Abstract
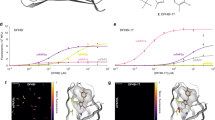
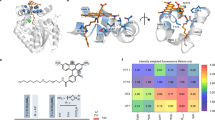
A grand challenge in biosensor design is to develop a single-molecule, fluorescent protein-based platform that can be easily adapted to recognize targets of choice. Here, we created a family of adaptable, turn-on maturation (ATOM) biosensors consisting of a monobody (circularly permuted at one of two positions) or a nanobody (circularly permuted at one of three positions) inserted into a fluorescent protein at one of three surface loops. Multiplexed imaging of live human cells coexpressing cyan, yellow and red ATOM sensors detected biosensor targets that were specifically localized to various subcellular compartments. Fluorescence activation involved ligand-dependent chromophore maturation with turn-on ratios of up to 62-fold in cells and 100-fold in vitro. Endoplasmic reticulum- and mitochondria-localized ATOM sensors detected ligands that were targeted to those organelles. The ATOM design was validated with three monobodies and one nanobody inserted into distinct fluorescent proteins, suggesting that customized ATOM sensors can be generated quickly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All raw and processed data, statistical analyses and images (with associated metadata) generated in this work have been deposited to the Figshare repository (https://doi.org/10.58120/upstate.23959239.v2). This work did not generate any code. Plasmids expressing ATOM biosensors have been deposited to Addgene (Addgene codes 209702–209721). All other resources used in this study will be made available upon request.
References
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 (2001).
Baird, G. S., Zacharias, D. A. & Tsien, R. Y. Circular permutation and receptor insertion within green fluorescent proteins. Proc. Natl Acad. Sci. USA 96, 11241–11246 (1999).
Nasu, Y., Shen, Y., Kramer, L. & Campbell, R. E. Structure- and mechanism-guided design of single fluorescent protein-based biosensors. Nat. Chem. Biol. 17, 509–518 (2021).
van der Linden, F. H. et al. A turquoise fluorescence lifetime-based biosensor for quantitative imaging of intracellular calcium. Nat. Commun. 12, 7159 (2021).
Inoue, M. et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 12, 64–70 (2015).
Inoue, M. et al. Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics. Cell 177, 1346–1360.e24 (2019).
Sun, X. R. et al. Fast GCaMPs for improved tracking of neuronal activity. Nat. Commun. 4, 2170 (2013).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Binz, H. K., Amstutz, P. & Plückthun, A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 23, 1257–1268 (2005).
Koide, A., Bailey, C. W., Huang, X. & Koide, S. The fibronectin type III domain as a scaffold for novel binding proteins. J. Mol. Biol. 284, 1141–1151 (1998).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Kroning, K. E. et al. A modular fluorescent sensor motif used to detect opioids, protein–protein interactions, and protease activity. ACS Chem. Biol. 17, 2212–2220 (2022).
Stumpp, M. T., Binz, H. K. & Amstutz, P. DARPins: a new generation of protein therapeutics. Drug Discov. Today 13, 695–701 (2008).
Amstutz, P. et al. Intracellular kinase inhibitors selected from combinatorial libraries of designed ankyrin repeat proteins. J. Biol. Chem. 280, 24715–24722 (2005).
Nord, K. et al. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 15, 772–777 (1997).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
John, A. M., Sekhon, H., Ha, J.-H. & Loh, S. N. Engineering a fluorescent protein color switch using entropy-driven β-strand exchange. ACS Sens. 7, 263–271 (2022).
Wojcik, J. et al. A potent and highly specific FN3 monobody inhibitor of the Abl SH2 domain. Nat. Struct. Mol. Biol. 17, 519–527 (2010).
Gupta, A. et al. Facile target validation in an animal model with intracellularly expressed monobodies. Nat. Chem. Biol. 14, 895–900 (2018).
Spencer-Smith, R. et al. Inhibition of RAS function through targeting an allosteric regulatory site. Nat. Chem. Biol. 13, 62–68 (2017).
Fridy, P. C. et al. A robust pipeline for rapid production of versatile nanobody repertoires. Nat. Methods 11, 1253–1260 (2014).
Do, K. & Boxer, S. G. GFP variants with alternative β-strands and their application as light-driven protease sensors: a tale of two tails. J. Am. Chem. Soc. 135, 10226–10229 (2013).
Tsien, R. Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Balleza, E., Kim, J. M. & Cluzel, P. Systematic characterization of maturation time of fluorescent proteins in living cells. Nat. Methods 15, 47–51 (2018).
Wang, P. et al. WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proc. Natl Acad. Sci. USA 115, 8581–8586 (2018).
Sirvent, A., Benistant, C. & Roche, S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol. Cell 100, 617–631 (2008).
Kim, J.-H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE https://doi.org/10.1371/journal.pone.0018556 (2011).
Shariati, K. et al. A superfolder green fluorescent protein-based biosensor allows monitoring of chloride in the endoplasmic reticulum. ACS Sens. 7, 2218–2224 (2022).
Oliinyk, O. S. et al. Single-domain near-infrared protein provides a scaffold for antigen-dependent fluorescent nanobodies. Nat. Methods 19, 740–750 (2022).
Tang, J. C. et al. Detection and manipulation of live antigen-expressing cells using conditionally stable nanobodies. eLife 5, e15312 (2016).
Feng, J. et al. A general strategy to construct small molecule biosensors in eukaryotes. eLife 4, e10606 (2015).
Navarro, R., Chen, L., Rakhit, R. & Wandless, T. J. A novel destabilizing domain based on a small-molecule dependent fluorophore. ACS Chem. Biol. 11, 2101–2104 (2016).
