Abstract
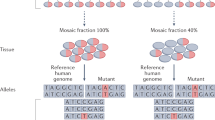
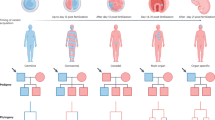
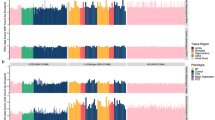
Traditionally, we have considered genetic mutations that cause neurodevelopmental diseases to be inherited or de novo germline mutations. Recently, we have come to appreciate the importance of de novo somatic mutations, which occur postzygotically and are thus present in only a subset of the cells of an affected individual. The advent of next-generation sequencing and single-cell sequencing technologies has shown that somatic mutations contribute to normal and abnormal human brain development. Somatic mutations are one important cause of neuronal migration and brain overgrowth disorders, as suggested by visible focal lesions. In addition, somatic mutations contribute to neurodevelopmental diseases without visible lesions, including epileptic encephalopathies, intellectual disability, and autism spectrum disorder, and may contribute to a broad range of neuropsychiatric diseases. Studying somatic mutations provides insight into the mechanisms underlying human brain development and neurodevelopmental diseases and has important implications for diagnosis and treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jónsson, H. et al. Parental influence on human germline de novo mutations in 1,548 trios from Iceland. Nature 549, 519–522 (2017).
Kong, A. et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488, 471–475 (2012).
O’Roak, B. J. et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585–589 (2011).
Veltman, J. A. & Brunner, H. G. De novo mutations in human genetic disease. Nat. Rev. Genet. 13, 565–575 (2012).
Larsen, F. W. & Mouridsen, S. E. The outcome in children with childhood autism and Asperger syndrome originally diagnosed as psychotic. A 30-year follow-up study of subjects hospitalized as children. Eur. Child Adolesc. Psychiatry 6, 181–190 (1997).
Power, R. A. et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry 70, 22–30 (2013).
Philp, A. J. et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 61, 7426–7429 (2001).
Biesecker, L. G. & Spinner, N. B. A genomic view of mosaicism and human disease. Nat. Rev. Genet. 14, 307–320 (2013).
Azevedo, F. A. et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541 (2009).
Workman, A. D., Charvet, C. J., Clancy, B., Darlington, R. B. & Finlay, B. L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 33, 7368–7383 (2013).
Bae, T. et al. Different mutational rates and mechanisms in human cells at pregastrulation and neurogenesis. Science 359, 550–555 (2018). This study used clonal cell expansion of fetal human neuronal progenitor cells to estimate the somatic mutation rate during neurogenesis.
Lodato, M. A. et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359, 555–559 (2018). This study used whole-genome sequencing of single human neurons to demonstrate that somatic SNVs in human neurons accumulate linearly with age.
Poduri, A., Evrony, G. D., Cai, X. & Walsh, C. A. Somatic mutation, genomic variation, and neurological disease. Science 341, 1237758 (2013).
D’Gama, A. M. et al. Somatic mutations activating the mTOR pathway in dorsal telencephalic progenitors cause a continuum of cortical dysplasias. Cell Rep. 21, 3754–3766 (2017).
Dou, Y. et al. Postzygotic single-nucleotide mosaicisms contribute to the etiology of autism spectrum disorder and autistic traits and the origin of mutations. Hum. Mutat. 38, 1002–1013 (2017). This study, along with refs 16–18, analyzed WES of people with ASD to estimate the contribution of somatic mutationsto ASD risk.
Freed, D. & Pevsner, J. The contribution of mosaic variants to autism spectrum disorder. PLoS Genet. 12, e1006245 (2016). This study, along with refs. 15,17,18, analyzed WES of people with ASD to estimate the contribution of somatic mutations to ASD risk.
Krupp, D. R. et al. Exonic mosaic mutations contribute risk for autism spectrum disorder. Am. J. Hum.Genet. 101, 369–390 (2017). This study, along with refs. 15,16,18, analyzed WES of people with ASD to estimate the contribution of somatic mutations to ASD risk.
Lim, E. T. et al. Rates, distribution and implications of postzygotic mosaic mutations in autism spectrum disorder. Nat. Neurosci. 20, 1217–1224 (2017). This study, along with refs 15–17, analyzed WES of people with ASD to estimate the contribution of somatic mutations to ASD risk.
Lodato, M. A. et al. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 350, 94–98 (2015). This study used whole-genome sequencing of single human neurons to estimate the number of somatic mutations in each postmitotic neuron.
Gennaro, E. et al. Somatic and germline mosaicisms in severe myoclonic epilepsy of infancy. Biochem. Biophys. Res. Commun. 341, 489–493 (2006).
