Abstract
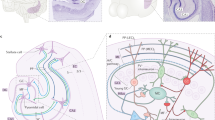
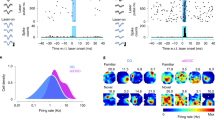
Complex spatial working memory tasks have been shown to require both hippocampal sharp-wave ripple (SWR) activity and dentate gyrus (DG) neuronal activity. We therefore asked whether DG inputs to CA3 contribute to spatial working memory by promoting SWR generation. Recordings from DG and CA3 while rats performed a dentate-dependent working memory task on an eight-arm radial maze revealed that the activity of dentate neurons and the incidence rate of SWRs both increased during reward consumption. We then found reduced reward-related CA3 SWR generation without direct input from dentate granule neurons. Furthermore, CA3 cells with place fields in not-yet-visited arms preferentially fired during SWRs at reward locations, and these prospective CA3 firing patterns were more pronounced for correct trials and were dentate-dependent. These results indicate that coordination of CA3 neuronal activity patterns by DG is necessary for the generation of neuronal firing patterns that support goal-directed behavior and memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xavier, G. F. & Costa, V. C. Dentate gyrus and spatial behaviour. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 762–773 (2009).
Leutgeb, J. K., Leutgeb, S., Moser, M. B. & Moser, E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007).
Neunuebel, J. P. & Knierim, J. J. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014).
Amaral, D. G. & Witter, M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 (1989).
Scharfman, H. E. The CA3 “backprojection” to the dentate gyrus. Prog. Brain Res. 163, 627–637 (2007).
Treves, A. & Rolls, E. T. Computational analysis of the role of the hippocampus in memory. Hippocampus 4, 374–391 (1994).
Lisman, J. E., Talamini, L. M. & Raffone, A. Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Netw. 18, 1191–1201 (2005).
Buzsáki, G. Hippocampal sharp waves: their origin and significance. Brain Res. 398, 242–252 (1986).
Csicsvari, J., Hirase, H., Mamiya, A. & Buzsáki, G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28, 585–594 (2000).
Oliva, A., Fernández-Ruiz, A., Buzsáki, G. & Berényi, A. Role of hippocampal CA2 region in triggering sharp-wave ripples. Neuron 91, 1342–1355 (2016).
Buzsáki, G., Horváth, Z., Urioste, R., Hetke, J. & Wise, K. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027 (1992).
Lee, A. K. & Wilson, M. A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002).
Foster, D. J. & Wilson, M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683 (2006).
Pfeiffer, B. E. & Foster, D. J. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79 (2013).
Diba, K. & Buzsáki, G. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242 (2007).
Karlsson, M. P. & Frank, L. M. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918 (2009).
Carr, M. F., Jadhav, S. P. & Frank, L. M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14, 147–153 (2011).
Dupret, D., O’Neill, J., Pleydell-Bouverie, B. & Csicsvari, J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 13, 995–1002 (2010).
Ego-Stengel, V. & Wilson, M. A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010).
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsáki, G. & Zugaro, M. B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223 (2009).
Jadhav, S. P., Kemere, C., German, P. W. & Frank, L. M. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012).
Sullivan, D. et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616 (2011).
Penttonen, M., Kamondi, A., Sik, A., Acsády, L. & Buzsáki, G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus 7, 437–450 (1997).
Bragin, A., Jandó, G., Nádasdy, Z., van Landeghem, M. & Buzsáki, G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J. Neurophysiol. 73, 1691–1705 (1995).
Senzai, Y. & Buzsáki, G. Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron 93, 691–704.e5 (2017).
Danielson, N. B. et al. In vivo imaging of dentate gyrus mossy cells in behaving mice. Neuron 93, 552–559.e4 (2017).
GoodSmith, D. et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron 93, 677–690.e5 (2017).
O’Neill, J., Senior, T. & Csicsvari, J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49, 143–155 (2006).
Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015).
Kay, K. et al. A hippocampal network for spatial coding during immobility and sleep. Nature 531, 185–190 (2016).
Ahmed, O. J. & Mehta, M. R. Running speed alters the frequency of hippocampal gamma oscillations. J. Neurosci. 32, 7373–7383 (2012).
Leutgeb, S. & Leutgeb, J. K. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn. Mem. 14, 745–757 (2007).
Singer, A. C., Carr, M. F., Karlsson, M. P. & Frank, L. M. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77, 1163–1173 (2013).
McLamb, R. L., Mundy, W. R. & Tilson, H. A. Intradentate colchicine disrupts the acquisition and performance of a working memory task in the radial arm maze. Neurotoxicology 9, 521–528 (1988).
Jung, M. W. & McNaughton, B. L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182 (1993).
Neunuebel, J. P. & Knierim, J. J. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J. Neurosci. 32, 3848–3858 (2012).
Myers, C. E. & Scharfman, H. E. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus 21, 1190–1215 (2011).
Colgin, L. L. Do slow and fast gamma rhythms correspond to distinct functional states in the hippocampal network? Brain Res. 1621, 309–315 (2015).
