Abstract
Homology-directed repair (HDR), a method for repair of DNA double-stranded breaks can be leveraged for the precise introduction of mutations supplied by synthetic DNA donors, but remains limited by low efficiency and off-target effects. In this study, we report HDRobust, a high-precision method that, via the combined transient inhibition of nonhomologous end joining and microhomology-mediated end joining, resulted in the induction of point mutations by HDR in up to 93% (median 60%, s.e.m. 3) of chromosomes in populations of cells. We found that, using this method, insertions, deletions and rearrangements at the target site, as well as unintended changes at other genomic sites, were largely abolished. We validated this approach for 58 different target sites and showed that it allows efficient correction of pathogenic mutations in cells derived from patients suffering from anemia, sickle cell disease and thrombophilia.
Similar content being viewed by others
Main
In CRISPR-mediated genome editing, CRISPR nucleases are used to introduce double-stranded breaks (DSBs) at genomic sites that are complementary to the spacer sequence of a guide (g)RNA1,2. Sometimes, DSBs can also occur at unintended ‘off-target’ sites that have sequence similarity to the target site3. Cellular repair of these DSBs often results in mutations and thus genome editing, while inability to repair the DSB will result in cell death4. DSB repair is mainly carried out by nonhomologous end joining (NHEJ) and by microhomology-mediated end joining (MMEJ), which can serve as a backup for NHEJ5. Both NHEJ and MMEJ frequently result in insertions and deletions (indels) of a few nucleotides, which are leveraged for targeted gene disruption6, but they can also cause larger deletions of several hundred nucleotides or chromosome rearrangements7,8. Another end-joining pathway is single-strand annealing (SSA), which requires long stretches (>10 base pairs (bp)) of sequence similarity at both sides of DSBs9. Finally, DSBs can be repaired by homology-directed repair (HDR) using sister chromatids as templates, referred to as homologous recombination (HR). HDR using a single-stranded exogenous DNA donor, which can be experimentally provided to the cells, sometimes referred to as single-strand template repair (SSTR), can be mediated by canonical HR genes, Fanconi anemia genes or DNA mismatch repair genes10,11,12.
DNA donors can be designed to introduce nucleotide changes or whole genes into the genome. This holds great promise for treating genetic diseases, as well as for genome-engineering strategies. However, this is difficult because HDR is inefficient compared to NHEJ and MMEJ and because unintended editing events often occur at the targeted genomic sites, as well as elsewhere in the genome13,14,15,16.
Many studies have therefore attempted to increase HDR efficiency by transiently inhibiting proteins central to NHEJ using short interfering (si) RNAs or small molecules17,18,19,20. For example, we have previously shown that small-molecule inhibition of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) results in predominant HDR-mediated DSB repair (>50%)20. However, this is a finely tuned interaction, as a full PRKDC knockout results in proportionally less HDR as it affects the levels of a different kinase (ATM) that is crucial for efficient HDR21,22. Others have achieved a modest increase in HDR by inhibiting DNA ligase IV, an enzyme necessary for NHEJ23.
Like HDR, MMEJ requires resected DSB ends with single-stranded overhangs. The only protein known to have a function exclusive to MMEJ is polymerase theta (Polθ), encoded by the gene POLQ. In polymerase theta-mediated end joining, Polθ aligns short nucleotide similarities before DNA synthesis24,25. Inhibition of Polθ has been found to slightly increase homologous recombination26. However, several other studies show no increase in HDR27,28. Other proteins critical for MMEJ are poly(ADP-ribose)-polymerase 1 (PARP1) and DNA ligase I/III, but these are also needed for nick repair and DNA replication. No protein exclusive for SSA has so far been described. For example, RAD52 is central to SSA by promoting annealing of complementary single-stranded DNA, but it also interacts with replication protein A complex (RPA) and RAD51 to stimulate HR29. SSTR has been described to be dependent on Rad52 in yeast12, while RAD52 is dispensable for SSTR in human cells30. However, ectopic expression of both RAD52 and a dominant-negative form of tumor protein p53-binding protein 1 improves HDR from single-stranded but not double-stranded DNA donors30.
To improve the efficiency of precise genome editing by HDR, we introduced mutations in genes necessary for NHEJ, MMEJ and SSA. We found that the combined inhibition of NHEJ by the K3753R mutation in DNA-PKcs and by Polθ V896* (stop codon introduction) in MMEJ results in DSB repair almost exclusively by HDR, while indels, large deletions/rearrangements and off-target editing events are largely abolished. We show that transient inhibition of the two repair pathways using the HDRobust substance mix yields similar results in unmodified human cells.
Results
Mutational inhibition of repair genes
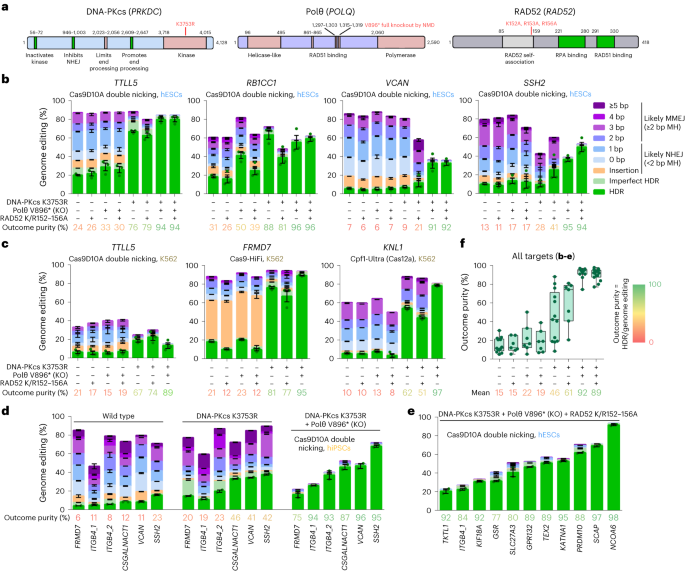
To prevent repair of DSBs by NHEJ, MMEJ and SSA while preserving HDR efficiency, we introduced mutations in genes to prevent end joining without affecting HR and thus HDR (Fig. 1a). To inhibit NHEJ, we inactivated the kinase function of DNA-PKcs (K3753R) while keeping other parts of the protein intact. To inhibit MMEJ, we introduced a stop codon (V896*) in POLQ that eliminates the DNA polymerase domain and RAD51 binding31, which may be detrimental for HR by sequestering RAD51. To inhibit SSA, we introduced the three mutations (K152A/R153A/R156A) in RAD52 that abolish DNA binding32 while keeping RAD51 binding, which stimulates HR, intact. We generated the DNA-PKcs K3753R, Polθ V896* and RAD52 K152A/R153A/R156A (K/R152–156A) mutations singly and in all four possible combinations in H9 human embryonic stem cells (hESCs) carrying an inducible (iCRISPR) Cas9D10A gene13,19. Cell population growth was normal for single mutants, reduced for a combination of DNA-PKcs K3753R with Polθ V896* and lowest for combinations containing DNA-PKcs K3753R with RAD52 K/R152–156A (Extended Data Fig. 1)
