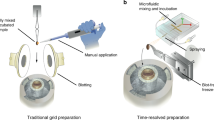
Abstract
Single-particle cryogenic electron microscopy (cryo-EM) allows reconstruction of high-resolution structures of proteins in different conformations. Protein function often involves transient functional conformations, which can be resolved using time-resolved cryo-EM (trEM). In trEM, reactions are arrested after a defined delay time by rapid vitrification of protein solution on the EM grid. Despite the increasing interest in trEM among the cryo-EM community, making trEM samples with a time resolution below 100 ms remains challenging. Here we report the design and the realization of a time-resolved cryo-plunger that combines a droplet-based microfluidic mixer with a laser-induced generator of microjets that allows rapid reaction initiation and plunge-freezing of cryo-EM grids. Using this approach, a time resolution of 5 ms was achieved and the protein density map was reconstructed to a resolution of 2.1 Å. trEM experiments on GroEL:GroES chaperonin complex resolved the kinetics of the complex formation and visualized putative short-lived conformations of GroEL–ATP complex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The protein models with the PDB accession codes 4AAQ, 4KI8, 7A4M, 1XS3, 1AON, 1DP0, and 4V40 were used in this study. The newly generated cryo-EM density maps and refined atomic models are being deposited in the PDB and EMDB databases under accession codes: apoferritin 5 ms 8BK9, EMD-16093; apoferritin 35 ms 8BKA, EMD-16094; apoferritin 205 ms 8BKB, EMD-16095; β-galactosidase 5 ms 8BK7, EMD-16091; β-galactosidase 35 ms 8BKG, EMD-16097; β-galactosidase 205 ms 8BK8, EMD-16092; GroEL–ADP 13 ms 8BL7, EMD-16102; GroEL–ATP–ADP 13 ms 8BLD, EMD-16107; GroEL–ATP 13 ms 8BLY, EMD-16115; GroEL–ADP 50 ms 8BLE, EMD-16108; GroEL–ATP–ADP 50 ms 8BLF, EMD-16109; GroEL–ATP 50 ms 8BLC, EMD-16106; GroEL–GroES–ATP 50 ms EMD-16154; GroEL–ATP 200 ms 8BL2, EMD-16100; GroEL–GroES–ATP 200 ms 8BM0, EMD-16116; GroE–GroES–ADP 200 ms EMD-6157; GroEL–2GroES–ATP 200 ms 8BMT, EMD-16125; GroEL–ATP 20 s 8BMD, EMD-16118; GroEL–GroES–ATP 20 s 8BM1, EMD-16117; GroEL–GroES–ADP 20 s 8BMO, EMD-16119; GroEL–2GroES–ATP 20 s 8BKZ, EMD-16099.
Raw micrographs for trEM data of the GroEL–GroES complex were deposited to EMPIAR database under the accession code: EMPIAR-11481. Source data are provided with this paper.
Code availability
The code for Arduino board and the LabView-based interface for the plunger setup need adjustment depending on the specific hardware used. We will provide software, help with building a similar setup, and assistance with adjusting the software for the specific device upon request.
References
Frauenfelder, H., Sligar, S. G. & Wolynes, P. G. The energy landscapes and motions of proteins. Science 254, 1598–1603 (1991).
Astumian, R. D., Mukherjee, S. & Warshel, A. The physics and physical chemistry of molecular machines. Chem. Phys. Chem. 17, 1719–1741 (2016).
Astumian, R. D. & Bier, M. Mechanochemical coupling of the motion of molecular motors to ATP hydrolysis. Biophys. J. 70, 637–653 (1996).
Motlagh, H. N., Wrabl, J. O., Li, J. & Hilser, V. J. The ensemble nature of allostery. Nature 508, 331–339 (2014).
Lu, S. et al. Activation pathway of a G protein-coupled receptor uncovers conformational intermediates as targets for allosteric drug design. Nat. Commun. 12, 4721 (2021).
Ulbricht, W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 85, 1271–1301 (2005).
Sharma, S. et al. Monitoring protein conformation along the pathway of chaperonin-assisted folding. Cell 133, 142–153 (2008).
Miller, P. S. & Smart, T. G. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol. Sci. 31, 161–174 (2010).