Nakamura, K. et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology 81, 992–998 (2013).
Vadlamudi, L. et al. Timing of de novo mutagenesis--a twin study of sodium-channel mutations. N. Engl. J. Med. 363, 1335–1340 (2010).
Xu, X. et al. Amplicon resequencing identified parental mosaicism for approximately 10% of “de novo” SCN1A mutations in children with Dravet syndrome. Hum. Mutat. 36, 861–872 (2015).
Zerem, A. et al. Paternal germline mosaicism of a SCN2A mutation results in Ohtahara syndrome in half siblings. Eur. J. Paediatr. Neurol. 18, 567–571 (2014).
D’Gama, A. M. et al. Targeted DNA sequencing from autism spectrum disorder brains implicates multiple genetic mechanisms. Neuron 88, 910–917 (2015).
Cai, X. et al. Single-cell, genome-wide sequencing identifies clonal somatic copy-number variation in the human brain. Cell Rep. 8, 1280–1289 (2014).
McConnell, M. J. et al. Mosaic copy number variation in human neurons. Science 342, 632–637 (2013). This study demonstrated that mosaic CNVs are common in human neurons.
Crowe, C. A., Schwartz, S., Black, C. J. & Jaswaney, V. Mosaic trisomy 22: a case presentation and literature review of trisomy 22 phenotypes. Am. J. Med. Genet. 71, 406–413 (1997).
Daber, R. et al. Mosaic trisomy 17: variable clinical and cytogenetic presentation. Am. J. Med. Genet. A. 155A, 2489–2495 (2011).
Gérard-Blanluet, M. et al. Mosaic trisomy 9 and lobar holoprosencephaly. Am. J. Med. Genet. 111, 295–300 (2002).
Laus, A. C. et al. Karyotype/phenotype correlation in partial trisomies of the long arm of chromosome 16: case report and review of literature. Am. J. Med. Genet. A. 158A, 821–827 (2012).
Pangalos, C. et al. Understanding the mechanism(s) of mosaic trisomy 21 by using DNA polymorphism analysis. Am. J. Hum. Genet. 54, 473–481 (1994).
Patel, C. et al. Mosaic trisomy 1q: The longest surviving case. Am. J. Med. Genet. A. 149A, 1795–1800 (2009).
Schrander-Stumpel, C. T. et al. Mosaic tetrasomy 8p in two patients: clinical data and review of the literature. Am. J. Med. Genet. 50, 377–380 (1994).
Muotri, A. R. et al. L1 retrotransposition in neurons is modulated by MeCP2. Nature 468, 443–446 (2010). This study showed the first association between a neurodevelopmental disease, Rett syndrome, and increased somatic L1 insertions.
Lindhurst, M. J. et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 365, 611–619 (2011).
Kurek, K. C. et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am. J. Hum. Genet. 90, 1108–1115 (2012).
Shirley, M. D. et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 368, 1971–1979 (2013).
Kingsmore, S. et al. Exome sequencing reveals de novo germline mutation of the mammalian target of rapamycin (MTOR) in a patient with megalencephaly and intractable seizures. Journal of Genomes and Exomes 2, 63–72 (2013).
Knudson, A. G. Jr. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 68, 820–823 (1971).
Maertens, O. et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum. Mutat. 27, 1030–1040 (2006).
Garcia-Linares, C. et al. Dissecting loss of heterozygosity (LOH) in neurofibromatosis type 1-associated neurofibromas: Importance of copy neutral LOH. Hum. Mutat. 32, 78–90 (2011).
Crino, P. B., Aronica, E., Baltuch, G. & Nathanson, K. L. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology 74, 1716–1723 (2010).
Qin, W. et al. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 20, 1096–1105 (2010).
Tyburczy, M. E. et al. A shower of second hit events as the cause of multifocal renal cell carcinoma in tuberous sclerosis complex. Hum. Mol. Genet. 24, 1836–1842 (2015).
Sheen, V. L. et al. Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum. Mol. Genet. 10, 1775–1783 (2001).
Guerrini, R. et al. Germline and mosaic mutations of FLN1 in men with periventricular heterotopia. Neurology 63, 51–56 (2004).
Parrini, E., Mei, D., Wright, M., Dorn, T. & Guerrini, R. Mosaic mutations of the FLN1 gene cause a mild phenotype in patients with periventricular heterotopia. Neurogenetics 5, 191–196 (2004).
Sicca, F. et al. Mosaic mutations of the LIS1 gene cause subcortical band heterotopia. Neurology 61, 1042–1046 (2003).