a, Protein domain structures of DNA-PKcs, Polθ and RAD52. Motifs or domains beneficial or detrimental for HR/HDR are colored green or rose, respectively. The amino acid positions where domains start and end are given and their functions indicated, the positions of mutations are in red. b, Genome editing efficiencies using Cas9D10A double nicking in H9 hESCs that carry either no repair gene mutation or combinations of DNA-PKcs K3753R, Polθ V896* and RAD52 K152A/R153A/R156A (K/R152–156A). Frequencies of deletions are presented on the basis of microhomology (MH) length. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. For HDR, replicates are depicted by dots. The mean outcome purity given below is the percentage of HDR of all editing events. KO, knockout. c, Genome editing efficiencies using Cas9D10A double nicking, Cas9 (HiFi) and Cas12a (Cpf1-Ultra) in K562 cells that carry either no repair gene mutation or combinations of DNA-PKcs K3753R, Polθ V896* and RAD52 K/R152–156A. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. d, Genome-editing efficiencies using Cas9D10A double nicking in 409B2 hiPSCs without and with combinations of repair gene mutants of targets for which we have previously shown that DNA-PKcs K3753R alone is not sufficient20. Independent biological replicates were performed (n = 2) and error bars show the s.e.m. e, Genome editing efficiencies of 11 additional targets using Cas9D10A double nicking in H9 hESCs that carry the mutations DNA-PKcs K3753R, Polθ V896* and RAD52 K/R152–156A. Independent biological replicates were performed (n = 2) and error bars show the s.e.m. f, Outcome purity for all targets in b–e for wild-type cells or cells with repair gene mutations. Each dot indicates the mean of one target, boxes the 25th to 75th percentile, lines medians and whiskers extend from minimum to maximum values.
To test the efficiency of HDR-mediated editing, we performed editing of single positions using single-stranded DNA donors together with transfected gRNA in protein-coding genes TTLL5, RB1CC1, VCAN and SSH2 (Fig. 1b). Donors were designed such that the mutation of interest also serves as a blocking mutation to prevent recutting. After isolation of DNA, PCR amplicons of the targeted regions were sequenced and HDR was scored as the fraction of amplified molecules that carry the intended nucleotide substitutions. Indels were scored as insertions and deletions with varying lengths of microhomology. When deletions occurred at sites where the sequence on one end of the deletion was identical to the undeleted sequence on the other end and was at least two nucleotides long, we scored this as likely a result of MMEJ, while other indels were attributed to NHEJ20. However, some deletions attributed to MMEJ could also be due to NHEJ by chance. Combinations of the targeted nucleotide substitutions and indels were scored as ‘imperfect HDR’. We further quantified the ratio of HDR-dependent intended edits to all genome editing events that differ from the wild-type sequence and refer to this as ‘outcome purity’.
RAD52 K/R152–156A alone, or its addition to Polθ V896* and/or DNA-PKcs K3753R, did not increase the efficiency of HDR relative to other outcomes (Fig. 1b). Polθ V896* alone did not clearly change HDR efficiency for VCAN and SSH2, while it increased efficiency for TTLL5 (21% to 29% HDR) and RB1CC1 (19% to 41%). DNA-PKcs K3753R alone clearly did not increase HDR efficiency for VCAN, slightly increased efficiency for SSH2 (10% to 16%) and strongly increased efficiency for TTLL5 (21% to 67%) and RB1CC1 (19% to 63%). For all targets, deletion patterns of cells carrying repair mutant combinations that include DNA-PKcs K3753R were different from the other cell lines (Extended Data Fig. 2a). The combination of Polθ V896* with DNA-PKcs K3753R strongly increased HDR efficiency for VCAN (7% to 33%) and SSH2 (16% to 37%), and further increased efficiency for TTLL5 (67% to 80%). Outcome purity was above 91% for all four genes, indicating that inhibition of NHEJ and MMEJ by the combination of Polθ V896* with DNA-PKcs K3753R causes CRISPR-induced DSBs to be repaired almost completely by HDR. This is supported by the observation that this double inhibition reduces mean indels from 82% to 1.7% and results in excessive cell death (at least 95%) when we edited three of the above targets without DNA donors as templates for HDR (Extended Data Fig. 3).
To test how mutations in the DNA repair genes affect the relative amount of HDR in a different cell type and when using CRISPR enzymes that produce different types of DNA breaks, we introduced the DNA-PKcs K3753R, Polθ V896* and RAD52 K/R152–156A mutations singly and in three combinations (no clones could be obtained for the triple mutant combination) in a human myelogenous leukemia line (K562) using the ribonucleoprotein (RNP) high fidelity Cas9 variant (Cas9-HiFi)33. In these lines, we edited TTLL5 with Cas9D10A RNP, FRMD7 with Cas9-HiFi RNP and KNL1 with a Cas12a variant (Cpf1-Ultra)34 RNP. Similar to the results in H9 hESCs and regardless of CRISPR enzyme used, deletion patterns of cells carrying DNA-PKcs K3753R alone or in combination were different from the other cell lines (Extended Data Fig. 2b), and the combination of Polθ V896* with DNA-PKcs K3753R resulted in predominant HDR in the three genes, albeit with reduced efficiency when two different Cas9D10A RNPs were transfected for TTLL5 double nicking (Fig. 1c). Cas9-HiFi editing of FRMD7 reached 89% and Cpf1-Ultra editing of KNL1 reached 78% HDR. Outcome purities ranged from 89 to 97%.
In H9 hESCs as well as K562 cells, inhibition of NHEJ by DNA-PKcs K3753R alone was sufficient to achieve almost complete HDR for the targets TTLL5, RB1CC1 and FRMD7, while there was a substantial proportion of deletions with microhomologies for VCAN, SSH2 and KNL1 (Fig. 1b,c). In line with the assumption that these deletions are due to MMEJ, the combination of Polθ V896* and DNA-PKcs K3753R resulted in virtually no deletions for VCAN, SSH2 and KNL1.