Orellana, L. Large-scale conformational changes and protein function: breaking the in silico barrier. Front. Mol. Biosci. 6, 117 (2019).
Wang, C., Polovitskaya, M. M., Delgado, B. D., Jentsch, T. J. & Long, S. B. Gating choreography and mechanism of the human proton-activated chloride channel ASOR. Sci. Adv. 8, 3942 (2022).
Fischer, N., Konevega, A. L., Wintermeyer, W., Rodnina, M. V. & Stark, H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature 466, 329–333 (2010).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Murphy, B. J. et al. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1–Fo coupling. Science 364, 1–10 (2019).
Dubochet, J. et al. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228 (1988).
Hofmann, S. et al. Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature 571, 580–583 (2019).
Parey, K. et al. Cryo-EM structure of respiratory complex I at work. eLife 7, e39213 (2018).
Guo, H. & Rubinstein, J. L. Structure of ATP synthase under strain during catalysis. Nat. Commun. 13, 2232 (2022).
Subramaniam, S. & Henderson, R. Molecular mechanism of vectorial proton translocation by bacteriorhodopsin. Nature 406, 653–657 (2000).
Kaledhonkar, S. et al. Late steps in bacterial translation initiation visualized using time-resolved cryo-EM. Nature 570, 400–404 (2019).
Berriman, J. & Unwin, N. Analysis of transient structures by cryo-microscopy combined with rapid mixing of spray droplets. Ultramicroscopy 56, 241–252 (1994).
Lu, Z. et al. Passive microfluidic device for submillisecond mixing. Sens. Actuators B Chem. 144, 301–309 (2010).
Feng, X. et al. A fast and effective microfluidic spraying-plunging method for high-resolution single-particle cryo-EM. Structure 25, 663–670 (2017).
Mäeots, M. E. et al. Modular microfluidics enables kinetic insight from time-resolved cryo-EM. Nat. Commun. 11, 3465 (2020).
Kontziampasis, D. et al. A cryo-EM grid preparation device for time-resolved structural studies. IUCrJ 6, 1024–1031 (2019).
Subramaniam, S., Gerstein, M., Oesterhelt, D. & Henderson, R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 12, 1–8 (1993).
Ménétret, J. F., Hofmann, W., Schröder, R. R., Rapp, G. & Goody, R. S. Time-resolved cryo-electron microscopic study of the dissociation of actomyosin induced by photolysis of photolabile nucleotides. J. Mol. Biol. 219, 139–144 (1991).
Frank, J. Time-resolved cryo-electron microscopy: recent progress. J. Struct. Biol. 200, 303–06 (2017).
Gołowicz, D., Kasprzak, P., Orekhov, V. & Kazimierczuk, K. Fast time-resolved NMR with non-uniform sampling. Prog. Nucl. Magn. Reson. Spectrosc. 116, 40–55 (2020).
Unwin, N. Acetylcholine receptor channel imaged in the open state. Nature 373, 37–43 (1995).
Lu, Z. et al. Monolithic microfluidic mixing–spraying devices for time-resolved cryo-electron microscopy. J. Struct. Biol. 168, 388–395 (2009).
Dandey, V. P. et al. Time-resolved cryo-EM using Spotiton. Nat. Methods 200, 1–12 (2020).
Lu, Z. et al. Gas-assisted annular microsprayer for sample preparation for time-resolved cryo-electron microscopy. J. Micromech. Microeng. 24, 115001 (2014).
Bringer, M. R., Gerdts, C. J., Song, H., Tice, J. D. & Ismagilov, R. F. Microfluidic systems for chemical kinetics that rely on chaotic mixing in droplets. Philos. Trans. A Math. Phys. Eng. Sci. 362, 1087–1104 (2004).
Hoath, S. D. Fundamentals of Inkjet Printing.(Wiley‐VCH Verlag GmbH & Co., 2015).
Hellman, A. N. et al. Laser-induced mixing in microfluidic channels. Anal. Chem. 79, 4484–4492 (2007).
Xu, B., Nguyen, N.-T. & Neng Wong, T. Droplet coalescence in microfluidic systems. Micro Nanosyst. 3, 131–136 (2011).
