Abstract
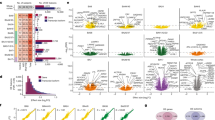
Transcriptomic analyses of postmortem brains have begun to elucidate molecular abnormalities in autism spectrum disorder (ASD). However, a crucial pathway involved in synaptic development, RNA editing, has not yet been studied on a genome-wide scale. Here we profiled global patterns of adenosine-to-inosine (A-to-I) editing in a large cohort of postmortem brains of people with ASD. We observed a global bias for hypoediting in ASD brains, which was shared across brain regions and involved many synaptic genes. We show that the Fragile X proteins FMRP and FXR1P interact with RNA-editing enzymes (ADAR proteins) and modulate A-to-I editing. Furthermore, we observed convergent patterns of RNA-editing alterations in ASD and Fragile X syndrome, establishing this as a molecular link between these related diseases. Our findings, which are corroborated across multiple data sets, including dup15q (genomic duplication of 15q11.2-13.1) cases associated with intellectual disability, highlight RNA-editing dysregulation in ASD and reveal new mechanisms underlying this disorder.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
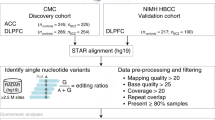
eCLIP-seq data on FMRP and FXR1P from postmortem human brain have been deposited in GEO with accession code GSE107895. RNA-seq data of Fragile X subjects, carriers and controls have been deposited in GEO with accession codes GSE107867 (NeuroBiobank data set) and GSE117776 (UC Davis FXTAS data set). Fastq files of RNA-seq from the idiopathic ASD, dup15q and control brains were obtained from our previous study9 and are available in the PsychENCODE website (https://www.synapse.org//#!Synapse:syn4921369/wiki/235539). Fastq files of RNA-seq data from the replicate ASD and control cohort are available in GEO (accession GSE51264 / GSE59288).
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn, text rev. (APA Publishing, Washington, DC, 2000).
Rojas, D. C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. (Vienna) 121, 891–905 (2014).
Guo, Y. P. & Commons, K. G. Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism Res. 10, 66–77 (2017).
Ha, S., Sohn, I. J., Kim, N., Sim, H. J. & Cheon, K. A. Characteristics of brains in autism spectrum disorder: structure, function and connectivity across the lifespan. Exp. Neurobiol. 24, 273–284 (2015).
Nelson, S. B. & Valakh, V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698 (2015).
Marchetto, M. C. et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry 22, 820–835 (2017).
de la Torre-Ubieta, L., Won, H., Stein, J. L. & Geschwind, D. H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22, 345–361 (2016).
Gupta, S. et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5, 5748 (2014).
Parikshak, N. N. et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 540, 423–427 (2016).
Irimia, M. et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 159, 1511–1523 (2014).
Wu, Y. E., Parikshak, N. N., Belgard, T. G. & Geschwind, D. H. Genome-wide, integrative analysis implicates microRNA dysregulation in autism spectrum disorder. Nat. Neurosci. 19, 1463–1476 (2016).
Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 17, 83–96 (2016).
Behm, M. & Öhman, M. RNA editing: a contributor to neuronal dynamics in the mammalian brain. Trends Genet. 32, 165–175 (2016).
Slotkin, W. & Nishikura, K. Adenosine-to-inosine RNA editing and human disease. Genome Med. 5, 105 (2013).
Khermesh, K. et al. Reduced levels of protein recoding by A-to-I RNA editing in Alzheimer’s disease. RNA 22, 290–302 (2016).
Eran, A. et al. Comparative RNA editing in autistic and neurotypical cerebella. Mol. Psychiatry 18, 1041–1048 (2013).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013).
Bahn, J. H. et al. Accurate identification of A-to-I RNA editing in human by transcriptome sequencing. Genome Res. 22, 142–150 (2012).
Porath, H. T., Carmi, S. & Levanon, E. Y. A genome-wide map of hyper-edited RNA reveals numerous new sites. Nat. Commun. 5, 4726 (2014).
Picardi, E., D’Erchia, A. M., Lo Giudice, C. & Pesole, G. REDIportal: a comprehensive database of A-to-I RNA editing events in humans. Nucleic Acids Res. 45,(D1), D750–D757 (2017).