We also generated repair mutants of iCRISPR–Cas9D10A 409B2 human induced pluripotent stem cells (hiPSCs) and tested VCAN, SSH2 as well as four other targets for which we had previously observed that DNA-PKcs K3753R alone is not sufficient to achieve outcome purities above 50% (ref. 20). In this case, the combination of DNA-PKcs K3753R with Polθ V896* increased HDR efficiency (4.9-fold) more than DNA-PKcs K3753R alone (3.1-fold) and predominant HDR could be achieved for all targets (mean outcome purity 90%) (Fig. 1d). Editing of 11 additional targets in iCRISPR–Cas9D10A H9 hESC triple repair mutant cells resulted in HDR efficiencies of 21–91% and outcome purities of 77–98% (Fig. 1e). Comparison of outcome purities of all edits in repair gene mutant cells shows that triple mutant cells are not superior to double mutant cells (Fig. 1f).
Transient repair pathway inhibition
We have recently shown that a small-molecule inhibitor of the active site of DNA-PKcs (M3814; synonyms: nedisertib, pebosertib) almost completely blocks NHEJ and transiently increases HDR to an extent comparable to the DNA-PKcs K3753R mutation20. To test whether it is possible to also transiently inhibit MMEJ and combine it with NHEJ inhibition, we used iCRISPR–Cas9D10A H9 hESCs carrying DNA-PKcs K3753R and a commercial combination of four siRNAs to silence the POLQ transcript. When we attempted to edit VCAN, for which inhibition of both NHEJ and MMEJ is needed to achieve predominant HDR, outcome purity increased from 38% to 74%, but absolute HDR efficiency did not increase (Extended Data Fig. 4a). However, only one of the four siRNAs used in this experiment targeted sequences upstream of RAD51-binding sites in the POLQ transcript35 (Extended Data Fig. 4b). We speculated that the siRNA-induced cleavage of the mRNA may result in a truncated protein that may sequester RAD51 and thereby inhibit HDR26.
To test whether the limitation in HDR efficiency is indeed due to Polθ functions different from its polymerase domain, we generated two mutations (D2540A/E2541A) to eliminate the polymerase activity of Polθ36 in the cell line while keeping RAD51 binding intact. This drastically increased the outcome purity to 93%, but only slightly increased HDR efficiency to 24% (Extended Data Fig. 4a). Addition of the RAD51 inhibitor B02 (ref. 37) resulted in a dose-dependent decrease of HDR, supporting the importance of RAD51 for HDR in our model system. We therefore tested a custom siRNA targeting exon 15, which is upstream of the RAD51-binding sites. This molecule increased HDR efficiency from 21% to 44% when tested alone. It reduced POLQ mRNA levels to 20% in 8 h and was undetectable in later time points until 48 h (Extended Data Fig. 4c). As expected, it was unable to increase HDR in a POLQ mutant engineered to express mRNA, with silent codon mutations that prevent binding of this particular siRNA, while amino acids of Polθ are unchanged (Extended Data Fig. 4a). When this siRNA was combined with the siRNA pool (Fig. 2a) the outcome purity after editing in H9 hESCs carrying DNA-PKcs K3753R for two sites in VCAN and six other loci was at least 92%, regardless of whether Cas9-HiFi, Cas9D10A double nicking or Cpf1-Ultra were used (Fig. 2b).
a, Strategy for transient repair pathway inhibition. Motifs or domains beneficial or detrimental for homologous recombination/HDR are colored green or rose, respectively. The amino acid positions where domains start and end are given, their functions indicated and the small-molecule inhibitor of the kinase domain of DNA-PKcs M3814 (ref. 76) and siRNAs targeting the POLQ mRNA are indicated in red. b, Genome editing efficiencies using either iCRISPR–Cas9D10A double nicking, Cas9-HiFi RNP or Cpf1-Ultra RNP (Cas12a) in H9 hESCs that carry DNA-PKcs K3753R and transfected with the POLQ siRNAs (‘semitransient inhibition’, that is, genetic NHEJ inhibition, transient MMEJ inhibition). Frequencies of deletions are presented on the basis of microhomology length. Independent biological replicates were performed (n = 2) and error bars show the s.e.m. For HDR, replicates are depicted by dots. The panel to the right gives mean outcome purities (percentage HDR of all editing events) for each target. Each dot indicates the mean of one target, the box the 25th to 75th percentile, lines medians and whiskers extend from minimum to maximum values. c, Genome editing efficiencies using Cas9D10A double nicking in H9 hESCs, as well as using Cas9D10A double nicking, Cas9-HiFi or Cpf1-Ultra in K562 cells and transient inhibition (inh.) of DNA-PKcs by M3814 and/or of POLQ by siRNA. Independent biological replicates were performed (n = 2) and error bars show the s.e.m. Mean outcome purities are given below the charts. d, Genome editing efficiencies of 30 targets using iCRISPR–Cas9 in 409B2 hiPSCs with transient end-joining inhibition by HDRobust (M3814 + POLQ siRNA mix). Independent biological replicates were performed (n = 3) and error bars show the s.e.m. Each dot in the right panel indicates the mean of one target, the box the 25th to 75th percentile, the line the median and whiskers extend to the minimum and maximum values. e, Repeated editing in H9 hESCs with HDRobust increases the percentage of precisely edited cells while maintaining outcome purity. Independent biological replicates were performed (n = 3) and error bars show the s.e.m.
In line with our observation that it is not sufficient to inactivate the polymerase domain of POLQ to maximize HDR, small-molecule Polθ polymerase inhibitor ART558 (ref. 38) increased outcome purity to 75%, but not absolute HDR (Extended Data Fig. 5a). Small-molecule Polθ ATPase inhibitor novobiocin39 had no clear effect (Extended Data Fig. 5b). We also tested two small-molecule inhibitors of PARP and two inhibitors of DNA ligase I/III, two proteins that are involved in MMEJ, but none of them increased HDR efficiency or outcome purity (Extended Data Fig. 5c–f).
Next, we edited genes previously targeted in the repair gene mutant cells (Fig. 1b) using transient inhibition of NHEJ by M3814 and/or MMEJ by the POLQ siRNAs in H9 hESCs and K562 cells expressing wild-type repair proteins. Transient inhibition of only NHEJ increased HDR fivefold, while inhibition of both NHEJ and MMEJ led to a 6.3-fold increase in HDR (Fig. 2c). Outcome purity in the latter case was 72–87%. The combination of M3814 and POLQ siRNAs, which we dubbed ‘HDRobust’, can thus robustly achieve HDR efficiencies comparable to genetic inhibition, albeit with few residual indels for some targets.
