Abstract
Cryogenic electron microscopy is widely used in structural biology, but its resolution is often limited by the dynamics of the macromolecule. Here we developed a refinement protocol based on Gaussian mixture models that integrates particle orientation and conformation estimation and improves the alignment for flexible domains of protein structures. We demonstrated this protocol on multiple datasets, resulting in improved resolution and resolvability, locally and globally, by visual and quantitative measures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The three datasets used in the paper are publicly available through EMPIAR: EMPIAR-10786 for the GPCR, EMPIAR-10492 for the SARS-CoV-2 spike, and EMPIAR-10374 for the ABC transporter. Structures produced in this paper are deposited in EMDatabank, and the corresponding refined models in the PDB: EMD-41840, 8U26 for the GPCR; EMD-41841, 8U28 for SARS-CoV-2 spike; and EMD-41845, 8U2C for the ABC transporter.
Code availability
All computational tools described here are implemented in EMAN2, a free and open source software for cryoEM/cryogenic electron tomography imaging processing. The code is available at github.com/cryoem/eman2, and a tutorial for these tools can be found through eman2.org/e2gmm_refine.
References
Cheng, Y. Single-particle cryo-EM—how did it get here and where will it go. Science 361, 876–880 (2018).
Zhang, K., Pintilie, G. D., Li, S., Schmid, M. F. & Chiu, W. Resolving individual atoms of protein complex by cryo-electron microscopy. Cell Res. 30, 1136–1139 (2020).
Nakane, T. et al. Single-particle cryo-EM at atomic resolution. Nature 587, 152–156 (2020).
Yip, K. M., Fischer, N., Paknia, E., Chari, A. & Stark, H. Atomic-resolution protein structure determination by cryo-EM. Nature 587, 157–161 (2020).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Nakane, T., Kimanius, D., Lindahl, E. & Scheres, S. H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife 7, e36861 (2018).
Zhong, E. D., Bepler, T., Berger, B. & Davis, J. H. CryoDRGN: reconstruction of heterogeneous cryo-EM structures using neural networks. Nat. Methods 18, 176–185 (2021).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Punjani, A. & Fleet, D.J. 3DFlex: determining structure and motion of flexible proteins from cryo-EM. Nat. Methods 20, 860–870 (2023).
Herreros, D. et al. Estimating conformational landscapes from Cryo-EM particles by 3D Zernike polynomials. Nat. Commun. 14, 154 (2023).
Chen, M. & Ludtke, S. J. Deep learning-based mixed-dimensional Gaussian mixture model for characterizing variability in cryo-EM. Nat. Methods 18, 930–936 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. Preprint at arXiv https://doi.org/10.48550/arXiv.1412.6980 (2017).
Chen, M., Toader, B. & Lederman, R. Integrating molecular models into CryoEM heterogeneity analysis using scalable high-resolution deep gaussian mixture models. J. Mol. Biol. https://doi.org/10.1016/j.jmb.2023.168014 (2023).
Martín~Abadi et al. TensorFlow: Large-scale machine learning on heterogeneous systems. Preprint at arXiv https://doi.org/10.48550/arXiv.1603.04467 (2016).
Harris, J. A. et al. Selective G protein signaling driven by substance P-neurokinin receptor dynamics. Nat. Chem. Biol. 18, 109–115 (2022).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Ke, Z. et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 588, 498–502 (2020).
Jackson, S. M. et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 25, 333–340 (2018).
Dong, Y. et al. Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature 565, 49–55 (2019).
Herreros, D. et al. ZART: a novel multiresolution reconstruction algorithm with motion-blur correction for single particle analysis. J. Mol. Biol. 435, 168088 (2023).
Asarnow, D., Palovcak, E. & Cheng, Y. asarnow/pyem: UCSF pyem v0.5. Zenodo https://doi.org/10.5281/zenodo.3576630 (2019).
Penczek, P. A. Reliable cryo-EM resolution estimation with modified Fourier shell correlation. IUCrJ 7, 995–1008 (2020).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Bell, J. M., Chen, M., Baldwin, P. R. & Ludtke, S. J. High resolution single particle refinement in EMAN2.1. Methods 100, 25–34 (2016).
Bartesaghi, A. et al. Atomic resolution cryo-EM structure of β-galactosidase. Structure 26, 848–856.e3 (2018).
Acknowledgements
This research has been supported by NIH grant R21MH125285 and R01GM150905 to M.C., and R01GM113195 and the Chan Zuckerberg Initiative fund from Silicon Valley Community Foundation (award no. 2021-234593) to W.C. Computational resources from SLAC Shared Scientific Data Facility (SDF) are used for the work. We thank G. Pintilie for helping with the final model refinement for PDB deposition.
Author information
Authors and Affiliations
Contributions
M.C. designed the algorithm. M.C. implemented the software and performed the experiments. M.C., M.F.S. and W.C. contributed to data interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Ellen Zhong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Arunima Singh, in collaboration with the Nature Methods team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
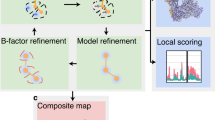
Extended Data Fig. 1 Workflow diagram for GMM-based particle orientation and conformation refinement.
Each block represents one step of processing, and the arrows indicate the sequence of the processes. The right side, DNN-based heterogeneity analysis is optional and is only recommended when large scale movement is present in the system. The result of the DNN-based refinement focusing on one region can be treated as one patch and merged into the patch-by-patch refinement results from the main workflow.