Haliburton, J. R. et al. Efficient extraction of oil from droplet microfluidic emulsions. Biomicrofluidics 11, 34111 (2017).
Tagawa, Y. et al. Highly focused supersonic microjets. Phys. Rev. X 2, 31002–31010 (2012).
Park, S.-Y., Wu, T.-H., Chen, Y., Teitell, M. A. & Chiou, P.-Y. High-speed droplet generation on demand driven by pulse laser-induced cavitation. Lab Chip 11, 1010–1013 (2011).
Baroud, C. N., Gallaire, F. & Dangla, R. Dynamics of microfluidic droplets. Lab Chip 10, 2032–2045 (2010).
Quinto-Su, P. A., Suzuki, M. & Ohl, C.-D. Fast temperature measurement following single laser-induced cavitation inside a microfluidic gap. Sci. Rep. 4, 1–6 (2014).
Noble, A. J. et al. Reducing effects of particle adsorption to the air–water interface in cryo-EM. Nat. Methods 15, 793–795 (2018).
Glaeser, R. M. Proteins, interfaces, and cryo-EM grids. Curr. Opin. Colloid Interface Sci. 34, 1–8 (2018).
Klebl, D. P. et al. Need for Speed: examining protein behavior during cryoEM grid preparation at different timescales. Structure 28, 1238–1248 (2020).
Clare, D. K. et al. ATP-triggered conformational changes delineate substrate-binding and -folding mechanics of the GroEL chaperonin. Cell 149, 113–123 (2012).
Horwich, A. L. & Fenton, W. A. Chaperonin-assisted protein folding: a chronologue. Q. Rev. Biophys. 53, e4 (2020).
Yifrach, O. & Horovitz, A. Transient kinetic analysis of adenosine 5′-triphosphate binding-induced conformational changes in the allosteric chaperonin GroEL. Biochemistry 37, 7083–7088 (1998).
Yifrach, O. & Horovitz, A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry 34, 5303–5308 (1995).
Kaledhonkar, S., Fu, Z., White, H. & Frank, J. Time-resolved cryo-electron microscopy using a microfluidic chip. Methods Mol. Biol. 1764, 59–71 (2018).
Monteiro, D. C. F., Amoah, E., Rogers, C. & Pearson, A. R. Using photocaging for fast time-resolved structural biology studies. Acta Crystallogr D. Struct. Biol. 77, 1218–1232 (2021).
Shaikh, T. R., Barnard, D., Meng, X. & Wagenknecht, T. Implementation of a flash-photolysis system for time-resolved cryo-electron microscopy. J. Struct. Biol. 165, 184–189 (2009).
Dubochet, J. & McDowall, A. W. Vitrification of pure water for electron microscopy. J. Microsc. 124, 3–4 (1981).
Klebl, D. P. et al. Sample deposition onto cryo-EM grids: from sprays to jets and back. Acta Crystallogr D. Struct. Biol. 76, 340–349 (2020).
Jain, T., Sheehan, P., Crum, J., Carragher, B. & Potter, C. S. Spotiton: a prototype for an integrated inkjet dispense and vitrification system for cryo-TEM. J. Struct. Biol. 179, 68–75 (2012).
Rubinstein, J. L. et al. Shake-it-off: a simple ultrasonic cryo-EM specimen-preparation device. Acta Crystallogr D. Struct. Biol. 75, 1063–1070 (2019).
Bock, L. V. & Grubmüller, H. Effects of cryo-EM cooling on structural ensembles. Nat. Commun. 13, 1709 (2022).
McDonald, J. C. et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21, 27–40 (2000).
Xia, Y. & Whitesides, G. M. Soft lithography. Angew. Chem. Int. Ed. 37, 550–575 (1998).
Efremov, R. G. & Stroobants, A. Coma-corrected rapid single-particle cryo-EM data collection on the CRYO ARM 300. Acta Crystallogr D. Struct. Biol. 77, 555–564 (2021).
Fislage, M., Shkumatov, A. V., Stroobants, A. & Efremov, R. G. Assessing the JEOL CRYO ARM 300 for high-throughput automated single-particle cryo-EM in a multiuser environment. IUCrJ 7, 707–718 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Casañal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 29, 1069–1078 (2020).