We then tested 30 targets with HDRobust using lipofection of gRNA and DNA donors in iCRISPR–Cas9 409B2 hiPSCs. Figure 2d shows that this resulted in predominant HDR for 29 targets (97%) with a mean HDR efficiency across targets of 60% and a mean outcome purity of 82%. When edited without DNA donors, these targets show a wide range of indel signatures, including those that have been described to be unfavorable for HDR due to high MMEJ deletion frequency40 (Extended Data Fig. 6 and Supplementary Data 1). Editing without DNA donors resulted in 75% to 94% cell survival (mean 87%), while editing using DNA donors and HDRobust resulted in a wider range of 35% to 96% (mean 59%) (Extended Data Fig. 7).
For one target where HDR was 50% after one edit with HDRobust, repeating the editing of the cell population twice with HDRobust increased HDR to 86% (Fig. 2e). When testing repeated cell bulk editing on four additional targets, absolute HDR ranged from 80% to 96%, while outcome purity ranged from 89% to 97% (Extended Data Fig. 8a). Further, cell survival was increased when more cells contain the desired substitution that also serves as a blocking mutation to prevent cleavage (Extended Data Fig. 8b). Thus, cell populations carrying high proportions of precisely edited cells can be produced using HDRobust without the isolation of cellular clones.
Prevention of unintended on-target effects
In addition to small indels, deletions of a few hundreds or thousands of bases, as well as complex chromosomal rearrangements at the target site, can occur during genome editing7,8,14,41. To investigate whether such effects might be prevented by inhibition of NHEJ and MMEJ, we edited two sites in SCAP and TEX2 that we had previously noted were often affected by copy number losses when edited in iCRISPR–Cas9D10A H9 hESCs. We isolated 46–88 cellular clones derived from single cells, sequenced the target site and estimated the copy number of the target sites by droplet digital PCR, to detect deletions, duplications, translocations and chromosome (arm) losses. When SCAP and TEX2 were edited in cells with wild-type repair genes, 8.3% and 13% of the cellular clones were affected by copy number losses, respectively (Fig. 3a). In contrast, when using HDRobust, no cellular clones with losses of target sites were detected among 164 clones analyzed (Fig. 3b). When editing the genes in cells where NHEJ and MMEJ have been inactivated by genetic mutations, similar results were achieved, confirming that the effects are due to the inhibition of the DNA-PKcs kinase function and of Polθ (Fig. 3c–e). Thus, inhibition of NHEJ and MMEJ prevents target copy number loss at the target sites.
a–e, Target site sequencing and droplet digital (dd) PCR copy number analysis of cellular clones after editing of different targets (SCAP left panels or TEX2 right panels) using Cas9D10A double nicking in H9 hESCs without repair gene mutations (a), transient end-joining inhibition using HDRobust (M3814 + POLQ siRNA mix) (b), DNA-PKcs K3753R (c), DNA-PKcs K3753R and Polθ V896* (d) or DNA-PKcs K3753R, Polθ V896* and RAD52 K152A/R153A/R156A (K/R152–156A) (e). The copy number of target sequences relative to the gene FOXP2 in cellular clones is plotted as a filled or open circle when one predominant DNA sequence (seq.) (apparent homozygous) or two DNA sequences with a similar frequency (apparent heterozygous) were obtained, respectively. The circles are in shades of green and blue to represent different combinations of unmodified chromosomes, chromosomes modified by HDR and chromosomes modified by NHEJ or MMEJ (summarized as end joining, EJ). Incorporation of the targeted substitution regardless of the presence of additional mutations is quantified as HDR. For cellular clones with one predominant DNA sequence, ‘pure HDR’ labels the exclusive presence of the targeted substitution. A black dot in a circle fill indicates an indel at the ddPCR primer/probe site that results in inability to amplify this locus for one chromosome. Cellular clones with copy number loss indicative of an on-target effect are labeled with red arrows. The measure of center for the error bars represents the ratio of the Poisson-corrected number of target to reference molecules multiplied by two for the diploid state of the reference gene. The error bars represent the 95% confidence interval of this measurement. The numbers of cellular clones analyzed and percentages of on-target effects are given. Pie charts give the percentage of genotypes of the cellular clones. WT, wild type.
Prevention of off-target effects
To investigate the extent to which inhibition of NHEJ and MMEJ might prevent unintended editing at off-target sites, we introduced nucleotide substitutions in KATNA1, OSBP2 and RAD52 in H9 hESCs, using gRNAs that in each case matched off-target sites with a single mismatch (Fig. 4a). Three days after editing using Cas9 RNP or Cas9-HiFi RNP with and without HDRobust we scored the editing efficiency at the intended targets as well as at the two most likely predicted off-target sites42,43 (Fig. 4b). As expected, Cas9-HiFi, which is engineered to reduce off-target editing, reduced the number of off-target deletions from about 60% for the single mismatch off-target sites to 0.3–20%. However, the outcome purity at the intended targets was 45–68%. When Cas9-HiFi is combined with HDRobust, outcome purity increases to 84–96% and off-target editing is further reduced up to tenfold (0.4–2%) (Fig. 4b). Similar results were achieved in cells where DNA-PKcs and Polθ have been inactivated by mutations (Fig. 4c). Out of the 69 on-target and six off-target sites investigated, we find only one site (O-OT-1) where NHEJ and MMEJ inhibition is not sufficient to prevent deletion formation (Extended Data Fig. 9).
a, Target site sequences of three different gRNAs that are predicted to be prone to off-target editing using CFD42 and MIT77 specificity scores. The sequences of the two off-targets with the highest CFD scores are shown below the on-target sites. Identical bases are given by dots. The CFD and MIT scores are in black frames. b, Genome editing efficiencies with Cas9, Cas9-HiFi or Cas9-HiFi with HDRobust at the on-target and top two CFD off-target sites in H9 hESCs without repair gene mutations. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. c, Genome editing efficiencies at the on-target and top two CFD off-target sites by Cas9 RNP or Cas9-HiFi RNP in H9 hESCs without repair gene mutations, as well as with combinations of DNA-PKcs K3753R, Polθ V896* and RAD52 K152A/R153A/R156A (K/R152–156A). Independent biological replicates were performed (n = 3) and error bars show the s.e.m. d, Cell survival after editing with Cas9 RNP or Cas9-HiFi RNP in H9 hESCs without repair gene mutations, in combinations of DNA-PKcs K3753R, Polθ V896* and RAD52 K/R152–156A, as well as with HDRobust and Cas9-HiFi in wild-type cells. Cell survival was quantified by a fluorescence resazurin assay with respect to mock electroporation without editing. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. Replicates are depicted by dots for HDR, NHEJ + MMEJ, or cell survival.