Extended Data Fig. 2 Detailed results of the GPCR dataset (EMPIAR-10786).
(a) ‘Gold-standard’ FSC curves of the reconstruction using initial orientation (gray), global GMM-based refinement (yellow) and patch-by-patch refinement (pink). (b) Q-score comparison of the three corresponding maps. (c-d) Overall structure of the three reconstructions, colored by local resolution, and comparison of local real space features. The cyan boxes in C highlight the location of features in the corresponding columns shown in D.
Extended Data Fig. 3 Detailed results of the SARS-COV2 dataset (EMPIAR-10492).
(a) ‘Gold-standard’ FSC curves of the reconstruction using initial orientation (gray), global GMM-based refinement (yellow) and patch-by-patch refinement (pink). (b) Q-score comparison of the three corresponding maps. (c-d) Overall structure of the three reconstructions, colored by local resolution, and comparison of local real space features. The cyan boxes in C highlight the location of features in the corresponding columns shown in D.
Extended Data Fig. 4 Detailed results of the ABC transporter dataset (EMPIAR-10374).
(a) ‘Gold-standard’ FSC curves of the reconstruction using initial orientation (gray), global GMM-based refinement (yellow) and patch-by-patch refinement (pink). (b) Q-score comparison of the three corresponding maps. (c-d) Overall structure of the three reconstructions, colored by local resolution, and comparison of local real space features. The cyan boxes in C highlight the location of features in the corresponding columns shown in D.
Extended Data Fig. 5 Visualization of GMMs.
(a) Reconstruction of the GPCR using the voxel map representation, determined at 3.3 Å resolution. 6 × 106 floating point values are required to represent the structure. (b) GMM representation of the A, shown in isosurface view. 5 × 104 floating point values are used to represent the structure. (c) FSC curve between A and B. The two structures are virtually identical up to 3.3 Å. (d) Visualization of the GMM from B using 3D scatter plot. Each point is colored by the amplitude of the Gaussian function and the size of the points correspond to the width of Gaussian functions. (e) Overlay of the coordinates of Gaussian functions in the corresponding density map.
Extended Data Fig. 6 Diagram for the patch-by-patch refinement.
(a) Input density maps and GMMs from the global orientation refinement. (b) Scatter plot of Gaussian coordinates, colored by clustering result. (c) Soft masks for each cluster. Each mask is a sphere covering all Gaussian coordinates of the corresponding cluster, with a soft falloff. (d) Focused refinement results using the soft masks, colored by local resolution. Note that the same refinement process is done independently for the even/odd subsets of particles using the corresponding half map/GMM as reference. (e) Masked out density from the individual focused refinement result. (f) Final composite map generated by weighted averaging.
Extended Data Fig. 7 Orientation and translation change before and after GMM-based refinement.
(a) Histogram of particle orientation assignment change after the GMM-based global refinement. Mean = 2.34, std = 1.37 degrees. (b) Histogram of particle translation change after the GMM-based global refinement. Mean = 0.66, std = 0.46 pixels. (c) Histogram of particle orientation assignment change after the GMM-based focused refinement. Mean = 3.74, std = 1.76 degrees. (d) Histogram of particle translation change after the GMM-based global refinement. Mean = 0.89, std = 0.62 pixels.
Extended Data Fig. 8 Performance of GMM-based refinement in datasets at lower resolution.
(a–c) Refinement of a small subset of the GPCR dataset. (d–f) Refinement of a small subset of the SARS-COV2 dataset. (a, d) ‘Gold-standard’ FSC curves of the reconstruction using voxel-based refinement (gray), global GMM-based refinement (yellow) and patch-by-patch refinement (pink). (b, f) Overall structure of the initial reconstruction and the final patch-by-patch refinement result, colored by local resolution. (c, f) Comparison of local features from the voxel-based, global GMM-based and patch-by-patch refinement.
Extended Data Fig. 9 Impact of flexible domains on the global FSC curve.
(a) Simulated density map of β-galactosidase, with two rigid subunits at 2.5 Å and two flexible ones resolved at 15 Å. (b) Same simulated map as A, with the two flexible subunits resolved at 7 Å. (c) Comparison of ‘gold-standard’ FSC curves of the two structures. Blue - A, red - B.
Supplementary information
Supplementary Video 1
Structure comparison of the GPCR dataset (EMPIAR-10786). Gray: reconstruction using initial orientation. Pink: reconstruction after GMM-based patch-by-patch refinement.
Supplementary Video 2
Structure comparison of the SARS-CoV-2 dataset (EMPIAR-10492). Gray: reconstruction using initial orientation. Pink: reconstruction after GMM-based patch-by-patch refinement.
Supplementary Video 3
Structure comparison of the ABC transporter dataset (EMPIAR-10374). Gray: reconstruction using initial orientation. Pink: reconstruction after GMM-based patch-by-patch refinement.
Supplementary Video 4
Converting structural heterogeneity to particle orientation focusing on the Fab of the ABC transporter (EMPIAR-10374).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Schmid, M.F. & Chiu, W. Improving resolution and resolvability of single-particle cryoEM structures using Gaussian mixture models. Nat Methods 21, 37–40 (2024). https://doi.org/10.1038/s41592-023-02082-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-02082-9