Afonine, P. V., Headd, J. J. & Terwilliger, T. C. New tool: phenix. real_space_refine. Computational Crystallogr. Newsl. 4, 43–44 (2013).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Hashmi, A. & Xu, J. On the quantification of mixing in microfluidics. J. Lab Autom. 19, 488–491 (2014).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Naydenova, K. & Russo, C. J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. 8, 629 (2017).
Acknowledgements
We thank H. De Greve for designing primers used to clone the lacZ gene. We are indebted to A. Manz (KIST, Saarbrüken) for helpful discussions, B. Schied (ULB, Brussels) for a practical introduction to microfluidics and help with setting up microfabrication laboratory, and M. Fislage for assistance with cryo-EM data collection. We would like to acknowledge the funding provided by Vlaams Instituut Voor Biotechnologie, Fonds Wetenschappelijk Onderzoek (grant nos. G0H5916N, G054617N to R.G.E.), and by the European Research Council (grant no. 726436 to R.G.E.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.T. developed the microfluidic chip for trEM, established conditions for the preparation of trEM samples, collected, and processed trEM data for apoferritin and β-galactosidase. R.G.E., S.T., and R.C. designed and constructed the plunger device. S.T. and M.D. optimized the treatment of EM grids for optimal droplet spreading. M.D. collected and analyzed trEM data for GroEL–GroES. A.S. produced and purified proteins. R.G.E. prepared the original manuscript draft. S.T., M.D., and R.G.E. prepared figures, reviewed, and edited the manuscript. R.G.E. conceived, managed, and supervised the project and acquired funding.
Corresponding author
Ethics declarations
Competing interests
R.G.E., S.T., and R.C. are inventors on the patent application WO 2022/148859 A1 disclosing the instrument for trEM, filed by Vlaams Instituut voor Biotechnologie and Vrije Universiteit Brussel. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling editor: Arunima Singh, in collaboration with the Nature Methods team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
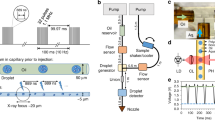
Extended Data Fig. 1 Time-resolved plunger setup.
a, Schematics of the time-resolved device. The key elements of the setup are labeled, and the laser (green) and white light beams used for high-speed recording (yellow) are shown. b, Photograph of the time-resolved plunger setup. c, Photograph of a microfluidic chip for trEM setup. The functional modules are indicated.
Extended Data Fig. 2 Evaluation of in-drop mixing efficiency.
a, Experimental design: serpentine channel with 16 sections was used to evaluate mixing efficiency. Position 0 is the position at which two solutions flow parallel to each other before droplet formation. Pressures applied to the oil and water phase from top to bottom were 80, 85; 120,115; and 160, 140 mbar. Positions used for evaluating the relative mixing index (RMI) are indicated. The images show representative snapshots from high-speed videos containing > 50 frames each. b, Pixel intensity histograms of the imaged droplets qualitatively indicate the mixing extent. The histograms represent pixel intensity distributions within identical areas of the water-in-oil droplet at the indicated positions. c, RMI for three flow conditions calculated at eight selected positions. Data are presented as mean values ± s.d. Each point of the graph was obtained analyzing three droplets.
Extended Data Fig. 3 Droplet velocity in the jets generated by LIC.
a, Snapshots of laser-induced jet extracted from a high-speed video recorded at 100,000 fps starting from the laser pulse. The images show the formation of a jet and its disintegration into droplets. b, Dependence of jet velocity on the laser power. The measurements were performed at a laser frequency of 3500 Hz. The energy per pulse was scaled by a factor of 12 to account for the splitting of the laser beam into 6 beams by holographic plate and power reduction by the beam splitter. The concentration of amaranth dye was 16 mM (10 mg/mL). Each point on the graph was obtained by averaging velocities of 10 airborne droplets. Data are presented as mean values ±s.d. t, time from laser pulse.
Extended Data Fig. 4 Influence of microfluidic chip and LIC on the activity of β-galactosidase.