After Cas9 editing, double inhibition of NHEJ and MMEJ strongly reduces HDR efficiency at the on-target site, from 34% to 1.3% for KATNA1, from 26% to 1% for RAD52 and from 53% to 20% for OSBP2 (Fig. 4c). This is contrary to the increases in HDR seen for other targets (Figs. 1–3), which were not selected for having off-target sites that are likely to be cleaved. This suggests that off-target cleavage results in cell death in cells lacking DNA end-joining repair.
In agreement with this, editing of targets prone to off-target editing using Cas9 RNP in unmodified H9 hESCs results in cell survival of 30–50%, while inhibition of both NHEJ and MMEJ by DNA-PKcs K3753R and Polθ V896* (double mutant) results in cell survival of only 10% (Fig. 4d). Using Cas9-HiFi, cell survival is increased to 80% for all targets in unmodified H9 hESCs, and up to 60% or 77% when both NHEJ and MMEJ are blocked by mutations or HDRobust, respectively. Thus, cell survival after editing with double inhibition cells is reduced in cases where off-target cleavage is frequent.
Importantly, the genome stability of proliferating cells is not compromised in double-repair mutant cells compared to wild-type cells after long-term culture with the drug bleomycin, which induces random DSBs (Supplementary Discussion and Supplementary Fig. 1).
Comparison to prime editing
Prime editing44 (PE) is currently the method of choice for many applications. It relies on the introduction of single-strand breaks by a Cas9H840A nickase, which is linked to a reverse transcriptase (RT), and that uses the cleaved strand of the target site as a primer to introduce edits from PE gRNA (pegRNA).
To compare the efficiency and outcome purity achieved by HDRobust with PE, we generated a H9 hESC line with an inducible PE system carrying a human codon optimized RT linked to iCRISPR–Cas9H840A (iPrime), and another H9 hESC line where the RT was linked to a Cas9 nuclease variant (iPrimeCut) (Supplementary Data 2). To further improve PE conditions, we used pegRNAs with 5′ and 3′ end phosphorothioate bonds and 2′-OMe residues to prevent degradation of the pegRNA45. We tested three targets in the genes CDKL5 (install c.1412delA), FANCF (+5G to T) and RNF2 (+1C to A) for which the pegRNAs have been optimized44. We achieved iPrime PE efficiencies of 1.1% for CDKL5, 11.2% for FANCF and 18.4% for RNF2 (Fig. 5a) with outcome purities of 34%, 59% and 78%, respectively. PE at the same positions in other cell types results in varying efficiencies and outcome purities44,46, but our iPrime PE results are comparable to published efficiencies in hESCs47 (Fig. 5b). iPrimeCut PE editing efficiencies were slightly higher than with iPrime, but resulted in more indel formation as described48, and therefore achieved a mean outcome purity of only 21% across the targets. However, when we combined iPrimeCut with HDRobust, PE efficiency increased 3.8-fold and outcome purities were similar to the iPrime results (Fig. 5a).
a, Genome editing efficiencies using iPrime (Cas9H840A nickase), iPrimeCut (Cas9 nuclease prime editor) or iPrimeCut with HDRobust (M3814 + POLQ siRNA mix) in H9 hESCs that carry no repair gene mutation. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. For PE, replicates are depicted by dots. The mean outcome purities (percentage PE of all editing events) are given. b, Published editing efficiencies of mutations at the same positions for CDKL5, FANCF and RNF2 in hiPSCs, hESCs, HEK293 or T cells44,45,46,47 are shown. c, Genome editing efficiencies of the same sites as in a with iCRISPR–Cas9D10A double nicking, or Cas9-HiFi RNP with or without HDRobust in H9 hESCs that carry no repair gene mutation. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. For HDR, replicates are depicted by dots. Mean outcome purities (percentage HDR of all editing events) are given. d, Genome editing efficiencies using Cas9-HiFi in primary CD4+ T cells with M3814 alone or HDRobust. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. e, Genome editing efficiencies using Cas9-HiFi in patient-derived LCLs with or without HDRobust. Independent biological replicates were performed (n = 2) and error bars show the s.e.m.
When electroporating DNA donors and gRNAs that have target sequences identical to the pegRNAs used above, Cas9-HiFi RNP editing with HDRobust achieved HDR efficiencies of 21% for CDKL5, 89% for FANCF and 91% for RNF2 with outcome purities of 90–96% (Fig. 5c). Thus, editing with a DNA donor49 and HDRobust performs better than prime editing (PE3) in terms of absolute precise editing efficiency as well as outcome purity in hESCs, although comparisons to enhanced prime editing methods remain to be performed, and when selecting an editing method, other metrics (for example, viability of a given cell model) may be important to consider as well.
Correction of disease mutations
To take a step towards investigating the feasibility of ex vivo gene therapy, we used Cas9-HiFi RNP to introduce a nucleotide substitution in LAG3, a gene often modified to optimize chimeric antigen receptor-T cells for cancer treatment50. While M3814 alone increased HDR efficiency 2.8-fold in primary CD4+ T cells, HDRobust increased HDR efficiency 22.2-fold (0.8 to 16%) and outcome purity from 14 to 51% (Fig. 5d).
We furthermore corrected three different mutations (R459L, R198C and S106C) in the gene encoding glucose-6-phosphate dehydrogenase (G6PD) in lymphoblastoid cells (LCLs) derived from patients suffering from anemia. We also corrected a sickle cell mutation in the hemoglobin gene (HBB E6V) and a mutation in the prothrombin gene resulting in thrombophilia (F2 c.*97G>A also known as c.20210G>A). HDRobust increased mean HDR efficiency across the five targets from 4–34% to 17–72% and increased outcome purity from 32–51% to 67–90% (Fig. 5e).
Generation of brain organoids
To assess whether editing in conjunction with inhibition of NHEJ and MMEJ might negatively influence the ability of stem cells to differentiate, we edited a nucleotide in the gene NOVA1 to change a valine at position 197 in the encoded protein to an isoleucine seen in Neandertals and nonhuman primates. In in vitro three-dimensional cultures, this change has been reported to result in bumpier and smaller brain organoids during the proliferation stage than those derived from unedited stem cells51.