Gleeson, J. G. et al. Somatic and germline mosaic mutations in the doublecortin gene are associated with variable phenotypes. Am. J. Hum. Genet. 67, 574–581 (2000).
Jamuar, S. S. et al. Somatic mutations in cerebral cortical malformations. N. Engl. J. Med. 371, 733–743 (2014).
Rohlin, A. et al. Parallel sequencing used in detection of mosaic mutations: comparison with four diagnostic DNA screening techniques. Hum. Mutat. 30, 1012–1020 (2009).
Blümcke, I. et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52, 158–174 (2011).
Poduri, A. et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 74, 41–48 (2012). This study, along with refs 59,61, used direct study of human brain tissue to identify a genetic cause for HME.
Hua, Y. & Crino, P. B. Single cell lineage analysis in human focal cortical dysplasia. Cereb. Cortex 13, 693–699 (2003).
Blumcke, I. et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N. Engl. J. Med. 377, 1648–1656 (2017).
Harvey, A. S., Cross, J. H., Shinnar, S., Mathern, G. W. & Taskforce, I. P. E. S. S. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 49, 146–155 (2008).
Jansen, L. A. et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain 138, 1613–1628 (2015).
Lee, J. H. et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly.Nat. Genet. 44, 941–945 (2012). This study, along with refs 54,61, used direct study of human brain tissue to identify a genetic cause for HME.
Lim, J. S. et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat. Med. 21, 395–400 (2015).
Rivière, J. B. et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44, 934–940 (2012). This study, along with refs 54,59, used direct study of human brain tissue to identify a genetic cause for HME.
D’Gama, A. M. et al. mTOR pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann. Neurol. 77, 720–725 (2015).
Lim, J. S. et al. Somatic mutations in TSC1 and TSC2 cause focal cortical dysplasia. Am. J. Hum. Genet. 100, 454–472 (2017).
Baulac, S. et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann. Neurol. 77, 675–683 (2015).
Sim, J. C. et al. Familial cortical dysplasia caused by mutation in the mammalian target of rapamycin regulator NPRL3. Ann. Neurol. 79, 132–137 (2016).
Scheffer, I. E. et al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann. Neurol. 75, 782–787 (2014).
Weckhuysen, S. et al. Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia 57, 994–1003 (2016).
Evrony, G. D. et al. Single-neuron sequencing analysis of L1 retrotransposition and somatic mutation in the human brain. Cell 151, 483–496 (2012).
Baek, S. T. et al. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat. Med. 21, 1445–1454 (2015).
Roy, A. et al. Mouse models of human PIK3CA-related brain overgrowth have acutely treatable epilepsy. eLife 4, e12703 (2015).
Yuskaitis, C. J. et al. A mouse model of DEPDC5-related epilepsy: neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility. Neurobiol. Dis. 111, 91–101 (2018).
Allen, A. S. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
Carvill, G. L. et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat. Genet. 45, 825–830 (2013).
Depienne, C. et al. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 5, e1000381 (2009).
Veeramah, K. R. et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia 54, 1270–1281 (2013).
Weckhuysen, S. et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 71, 15–25 (2012).
Stosser, M. B. et al. High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genet. Med. 20, 403–410 (2018).
Perez, D., Hsieh, D. T. & Rohena, L. Somatic mosaicism of PCDH19 in a male with early infantile epileptic encephalopathy and review of the literature. Am. J. Med. Genet. A. 173, 1625–1630 (2017).
Pederick, D. T. et al. Abnormal cell sorting underlies the unique X–linked inheritance of PCDH19 epilepsy. Neuron 97, 59–66 (2018).
Fu, C. et al. GABAergic interneuron development and function is modulated by the Tsc1 gene. Cereb. Cortex 22, 2111–2119 (2012).
American Psychiatric Association & DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. (American Psychiatric Association, Washington, D.C., 2013).
Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 63, 1–21 (2014).
Kanner, L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 35, 100–136 (1968).
Bailey, A. et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol. Med. 25, 63–77 (1995).
Steffenburg, S. et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J. Child Psychol. Psychiatry 30, 405–416 (1989).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Levy, D. et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70, 886–897 (2011).
Lim, E. T. et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron 77, 235–242 (2013).
Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
O’Roak, B. J. et al. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat. Commun. 5, 5595 (2014).
O’Roak, B. J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622 (2012).
O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012).
Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372 (2010).
Sanders, S. J. et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70, 863–885 (2011).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science 316, 445–449 (2007).
Yu, T. W. et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron 77, 259–273 (2013).
Bourdon, V. et al. Evidence of somatic mosaicism for a MECP2 mutation in females with Rett syndrome: diagnostic implications. J. Med. Genet. 38, 867–871 (2001).