Atallah, B. V. & Scanziani, M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62, 566–577 (2009).
Tort, A. B., Komorowski, R. W., Manns, J. R., Kopell, N. J. & Eichenbaum, H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl Acad. Sci. USA 106, 20942–20947 (2009).
Carr, M. F., Karlsson, M. P. & Frank, L. M. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75, 700–713 (2012).
Hsiao, Y. T., Zheng, C. & Colgin, L. L. Slow gamma rhythms in CA3 are entrained by slow gamma activity in the dentate gyrus. J. Neurophysiol. 116, 2594–2603 (2016).
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B. & Moser, E. I. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 510, 143–147 (2014).
Martin, C., Beshel, J. & Kay, L. M. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J. Neurophysiol. 98, 2196–2205 (2007).
Rangel, L. M., Chiba, A. A. & Quinn, L. K. Theta and beta oscillatory dynamics in the dentate gyrus reveal a shift in network processing state during cue encounters. Front. Syst. Neurosci. 9, 96 (2015).
McNaughton, B. L., Barnes, C. A., Meltzer, J. & Sutherland, R. J. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 76, 485–496 (1989).
Hofer, K. T. et al. The hippocampal CA3 region can generate two distinct types of sharp wave-ripple complexes, in vitro. Hippocampus 25, 169–186 (2015).
Pfeiffer, B. E. & Foster, D. J. Place cells. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science 349, 180–183 (2015).
Walsh, T. J., Schulz, D. W., Tilson, H. A. & Schmechel, D. E. Colchicine-induced granule cell loss in rat hippocampus: selective behavioral and histological alterations. Brain Res. 398, 23–36 (1986).
Goldschmidt, R. B. & Steward, O. Preferential neurotoxicity of colchicine for granule cells of the dentate gyrus of the adult rat. Proc. Natl Acad. Sci. USA 77, 3047–3051 (1980).
Goldschmidt, R. B. & Steward, O. Neurotoxic effects of colchicine: differential susceptibility of CNS neuronal populations. Neuroscience 7, 695–714 (1982).
Brandon, M. P., Koenig, J., Leutgeb, J. K. & Leutgeb, S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron 82, 789–796 (2014).
Redish, A.D. MClust 3.5, freeware spike sorting. http://redishlab.neuroscience.umn.edu/MClust/MClust.html (2009).
Skaggs, W. E., McNaughton, B. L., Wilson, M. A. & Barnes, C. A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Koenig, J., Linder, A. N., Leutgeb, J. K. & Leutgeb, S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595 (2011).
Cavazos, J. E., Golarai, G. & Sutula, T. P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J. Neurosci. 11, 2795–2803 (1991).
Buckmaster, P. S. & Dudek, F. E. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 77, 2685–2696 (1997).
Schlesiger, M. I. et al. The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity. Nat. Neurosci. 18, 1123–1132 (2015).
Acknowledgements
We thank M. Wong, B.L. Boublil, N. Beer and A.-L. Schlenner for technical assistance; and we thank K.B. Fischer and L.A. Ewell for SWR detection analysis code. We also thank the following UCSD students for help with behavioral testing and microscopy: R. Brar, M. Josic, A. Kappe, S. Lum, C. Miller, D. Moller and L. Piper. This research was supported by NIH grant MH102841 to J.E.L. and J.L.; NIH grant MH100349 and a Walter F. Heiligenberg Professorship to J.L.; NIH grants MH100354, NS084324, NS086947, NS097772 and NS102915 to S.L.; a JSPS Postdoctoral Fellowship for Research Abroad, Precursory Research for Embryonic Science and Technology (PRESTO) from Japan Science and Technology Agency (JST) and Kaken-hi grants 17H05939, and 17H05551 to T.S.; and a PEW Postdoctoral Fellowship to V.P.
Author information
Authors and Affiliations
Contributions
T.S., V.C.P., S.L. and J.K.L. designed experiments. V.C.P. developed the lesion and performed the behavioral testing and dentate electrophysiological recording experiments. S.A. performed preliminary analyses of dentate recordings. T.S. performed CA3 electrophysiological recordings and behavior in DG-lesioned animals, with assistance from E.H. with behavior, histology and microscopy. T.S. performed all reported analyses. J.E.L. provided conceptual discussions. T.S., S.L. and J.K.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17
Rights and permissions
About this article
Cite this article
Sasaki, T., Piatti, V.C., Hwaun, E. et al. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat Neurosci 21, 258–269 (2018). https://doi.org/10.1038/s41593-017-0061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0061-5
This article is cited by
-
Awake ripples enhance emotional memory encoding in the human brain
Nature Communications (2024)
-
Interference of the inclusive language in the creation of the memory trace during the reading comprehension of texts
Journal of Cultural Cognitive Science (2024)
-
Assessments of dentate gyrus function: discoveries and debates
Nature Reviews Neuroscience (2023)
-
Oligodendrocyte dynamics dictate cognitive performance outcomes of working memory training in mice
Nature Communications (2023)
-
A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology
Molecular Psychiatry (2022)