not HD-tACS, impacts posterior alpha power suppression.
We questioned whether improvements in maintenance-related neural interactions and working-memory behavior might be due to the stimulation having enhanced perceptual attention immediately after target onset, which could in turn boost the fidelity of the target representation stored in working memory. To test this idea, we examined alpha power suppression immediately after target presentation because this EEG signature is thought to index the release of inhibition to facilitate task-relevant processing 92. If the exogenous stimulation enhanced working memory via a gating-by-inhibition attention mechanism, we should find greater target-locked posterior alpha suppression after active relative to sham stimulation in older adults. a, Target-locked time-frequency representations of total power from occipital and parietooccipital electrodes during memory blocks following sham stimulation in younger minus older adults reveal a significant age-related deficit in alpha suppression (t82 = 3.154, P = 0.002, dz = 0.688), suggesting that older adults were unable to functionally disinhibit task-relevant areas as efficiently as younger adults during the perceptual analysis of real-world objects. b, However, this age-related deficit in alpha suppression remained significant even after older adults received active stimulation (t82 = 2.766, P = 0.007, dz = 0.604). c, No changes to alpha power were observed between sham and active stimulation in older adults (t41 = 0.733, P = 0.468, dz = 0.113). The results showing age-related dysfunction in alpha rhythms during perceptual analysis align with work on the selective attention deficits in aging 96–98. However, unlike the neural interactions of theta-gamma PAC and theta phase synchronization during memory maintenance, this earlier signal during stimulus processing was not influenced by the HD-tACS and is thus an unlikely candidate for underlying the stimulation-induced performance benefit observed in older adults. Topographies show the spatial distribution of the power responses during the 8–14 Hz, 100–400 ms post-target analytic window, indicated by the white dashed box. Between-group comparisons used independent two-tailed t-tests (n = 84). Within-group comparisons used paired sample two-tailed t-tests (n = 42).
Supplementary Figure 2 Experiment 3: Reversing the phase angle of HD-tACS impairs working-memory performance.
We interpret the findings from experiments 1 and 2 showing improved working-memory performance following in-phase stimulation as resulting from the increased temporal synchronization of large-scale cortical interactions. However, if working-memory performance is truly mediated by a phase-sensitive network mechanism, then we should find that changing the phase angle direction of alternating current applied to frontotemporal regions should change the direction of the effects on behavioral performance. Unlike experiments 1 and 2 where we sought to synchronize neural activity with in-phase stimulation and enhance working memory, experiment 3 tested whether a desynchronizing or anti-phase montage could impair working memory. We predicted that delivering theta-tuned HD-tACS simultaneously to frontal and temporal regions with a relative 180° phase difference between targeted areas would impede frontotemporal neural integration and induce impairments in working-memory performance. We tested this prediction in a new cohort of young adults using a double blind, sham-controlled, within-subjects design (Methods), in which working-memory behavior on the change-detection task was evaluated (Fig. 1b). Experiment 3 showed that by shifting the phase angle of alternating current, we could switch the direction of the causal effects on working-memory performance. Box plots of RT and accuracy in young adults from memory blocks show anti-phase stimulation significantly decreased accuracy (t17 = 3.359, P = 0.004, dz = 0.792) and increased RT (t17 = 2.928, P = 0.009, dz = 0.690), relative to sham. These results, combined with those from experiments 1 and 2, suggest that we may be able to obtain rapid, bidirectional control over phase-sensitive memory mechanisms using frequency-tuned HD-tACS. Paired sample two-tailed t-tests (n = 18). Box-plot center, median; box limits, lower and upper quartiles; whiskers, lower and upper extreme values; points, outliers. ** P < 0.01.
Supplementary Figure 3 Experiment 4: HD-tACS improves working-memory success in poor performing young adults.
We asked whether the HD-tACS behavioral improvement observed in older adults across experiments 1 and 2 could be extended to younger individuals with relatively poor working-memory performance and weak maintenance-related PAC (Fig. 3e). For experiment 4, we re-recruited the poorest performing younger adults from experiment 1 to participate in a single test day involving frequency-tuned in-phase frontotemporal HD-tACS (Methods). Subjects’ behavior on the change-detection task (Fig. 1b) was collected before, during, and after stimulation. Box plots of RT and accuracy of poor performing younger adults from memory blocks show preferential enhancement of working-memory accuracy following stimulation, relative to the pre-stimulation baseline (t13 = 3.610, P = 0.003, dz = 0.965) and relative to subjects’ sham stimulation day from experiment 1 (t13 = 3.571, P = 0.003, dz = 0.954). Consistent with previous findings from older adults, the in-phase stimulation did not affect RT in these poor performing younger subjects compared to either baseline condition (all t13 < 0.656, P > 0.523, dz < 0.175). Further analysis showed that post-stimulation accuracy gains were significant for each 4 min time bin, relative to either baseline control condition (all t13 > 3.520, P < 0.005, dz > 0.941). The results suggest that the HD-tACS improvement may be applicable to younger individuals with poor working-memory function. Paired sample two-tailed t-tests (n = 14). Box-plot center, median; box limits, lower and upper quartiles; whiskers, lower and upper extreme values; points, outliers. ** P < 0.01.
Supplementary information
Rights and permissions
About this article
Cite this article
Reinhart, R.M.G., Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci 22, 820–827 (2019). https://doi.org/10.1038/s41593-019-0371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0371-x
This article is cited by
-
The effect of a cognitive training therapy based on stimulation of brain oscillations in patients with mild cognitive impairment in a Chilean sample: study protocol for a phase IIb, 2 × 3 mixed factorial, double-blind randomised controlled trial
Trials (2024)
-
Induced neural phase precession through exogenous electric fields
Nature Communications (2024)
-
Harmonic memory signals in the human cerebral cortex induced by semantic relatedness of words
npj Science of Learning (2024)
-
Causal phase-dependent control of non-spatial attention in human prefrontal cortex
Nature Human Behaviour (2024)
-
Aberrant oscillatory activity in neurofibromatosis type 1: an EEG study of resting state and working memory
Journal of Neurodevelopmental Disorders (2023)