Sekhon, H., Ha, J.-H. & Loh, S. N. Enhancing response of a protein conformational switch by using two disordered ligand binding domains. Front. Mol. Biosci. 10, 1114756 (2023).
Zheng, H., Bi, J., Krendel, M. & Loh, S. N. Converting a binding protein into a biosensing conformational switch using protein fragment exchange. Biochemistry 53, 5505–5514 (2014).
Dingus, J. G., Tang, J. C., Amamoto, R., Wallick, G. K. & Cepko, C. L. A general approach for stabilizing nanobodies for intracellular expression. eLife 11, e68253 (2022).
Nasu, Y. et al. A genetically encoded fluorescent biosensor for extracellular l-lactate. Nat. Commun. 12, 7058 (2021).
Wu, S.-Y. et al. A sensitive and specific genetically-encoded potassium ion biosensor for in vivo applications across the tree of life. PLoS Biol. 20, e3001772 (2022).
Blanden, A. R. et al. Zinc shapes the folding landscape of p53 and establishes a pathway for reactivating structurally diverse cancer mutants. eLife 9, e61487 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We thank X. Wang and X.-J. Chen for advice on mitochondrial imaging and for the gift of HEK 293T and HeLa cells, R. Oot and S. Wilkens for the gift of MDA-MB-231 cells, M. Mollapour for the gift of COS-7 cells, M. Cosgrove for the gift of the WDR5 expression plasmid and S. Koide for the gift of the MBSH2 and SH2 genes. This work was supported by National Institutes of Health grant nos. F30 GM146428 to H.S. and R01 GM148448 to S.N.L.
Author information
Authors and Affiliations
Contributions
J.-H.H., H.S. and S.N.L. conceived and designed the study. H.S. validated the ATOM biosensors in mammalian cells. J.-H.H. compared ATOM to relating biosensors. H.S. and J.-H.H. performed all cloning. A.R.J., M.F.P., S.B.P., P.O.M. and S.N.L. purified and evaluated ATOM performance in vitro. H.S., J.-H.H. and S.N.L. wrote the paper, with contributions from all authors including A.M.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Moritoshi Sato, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Ligand-dependent ATOM turn-on in cells is not due to increased biosensor levels.
HEK 293T cells were co-transfected with one plasmid encoding y-ATOMWDR5, y-ATOMSH2, y-ATOMRAS, or y-ATOMmCh and a second plasmid expressing WDR5, SH2, hRAS, or mCh. After 48 h, cells were fixed, stained with an anti-GFP antibody conjugated with Alexa594, and imaged in the red channel (antibody; data depicted as gray points) and yellow channel (biosensor; data depicted in indicated colors). Each data point represents a cell (50–100 per experiment). Scale bars are 200 µm. The results are representative of three BR. Number of cells, means, standard deviations, and P values obtained by a two-tailed t-test for all valid comparisons are included in Extended Data Table 1.
Extended Data Fig. 2 r-ATOMWDR5, y-ATOMSH2, and c-ATOMRAS, co-expressed in the same cells, exhibit high turn-on values in the presence of their target ligands but not in the presence of noncognate ligands.
To match Fig. 4, the cyan channel (hRAS) is pseudocolored red, the yellow channel (SH2) is pseudocolored green, and the red channel (WDR5) is pseudocolored blue. HEK 293T cells were co-transfected with an equimolar mixture of three biosensor plasmids and a fourth plasmid encoding WDR5 (first row), SH2 (second row), and hRAS (third row). The fourth row shows the same experiment, but the fourth plasmid expressed all three ligands as described in the text. Scale bars are 200 µm. Turn-on ratios in each color channel (bottom plots) were calculated by dividing the average intensity of cells expressing the biosensor and its cognate ligand by the average of the intensities of cells expressing the same biosensor and the two negative control ligands. Each data point represents a cell (40–60 per experiment). P < 10−4 for all comparisons shown. The results are representative of three BR. Number of cells, means, standard deviations, and P values obtained by a two-tailed t-test for all valid comparisons are included in Extended Data Table 1.
Extended Data Fig. 3 y-ATOMWDR5 detects endogenous WDR5 and identifies nuclear localization in HEK 293T cells.
Staining with anti-GFP antibody (red) indicated that WT and R80A mutant y-ATOMWDR5 were both expressed in cytoplasm and nucleus, with highest levels in the cytoplasm. Imaging in the yellow channel (pseudocolored green) showed the WT biosensor was activated in the nucleus but in the OFF state in the cytoplasm. R80A y-ATOMWDR5 was dark in both cytoplasm and nucleus. Scale bars are 20 µm. Each data point represents one cell (61 cells per experiment). ****, P < 10−4; ns, not significant. Results are representative of 3 BR. Number of cells, means, standard deviations, and P values obtained by a two-tailed t-test for all valid comparisons are included in Extended Data Table 1.
Extended Data Fig. 4 Performance of NIR-FbGFP localized to cytoplasm, mitochondria, and ER of HEK 293T cells.
Cells were co-transfected with plasmids encoding NIR-FbGFP and Clover (tagged with indicated localization sequences) and imaged in the far-red channel to quantify compartment-specific turn-on. (b) The same cells were imaged in the green channel to determine relative Clover expression. Scale bars are 200 µm. Results are representative of 3 BR.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1 and 2.
Supplementary Table 1
Excel table with primers used in this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sekhon, H., Ha, JH., Presti, M.F. et al. Adaptable, turn-on maturation (ATOM) fluorescent biosensors for multiplexed detection in cells. Nat Methods 20, 1920–1929 (2023). https://doi.org/10.1038/s41592-023-02065-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-02065-w
This article is cited by
-
Year in review 2023
Nature Methods (2024)