Levanon, E. Y. et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 22, 1001–1005 (2004).
Oakes, E., Anderson, A., Cohen-Gadol, A. & Hundley, H. A. Adenosine deaminase that acts on RNA 3 (ADAR3) binding to glutamate receptor subunit B pre-mRNA inhibits RNA editing in glioblastoma. J. Biol. Chem. 292, 4326–4335 (2017).
Rakic, P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735 (2009).
Abrahams, B. S. et al. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 4, 36 (2013).
Hwang, T. et al. Dynamic regulation of RNA editing in human brain development and disease. Nat. Neurosci. 19, 1093–1099 (2016).
Liu, X. et al. Disruption of an evolutionarily novel synaptic expression pattern in autism. PLoS Biol. 14, e1002558 (2016).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Davis, J. K. & Broadie, K. Multifarious functions of the Fragile X mental retardation protein. Trends Genet. 33, 703–714 (2017).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Zhang, Y. et al. The Fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J. 14, 5358–5366 (1995).
Van Nostrand, E. L. et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat. Methods 13, 508–514 (2016).
Ascano, M. et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386 (2012).
Vasudevan, S. & Steitz, J. A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 128, 1105–1118 (2007).
Van Nostrand, E. L. et al. A large-scale binding and functional map of human RNA binding proteins. Preprint at biorXiv https://www.biorxiv.org/content/early/2017/08/23/179648 (2017).
Hagerman, R., Hoem, G. & Hagerman, P. Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol. Autism 1, 12 (2010).
Abbeduto, L., McDuffie, A. & Thurman, A. J. The fragile X syndrome-autism comorbidity: what do we really know? Front. Genet. 5, 355 (2014).
Pinto, Y., Cohen, H. Y. & Levanon, E. Y. Mammalian conserved ADAR targets comprise only a small fragment of the human editosome. Genome. Biol. 15, R5 (2014).
Irimia, M. et al. Evolutionarily conserved A-to-I editing increases protein stability of the alternative splicing factor Nova1. RNA Biol. 9, 12–21 (2012).
DiStefano, C. et al. Identification of a distinct developmental and behavioral profile in children with Dup15q syndrome. J. Neurodev. Disord. 8, 19 (2016).
Battaglia, A. et al. The inv dup(15) syndrome: a clinically recognizable syndrome with altered behavior, mental retardation, and epilepsy. Neurology 48, 1081–1086 (1997).
Frith, C. & Dolan, R. The role of the prefrontal cortex in higher cognitive functions. Brain. Res. Cogn. Brain Res. 5, 175–181 (1996).
Jansen, A. et al. Gene-set analysis shows association between FMRP targets and autism spectrum disorder. Eur. J. Hum. Genet. 25, 863–868 (2017).
Pinto, D. et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 94, 677–694 (2014).
Fatemi, S. H. & Folsom, T. D. Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of individuals with autism: a postmortem brain study. Mol. Autism 2, 6 (2011).
Patzlaff, N. E., Nemec, K. M., Malone, S. G., Li, Y. & Zhao, X. Fragile X related protein 1 (FXR1P) regulates proliferation of adult neural stem cells. Hum. Mol. Genet. 26, 1340–1352 (2017).
Charman, T. et al. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med. 41, 619–627 (2011).
Finucane, B. M. et al. 15q duplication syndrome and related disorders. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, Seattle, 2016).
Ahn, J. & Xiao, X. RASER: reads aligner for SNPs and editing sites of RNA. Bioinformatics 31, 3906–3913 (2015).
Lee, J. H., Ang, J. K. & Xiao, X. Analysis and design of RNA sequencing experiments for identifying RNA editing and other single-nucleotide variants. RNA 19, 725–732 (2013).
Feldmeyer, D. et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat. Neurosci. 2, 57–64 (1999).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Josse, J. & Husson, F. missMDA: a package for handling missing values in multivariate data analysis. J. Stat. Softw. 70, 31 (2016).
Langfelder, P., Zhang, B. & Horvath, S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719–720 (2008).
Langfelder, P. & Horvath, S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst. Biol. 1, 54 (2007).