We used the gRNA target, DNA donor and Cas9 as in the published work51, as well as Cas9-HiFi RNP and HDRobust, to achieve efficient HDR efficiency and prevent unintended on-target and off-target effects, to edit 409B2 hiPSCs. Cas9-HiFi and HDRobust increased HDR efficiency from 34% to 83% and reduced the percentage of cellular clones with aberrant NOVA1 copy number from 69% to 3% (Fig. 6a–d). We also amplified and sequenced heterozygous single-nucleotide polymorphisms upstream and downstream of the target site to check for loss of heterozygosity that occurs when sister chromatids are used to repair DSBs (Fig. 6e). Subsequent organoid differentiation worked equally well in the wild type and in the edited cells suggesting that HDRobust does not affect the ability of the cells to differentiate to organoids. Organoid shape and size were not affected by the ancestral NOVA1 mutation (Fig. 6f,g and Extended Data Fig. 10), compatible with the conclusion that the morphological and other organoid alterations previously observed51 might be due to unintended on-targets effects52,53. In conclusion, our results demonstrate that genome editing in conjunction with HDRobust does not negatively influence the ability of 409B2 hiPSCs to differentiate into brain organoids.
a, Standard genome editing of NOVA1 as described in51. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. For HDR, replicates are depicted by dots. The right panel shows the percentage of single cell-derived colonies that have one predominant wild-type DNA sequence (apparent homozygous wild type, gray), one predominant ‘pure’ HDR sequence (apparent homozygous exclusive ancestral edit, green) or any other genotype (white). The total number of analyzed cellular clones is given. b, Genome editing efficiencies and genotypes of single cell-derived colonies after editing of NOVA1 as done in a, but with HDRobust and Cas9-HiFi RNP. Independent biological replicates were performed (n = 3) and error bars show the s.e.m. c, Copy number (relative to the FOXP2 gene) of the target site of the single cell-derived cellular clones from a that appear homozygous at the target site for wild type and ‘pure’ HDR on the basis of sequencing of the target site. Copy number estimates are plotted as gray and green circles for wild type and ‘pure’ HDR clones, respectively. The measure of center for the error bars represents the ratio of the Poisson-corrected number of target to reference molecules multiplied by two for the diploid state of the reference gene. The error bars represent the 95% confidence interval of this measurement. The number of analyzed cellular clones and percentage of clones with aberrant copy numbers of the target site are given. d, Copy number of the target site of the cellular clones from b that appear homozygous at the target site for wild type and ‘pure’ HDR on the basis of sequencing of the target site. The measure of center for the error bars represents the ratio of the Poisson-corrected number of target to reference molecules multiplied by two for the diploid state of the reference gene. The error bars represent the 95% confidence interval of this measurement. e, Genotypes of SNPs upstream (rs17111434) and downstream (rs8006267) of the target site from the cellular clones in d. f, Phase-contrast images of three typical cellular clones with the modern human (Hum-1–3, gray circles) or ancestral (Anc-1–3, green circles) NOVA1 during early proliferation (day 7), late proliferation (day 25) and maturation (day 33). g, Organoid size and shape descriptors of circularity, solidity and roundness during brain organoid development from proliferation to maturation (days 7–33). Data for three different cellular clones for human (gray circles) and ancestral (green circles) are given. Circles show the mean and error bars show the s.e.m. of measurements of four different organoids for each day and clone.
Discussion
Methods that enable the introduction of precise changes in genomes can be powerful tools in our genome editing arsenal for both research and clinical applications. For example, the introduction of deletions to inactivate genes is now implemented in many organisms to study the roles of specific genes54. In medicine, it holds great promise as a potential treatment for genetic diseases.
However, precise single-nucleotide genome editing has so far been limited by two technical problems. First, DNA breaks introduced by CRISPR–Cas9 need to be repaired by HDR, which allows nucleotide substitutions to be introduced from a template provided to the cells. However, NHEJ and MMEJ, which tend to introduce deletions, occur much more frequently than HDR. While this has been partly overcome by the development of base editors55,56, which convert one base to another by deamination, without introduction of DNA DSBs, it can only be applied to cytosines and adenines. Base editing efficiency is also limited and currently only 46% of pathogenic T>C variants and 34% of pathogenic G>A variants can be precisely edited by C>T base editors and A>G base editors, respectively57. Second, unintended genomic changes at the target site, as well as other sites in the genome that have sequence similarity to the intended targets, often occur. This can create problems within experimental systems and are a serious concern for therapeutic applications.
Here, we have developed HDRobust to overcome these challenges. We have shown that inhibition of DNA-PKcs kinase activity (needed for NHEJ) and Polθ (needed for MMEJ), either by nucleotide substitutions or by small molecules and siRNA, strongly improves single-nucleotide editing by HDR.
It may appear surprising that simultaneous delivery of siRNA with CRISPR reagents is sufficient to quantitatively inhibit MMEJ of CRISPR-induced DSBs. Repair of DSBs often lasts for more than 20 h in mammalian cells, and MMEJ shows delayed activity compared to other repair pathways58,59. Therefore, the high efficacy of the POLQ siRNA mix to prevent MMEJ is probably due to fast and long-lasting POLQ mRNA knockdown, fast turnover of Polθ protein60, and strong and persistent binding of the Cas9–gRNA complex to its target, for hours49,61, before DSBs are accessible to DNA repair.
Our observations that POLQ siRNA results in higher absolute HDR than small-molecule inhibition of the polymerase function of Polθ, and that small-molecule inhibition of RAD51 decreases HDR, is compatible with the hypothesis that RAD51-binding sites in Polθ limit HDR26. Notably, we and others have identified RAD51 to be required for HDR when single-stranded DNA donors are provided10,19,20, while several studies find RAD51 to be dispensable for SSTR11,12,62,63. This might be due to cell type-specific differential reliance on repair subpathways, or initial RAD51-independent SSTR of one chromosome and subsequent RAD51-dependent HR utilizing the already repaired chromosome as template. Further studies will be needed to clarify this.
Although HDRobust is a powerful tool, there remain some limitations. In some rare cases, as we show for one off-target site, NHEJ and MMEJ inhibition are not sufficient to prevent deletion formation. The reason is likely to be that in this case both sides of the cut have long stretches of sequence similarity, resulting in ≥16 bp sequences on one end of deletions that are identical to the undeleted sequences. Such long stretches of sequence similarity may allow annealing of the DNA strands without the help of repair proteins, since even inhibition of SSA in addition to NHEJ and MMEJ is unable to reduce deletions at this site. Another limitation to HDRobust is that HR and thus HDR are restricted to dividing cells. However, activation of HR in G1 phase of the cell cycle may be possible64 and hESCs and hiPSCs can obviously be differentiated into nondividing cell types of interest after editing65. Finally, inhibition of NHEJ and MMEJ is suitable for cells, but not for editing in organisms, except perhaps in some animal models.