Clayton-Smith, J., Watson, P., Ramsden, S. & Black, G. C. Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet 356, 830–832 (2000).
Helderman-van den Enden, A. T. et al. Monozygotic twin brothers with the fragile X syndrome: different CGG repeats and different mental capacities. J. Med. Genet. 36, 253–257 (1999).
Tinschert, S. et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur. J. Hum. Genet. 8, 455–459 (2000).
Verhoef, S. et al. High rate of mosaicism in tuberous sclerosis complex. Am. J. Hum. Genet. 64, 1632–1637 (1999).
Vogt, J. et al. Monozygotic twins discordant for neurofibromatosis type 1 due to a postzygotic NF1 gene mutation. Hum. Mutat. 32, E2134–E2147 (2011).
Castermans, D. et al. Position effect leading to haploinsufficiency in a mosaic ring chromosome 14 in a boy with autism. Eur. J. Hum. Genet. 16, 1187–1192 (2008).
Havlovicova, M. et al. A girl with neurofibromatosis type 1, atypical autism and mosaic ring chromosome 17. Am. J. Med. Genet. A. 143A, 76–81 (2007).
Kakinuma, H., Ozaki, M., Sato, H. & Takahashi, H. Variation in GABA-A subunit gene copy number in an autistic patient with mosaic 4 p duplication (p12p16). Am. J. Med. Genet. B. Neuropsychiatr. Genet. 147B, 973–975 (2008).
Meyer, K. J., Axelsen, M. S., Sheffield, V. C., Patil, S. R. & Wassink, T. H. Germline mosaic transmission of a novel duplication of PXDN and MYT1L to two male half-siblings with autism. Psychiatr. Genet. 22, 137–140 (2012).
Oliveira, G. et al. Partial tetrasomy of chromosome 3q and mosaicism in a child with autism. J. Autism Dev. Disord. 33, 177–185 (2003).
Papanikolaou, K. et al. A case of partial trisomy of chromosome 8p associated with autism. J. Autism Dev. Disord. 36, 705–709 (2006).
Sauter, S. et al. Autistic disorder and chromosomal mosaicism 46,XY[123]/46,XY,del(20)(pter --> p12.2)[10]. Am. J. Med. Genet. A. 120A, 533–536 (2003).
Yurov, Y. B. et al. Unexplained autism is frequently associated with low-level mosaic aneuploidy. J. Med. Genet. 44, 521–525 (2007).
Hoischen, A., Krumm, N. & Eichler, E. E. Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nat. Neurosci. 17, 764–772 (2014).
Wintle, R. F. et al. A genotype resource for postmortem brain samples from the Autism Tissue Program. Autism Res. 4, 89–97 (2011).
Casanova, M. F. et al. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol. Commun. 1, 67 (2013).
Stoner, R. et al. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 370, 1209–1219 (2014).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007 (2013).
Castronovo, P. et al. Somatic mosaicism in Cornelia de Lange syndrome: a further contributor to the wide clinical expressivity? Clin. Genet. 78, 560–564 (2010).
Gervasini, C. et al. Molecular characterization of a mosaic NIPBL deletion in a Cornelia de Lange patient with severe phenotype. Eur. J. Med. Genet. 56, 138–143 (2013).
Vissers, L. E., Gilissen, C. & Veltman, J. A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 17, 9–18 (2016).
Gilissen, C. et al. Genome sequencing identifies major causes of severe intellectual disability. Nature 511, 344–347 (2014).
Acuna-Hidalgo, R. et al. Post-zygotic point mutations are an underrecognized source of de novo genomic variation. Am. J. Hum. Genet. 97, 67–74 (2015). This study reanalyzed whole-genome sequencing from an ID cohort to demonstrate that somatic mutations are under-recognized and miscategorized as germline de novo mutations.
King, D. A. et al. Mosaic structural variation in children with developmental disorders. Hum. Mol. Genet. 24, 2733–2745 (2015).
Cannon, T. D., Kaprio, J., Lönnqvist, J., Huttunen, M. & Koskenvuo, M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch. Gen. Psychiatry 55, 67–74 (1998).
Yurov, Y. B. et al. The schizophrenia brain exhibits low-level aneuploidy involving chromosome 1. Schizophr. Res. 98, 139–147 (2008).
Yurov, Y. B., Vostrikov, V. M., Vorsanova, S. G., Monakhov, V. V. & Iourov, I. Y. Multicolor fluorescent in situ hybridization on post-mortem brain in schizophrenia as an approach for identification of low-level chromosomal aneuploidy in neuropsychiatric diseases. Brain Dev. 23(Suppl 1), S186–S190 (2001).