Wheeler, E. C., Van Nostrand, E. L. & Yeo, G. W. Advances and challenges in the detection of transcriptome-wide protein-RNA interactions. Wiley Interdiscip. Rev. RNA https://doi.org/10.1002/wrna.1436 (2018).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bailey, T. L., Johnson, J., Grant, C. E. & Noble, W. S. The MEME Suite. Nucleic Acids Res. 43(W1), W39–W49 (2015).
Aken, B. L. et al. The Ensembl gene annotation system. Database (Oxford) https://doi.org/10.1093/database/baw093 (2016).
Nojima, T., Gomes, T., Carmo-Fonseca, M. & Proudfoot, N. J. Mammalian NET-seq analysis defines nascent RNA profiles and associated RNA processing genome-wide. Nat. Protoc. 11, 413–428 (2016).
Sundararaman, B. et al. Resources for the comprehensive discovery of functional RNA elements. Mol. Cell 61, 903–913 (2016).
Bahn, J. H. et al. Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways. Nat. Commun. 6, 6355 (2015).
Acknowledgements
Postmortem brain samples used in this study were obtained from the University of Maryland Brain and Tissue Bank, which is a component of the US National Institutes of Health (NIH) NeuroBioBank. We are grateful to the subjects and families who participate in the tissue donation programs. This work was funded by grants from the NIH to X.X. (HG009417 and HG006264), G.W.Y. (HG004659, HG009417, HG007005 and MH107367), S.T. (T32HG002536), E.L.V.N. (HG009530), V.M.C. (MH094681) and R.J.H. (HD 036071). S.T is supported by the UCLA Eureka Scholarship. E.L.V.N. is a Merck Fellow of the Damon Runyon Cancer Research Foundation (DRG-2172-13). G.A.P. is supported by the National Science Foundation Graduate Research Fellowship. G.R. is supported by NIH fellowship 1F32MH114620.
Author information
Authors and Affiliations
Contributions
S.S.T. carried out data analyses with input from G.R. H.I.J., J.H.B. and A.A. performed molecular biology experiments. E.L.V.N., T.B.N., G.A.P. and G.W.Y carried out eCLIP experiments and data processing. Y.H.E.H. contributed to data visualization. C.L. carried out ASD RNA-seq data generation. V.M.C. and R.J.H. provided postmortem Fragile X and control samples. S.S.T, D.H.G. and X.X. designed the study, interpreted the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.W.Y. is a cofounder of Locana and Eclipse Bioinnovations and member of the scientific advisory boards of Locana, Eclipse Bioinnovations and Aquinnah Pharmaceuticals. E.V.N. is a cofounder and member of the scientific advisory board of Eclipse BioInnovations. The terms of these arrangements have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figures 1–31
Supplementary Figures 1–31
Supplementary Table 1
Meta data of brain samples used in this study.
Supplementary Table 2
Differential RNA editing sites identified from ASD-control samples in three brain regions.
Supplementary Table 3
List of primer sequences used in this study.
Supplementary Table 4
Differential editing sites in frontal cortex that correlate with expression of harboring gene.
Supplementary Table 5
Module memberships of editing sites from WGCNA.
Supplementary Table 6
List of FMPR and FXR1P eCLIP peaks.
Supplementary Table 7
Differential RNA editing sites identified in Fragile X samples.
Supplementary Table 8
Genes with brain-region-specific differential editing.
Supplementary Software
Supplementary Software.
Rights and permissions
About this article
Cite this article
Tran, S.S., Jun, HI., Bahn, J.H. et al. Widespread RNA editing dysregulation in brains from autistic individuals. Nat Neurosci 22, 25–36 (2019). https://doi.org/10.1038/s41593-018-0287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0287-x
This article is cited by
-
RNA modifications in physiology and disease: towards clinical applications
Nature Reviews Genetics (2024)
-
The multifaceted role of Fragile X-Related Protein 1 (FXR1) in cellular processes: an updated review on cancer and clinical applications
Cell Death & Disease (2024)
-
Transcriptomic analysis reveals associations of blood-based A-to-I editing with Parkinson’s disease
Journal of Neurology (2024)
-
The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Transcriptome driven discovery of novel candidate genes for human neurological disorders in the telomer-to-telomer genome assembly era
Human Genomics (2023)