Nevertheless, the precision of HDRobust opens a plethora of opportunities. We have shown that HDRobust performs well with different CRISPR enzymes (Cas9, Cas9D10A, Cas12a/Cpf1), cell types (hESCs, hiPSCs, K562 cells, primary CD4+ T cells, LCLs) and modes of delivery (electroporation and lipofection). It is also encouraging that the percentage of edited cells can be increased by two or three consecutive rounds of edits without any increase in the frequency of deletions. This opens the possibility of generating populations of cells where the majority are edited without the need to generate cellular clones from single cells, not only reducing work load, but also preventing clone-to-clone variation, which often complicates analyses of edited cells66.
Next, similar to prime editing44, HDRobust can introduce all 12 types of point mutations, as well as insertions and deletions, when provided with a suitable DNA donor. Thus, it has the potential to correct 89% of the pathogenic variants associated with human diseases in the ClinVar database44,67. Although not tested here, HDRobust is also likely to increase outcome purity and further increase already high efficiencies achieved when using AAV6 donors, which hold great promise for therapeutic gene editing33,68,69,70. However, HDRobust has not yet been validated for clinical development.
Because dual inhibition of NHEJ and MMEJ prevents copy number loss and off-target editing, we speculate that cells lacking these end-joining pathways cannot repair DSBs in the absence of a suitable DNA donor, resulting in the fact that they can only repair breaks using the exogenous DNA donor or sister chromatids at the on-target site, and solely sister chromatids at off-target sites. When DSBs cannot be repaired in one of these ways, especially when excessive off-target cleavage occurs, cells will die, resulting in a population of precisely edited and wild-type cells. Thus, both unintended indels and large-scale modifications associated with CRISPR cleavage (large deletions, duplications, inversions, translocations, chromothripsis7,41,71) can be prevented. This is supported by the observation that dual loss of Polθ and ligase IV abolishes integration of exogenous DNA in human cells72,73. Interestingly, double inhibition could potentially be used to screen for low-specificity gRNAs without actually having to identify the potential off-target sites.
Finally, HDRobust can be extended to cells from many other species, as the lysine residue at position 3753 in DNA-PKcs is conserved among vertebrates, and DNA-PKcs itself is widely distributed among invertebrates, fungi, plants and protists74. Similarly, homologs of Polθ exist in all or most multicellular eukaryotes75, making HDRobust widely applicable as a genome editing approach.
Methods
Cell culture
Stem cell lines used were: hESCs (WiCell Research Institute, catalog no. WA09, ethics permit AZ 3.04.02/0118), modified H9 hESCs carrying iCRISPR–Cas9D10A13,78 and 409B2 hiPSC (Riken BioResource Center, catalog no. HPS0076, GMO permit AZ 54-8452/26) carrying either iCRISPR–Cas9 or iCRISPR–Cas9D10A19. We modified iCRISPR H9 hESCs to carry a reverse transcriptase adjacent to Cas9H840A44 (iPrime) or Cas9 (ref. 48) (iPrimeCut) (Supplementary Data 2). Stem cells were grown on Matrigel Matrix (Corning, catalog no. 35248) in mTeSR1 medium (StemCell Technologies, catalog no. 05851) with supplement (StemCell Technologies, catalog no. 05852) that was replaced daily. At ∼80% confluence, stem cells were dissociated using EDTA (VWR, catalog no. 437012C) and split 1:6 to 1:10 in medium supplemented with 10 μM Rho-associated protein kinase (ROCK) inhibitor Y-27632 (Calbiochem, catalog no. 688000) for one day after replating. To generate cellular clones, H9 hESCs were treated with TrypLE (Gibco, catalog no. 12605010) for 5 min at 37 °C and triturated before seeding 1:100 to 1:500 in mTeSR1 containing (ROCK) inhibitor Y-27632. After at least 7 d, colonies were picked.
Human immortalized myelogenous leukemia cells (K562) (ECACC, catalog no. 89121407) were grown in Iscove’s modified Dulbecco’s media (ThermoFisher, catalog no. 12440053) with 10% FBS. CD4+ T cells (HemaCare, catalog no. PB04C-1) were grown in RPMI 1640 (ThermoFisher, catalog no. 11875-093) with 10% FBS and activated with Dynabeads Human T-Activator (CD3/CD28) (ThermoFisher, catalog no. 11131D). LCLs (Coriell Institute, catalog nos. GM14890, HG02367, GM16265, GM08369) were cultured in RPMI 1640 with 15% FBS at 37 °C in a humidified incubator with 5% CO2. Media was replaced every second day and cells were split 1:6 to 1:10 once per week.
All cell lines were authenticated by the supplier via certificate of analysis and additionally in-house by checking morphology. All cell lines were tested negative for mycoplasma contamination before and after the experiments.
Small molecules and oligonucleotides
Commercially available small molecules used were: M3814 (MedChemExpress, catalog no. HY-101570), Rucaparib (MedChemExpress, catalog no. HY-10617A), AG-14361 (MedChemExpress, catalog no. HY-12032), L67 (MedChemExpress, catalog no. HY-15586), L189 (MedChemExpress, catalog no. HY-15588), ART558 (MedChemExpress, catalog no. HY-141520), novobiocin (MedChemExpress, catalog no. HY-B0425), B02 (Sigma, catalog no. SML0364) and bleomycin (Sigma, catalog no. B8416). All gRNAs, DNA donors and primers were from Integrated DNA Technologies (Supplementary Data 2). SiRNAs were the predesigned smart pool-containing siRNAs 485, 1390, 1397 and 2460 (Horizon Discovery, ON-TARGET plus Human POLQ siRNA - SMART 10721) and the siRNA 765 (Integrated DNA Technologies, DsiRNA hs.Ri.POLQ.13.8).
Electroporation
Adherent stem cells as well as LCLs with a tendency to clump were treated with TrypLE (Gibco, catalog no. 12605010) for 5 min at 37 °C and triturated to obtain single cells, before addition of preheated media. Cells were counted using the Countess Automated Cell Counter (Invitrogen) and cell suspensions were centrifuged at 300g for 3 min at room temperature. iCRISPR cells were incubated in medium containing 2 μg ml−1 doxycycline (Clontech, catalog no. 631311) 3 d before editing to express Cas9, Cas9D10A or the prime editors, respectively. For stem cells without integrated iCRISPR, we used recombinant Streptococcus pyogenes Cas9, Cas9-HiFi (R691A) and Cas9D10A proteins, as well as the Acidaminococcus sp. BV3L6 Cas12a (Cpf1-Ultra) protein from Integrated DNA Technologies. Electroporation for all cell types (except LCLs) was done using the B-16 program of the Nucleofector 2b device (Lonza) in cuvettes for 100 μl Human Stem Cell nucleofection buffer (Lonza, catalog no. VVPH-5022), containing 1 million cells, 100 pmol electroporation enhancer, 320 pmol gRNA (crRNA/tracR duplex for Cas9 and its variants and crRNA for Cas12a) (or 640 pmol pegRNA and 214 pmol nicking gRNA for prime editing) and 200 pmol of each single-stranded DNA donor. Where applicable, we added 252 pmol CRISPR enzyme, 160 pmol of POLQ siRNA predesigned pool and 320 pmol of POLQ siRNA 765.