Sakai, M. et al. Assessment of copy number variations in the brain genome of schizophrenia patients. Mol. Cytogenet. 8, 46 (2015).
Ruderfer, D. M. et al. Mosaic copy number variation in schizophrenia. Eur. J. Hum. Genet. 21, 1007–1011 (2013).
Bundo, M. et al. Increased L1 retrotransposition in the neuronal genome in schizophrenia. Neuron 81, 306–313 (2014). This study used L1 copy number quantification to demonstrate increased L1 insertions in neurons from schizophrenia patients.
Doyle, G. A. et al. Analysis of LINE-1 elements in DNA from postmortem brains of individuals with schizophrenia. Neuropsychopharmacology 42, 2602–2611 (2017).
McConnell, M. J. et al. Intersection of diverse neuronal genomes and neuropsychiatric disease: the Brain Somatic Mosaicism Network. Science 356, eaal1641 (2017).
Yang, Y. et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 369, 1502–1511 (2013).
Campbell, I. M. et al. Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am. J. Hum. Genet. 95, 173–182 (2014). This study screened children with genomic disorders and their parents to demonstrate that parental somatic mosaicism is under-recognized and that mutations initially categorized as germline de novo mutations are sometimes detectable in DNA from parental blood.
Dey, S. S., Kester, L., Spanjaard, B., Bienko, M. & van Oudenaarden, A. Integrated genome and transcriptome sequencing of the same cell. Nat. Biotechnol. 33, 285–289 (2015).
Janiszewska, M. et al. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat. Genet. 47, 1212–1219 (2015).
Mariani, J. et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 (2015).
Abyzov, A. et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492, 438–442 (2012).
Hollingsworth, E. W. et al. iPhemap: an atlas of phenotype to genotype relationships of human iPSC models of neurological diseases. EMBO Mol. Med. 9, 1742–1762 (2017).
Lehner, T., Miller, B. L. & State, M. W. Genomics, Circuits, and Pathways in Clinical Neuropsychiatry. (Academic Press, Boston, MA, USA, 2016).
Bijlsma, E. K., Wallace, A. J. & Evans, D. G. Misleading linkage results in an NF2 presymptomatic test owing to mosaicism. J. Med. Genet. 34, 934–936 (1997).
Halliday, D. et al. Genetic severity score predicts clinical phenotype in NF2. J. Med. Genet. 54, 657–664 (2017).
Heyries, K. A. et al. Megapixel digital PCR. Nat. Methods 8, 649–651 (2011).
Dean, F. B., Nelson, J. R., Giesler, T. L. & Lasken, R. S. Rapid amplification of plasmid and phage DNA using Phi 29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res. 11, 1095–1099 (2001).
Zong, C., Lu, S., Chapman, A. R. & Xie, X. S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Hiatt, J. B., Pritchard, C. C., Salipante, S. J., O’Roak, B. J. & Shendure, J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome Res. 23, 843–854 (2013).
Erwin, J. A. et al. L1-associated genomic regions are deleted in somatic cells of the healthy human brain. Nat. Neurosci. 19, 1583–1591 (2016).
Evrony, G. D., Lee, E., Park, P. J. & Walsh, C. A. Resolving rates of mutation in the brain using single-neuron genomics. eLife 5, e12966 (2016).
Upton, K. R. et al. Ubiquitous L1 mosaicism in hippocampal neurons. Cell 161, 228–239 (2015).
Gole, J. et al. Massively parallel polymerase cloning and genome sequencing of single cells using nanoliter microwells. Nat. Biotechnol. 31, 1126–1132 (2013).
Acknowledgements
The authors thank members of the Walsh laboratory for helpful discussions. A.M.D. was supported by the NIGMS (T32GM007753). C.A.W. was supported by the NINDS (R01NS079277), the NIMH (U01MH106883) through the Brain Somatic Mosaicism Network, the Allen Discovery Center program through The Paul G. Allen Frontiers Group, and the Manton Center for Orphan Disease Research. C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Gama, A.M., Walsh, C.A. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci 21, 1504–1514 (2018). https://doi.org/10.1038/s41593-018-0257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0257-3
This article is cited by
-
Meta-analysis of 46,000 germline de novo mutations linked to human inherited disease
Human Genomics (2024)
-
Genetic architecture of childhood speech disorder: a review
Molecular Psychiatry (2024)
-
Cytogenomic epileptology
Molecular Cytogenetics (2023)
-
The genetics and pathogenesis of CAKUT
Nature Reviews Nephrology (2023)
-
Genomic Mosaicism of the Brain: Origin, Impact, and Utility
Neuroscience Bulletin (2023)