The activity of β-galactosidase was measured after passing through the microfluidic chip under conditions used for trEM plunging, that is using a similar concentration of amaranth dye, droplet-based mixer, and LIC. The sprayed solution was collected, and its enzymatic activity was measured. The control sample was not passed through the chip but contained amaranth dye in the buffer. The activity values were scaled by average control activity. All measurements were repeated 3 times. Values for the individual measurements, average, and standard deviation scaled by the average of control are shown.
Extended Data Fig. 5 Spreading of microdroplets on holey carbon grids.
a, b, Examples of spreading of LIC-generated droplets on a holey carbon grid (tplunge = 100 ms) and c, d, on a holey carbon grid coated with 3 nm thick carbon layer (tplunge = 200 ms). On holey grids, many holes remained empty even though the liquid spread over the carbon. On grids with an additional continuous carbon layer, many holes were covered with a thin layer of vitreous ice. All the images are representative from a set of more than 20 grids.
Extended Data Fig. 6 Processing of trEM data for apoferritin and β-galactosidase.
a, 2D class averages. Classes were separated by their appearance on the classes corresponding to apoferritin and to β-galactosidase particles. b, Reconstructions colored according to local resolution, and distribution of particle orientations. c, Fourier Shell Correlation (FSC) plots for half-maps (solid lines), phase randomized FSC curves (dashed lines) and model-map FSCs (thin lines).
Extended Data Fig. 7 Properties of trEM data and reconstructions for GroEL:GroES data.
a, Representative cryo-EM micrographs shown for individual reaction time points (from a set with n > 1700), b, 2D class averages. c, Reconstructions colored according to local resolution. The distribution of particle orientations is shown for each reconstruction. d, Half-maps FSC (solid lines), and phase-randomised FSC plots (dashed lines).
Extended Data Fig. 8 Processing scheme for trEM GroEL-GroES data.
a, Masked 3D classification was performed after consensus 3D refinement of pooled data for time points 50, 200 ms, and 20 s. The particles were classified into 20 classes. Ten classes with a substantial number of particles are shown out of which classes displaying high-resolution features were retained. Each of these classes was split into subsets corresponding to individual timepoint. b-e, Reconstructions from data subsets corresponding to individual time points for panel a, classes 4, 9, 14, and 15, respectively. f, Processing scheme for 13 ms timepoint. A consensus volume map was 3D classified with a mask into 6 classes. g, Data from 13 and 50 ms timepoints were merged, a consensus map was auto-refined with C7 symmetry, and particles were classified into 8 classes. Three classes converged to high resolution and are shown in the scheme. Class 1 and class 8 were split into 13 and 50 ms subsets to generate time point-specific individual volumes.
Supplementary information
Supplementary Information
Supplementary Figure 1 and Supplementary Tables 1–3
Supplementary Video
High-speed imaging of functioning trEM setup. The video is composed of 4 parts showing how microfluidic chip and plunger work. Part 1. Generation of microjets by LIC. The formation of airborne jets after each laser pulse is visualized using a microfluidic chip consisting of one single channel with a nozzle dimension of ≈50 × 50 µm (width × depth). Part 2. Operation of the complete device shows how the two sample solutions are encapsulated in droplets mixed, then merged in a single stream, and microjets are generated from three nozzles by LIC. The ejected droplets are applied on the EM grid moving towards the liquid ethane vial. Part 3 shows the trajectory of the plunger arm and grid during plunging. First, the grid is slowly brought close to the nozzle where it is accelerated towards the ethane vial and decelerated to avoid the arm recoil. Time is indicated relative to the moment when the grid passes in front of the nozzles. In part 4, the operation of the device in pulsed mode is shown.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 4
Plot source data
Source Data Extended Data Fig./Table 2
Statistical source data
Source Data Extended Data Fig./Table 3
Statistical source data
Source Data Extended Data Fig./Table 4
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torino, S., Dhurandhar, M., Stroobants, A. et al. Time-resolved cryo-EM using a combination of droplet microfluidics with on-demand jetting. Nat Methods 20, 1400–1408 (2023). https://doi.org/10.1038/s41592-023-01967-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01967-z
This article is cited by
-
ATP hydrolysis captured in atomic detail
Nature Chemistry (2024)