For LCLs, electroporation of LCLs was done using the T-020 program79 of the Nucleofector 2b Device (Lonza) in cuvettes for 100 μl Kit V buffer (Lonza, catalog no. VCA-1003) containing 4 million cells, 100 pmol electroporation enhancer, 640 pmol of gRNA (crRNA/tracR duplex), 450 pmol of each single-stranded DNA donor and 252 pmol CRISPR–Cas9-HiFi. Where applicable, we added 320 pmol of POLQ siRNA predesigned pool, and 640 pmol of POLQ siRNA 765. For transient NHEJ inhibition, 2 µM M3814 was added for 2 d after electroporation.
Lipofection
409B2 iCRISPR–Cas9 hiPSCs were incubated in medium containing 2 μg ml−1 doxycycline (Clontech, catalog no. 631311) 3 d before lipofection to express Cas9. Lipofection was done with a final concentration of 15 nM of gRNA (crRNA/tracR duplex), 7.5 nM of POLQ siRNA predesigned pool, 15 nM of POLQ siRNA 765 and 10 nM of single-stranded DNA donor. In brief, 0.75 μl RNAiMAX (Invitrogen, catalog no. 13778075) and the respective oligonucleotides were separately diluted in 25 μl OPTI-MEM (Gibco, catalog no. 1985-062) and incubated at room temperature for 5 min. Both the RNAiMAX and the oligonucleotide dilutions were mixed to yield 50 μl of OPTI-MEM including RNAiMAX, gRNAs and single-stranded DNA donor. The lipofection mix was incubated for 20–30 min at room temperature. Cells were dissociated using EDTA for 5 min and counted using the Countess Automated Cell Counter (Invitrogen). The lipofection mix, 100 μl containing 25,000 dissociated cells in mTeSR1 supplemented with Y-27632, 2 μg ml−1 doxycycline and 2 µM M3814 were put in one well of a 96-well plate covered with Matrigel Matrix (Corning, catalog no. 35248). After 24 h, the medium was replaced with mTeSR1 containing 2 µM M3814 and after one additional day with mTesR1 without M3814.
Illumina library preparation and sequencing
Five days or more after transfection cells were dissociated using TrypLE (Gibco, catalog no. 12605010), pelleted and resuspended in 15 μl QuickExtract DNA extraction solution (Lucigen, catalog no. QE09050). Incubation at 65 °C for 10 min, 68 °C for 5 min and finally 98 °C for 5 min was performed to yield single-stranded DNA. PCR was done in a T100 Thermal Cycler (Bio-Rad) using the KAPA2G Robust PCR Kit (Sigma, catalog no. KK5024) with supplied buffer B and 3 μl of cell extract in a total volume of 25 μl. The thermal cycling was: 95 °C 3 min; 34× (95 °C 15 s, 65 °C 15 s, 72 °C 15 s); 72 °C 60 s. Illumina adapters (P5 and P7) with sample-specific indices were added in a second PCR reaction80 using Phusion HF MasterMix (Thermo Scientific, catalog no. F-531L), 0.3 μl of the first PCR product and cycling was: 98 °C 30 s; 25× (98 °C 10 s, 58 °C, 10 s, 72 °C 20 s); 72 °C 5 min. Amplifications were analyzed using 2% EX agarose gels (Invitrogen, catalog no. G4010–11), indexed amplicons were purified using solid phase reversible immobilization beads in a 1:1 ratio of beads to PCR solution81. Double-indexed libraries were sequenced on a MiSeq (Illumina) giving paired-end sequences of 2 × 150 bp (+7 bp index). After base calling using Bustard (Illumina), adapters were trimmed using leeHom82.
Amplicon sequence analysis
Bam files were demultiplexed and converted into fastq files using SAMtools83. Fastq files were used as input for CRISPResso84 to analyze sequencing read percentage of wild type (unedited), targeted nucleotide substitution (HDR or PE in case of prime editing), indels (NHEJ and MMEJ) and mix of both (imperfect HDR or imperfect PE in case of prime editing). Analysis was restricted to amplicons with a minimum of 70% similarity to the wild-type sequence and to a window of 20 bp from each gRNA. Sequence similarity for an HDR occurrence was set to 95%. Unexpected substitutions were ignored as putative sequencing errors. We further employed a Python script to identify sequencing reads with indels to be a likely a result of NHEJ (<2 bp microhomology at deletion) or MMEJ (≥2 bp microhomology at deletion)20. Sequencing data from single cell-derived cellular clones was analyzed using SAMtools.
Droplet digital and quantitative PCR
Copy numbers of target sequences were estimated by quantitative ddPCR. Primers were designed flanking the cut site and the probe was designed excluding edited sites. The gene FOXP2 was used as copy number reference. The ddPCR amplification was done in 1× ddPCR Supermix for probes (no dUTP, Bio-Rad, catalog no. 1863024), 0.2 μM primer and 0.2 μM probe for target and reference, together with 1 μl genomic DNA in QuickExtract DNA extraction solution (Lucigen, catalog no. QE09050). After droplet generation, the PCR reaction for SCAP, TEX2/NOVA1 was run for 5/10 min at 95 °C, followed by 42/40 cycles of 35/30 s at 95 °C (at a ramp rate of 1.5/2 °C s−1) and 65/60 s at 60/59 °C (at a ramp rate of 1.5/2 °C s−1) and 5 min at 98 °C. Droplets were read in a QX200 Droplet reader (Bio-Rad) and allele copy numbers were determined relative to a different fluorophore for the FOXP2 reference and unedited control.
For the siRNA knockdown time course, RNA was extracted using ice-cold QuickExtract RNA extraction solution (Lucigen, catalog no. QER09015) and reverse transcribed into cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, catalog no. 4368814) and the thermal profile: 25 °C 10 min, 50 °C 30 min, 85 °C 5 min. Quantitative PCR (qPCR) was done using CFX96 Real-Time-System C1000Touch (Bio-Rad) and the Maxima SYBR Green qPCR Master mix no ROX (Thermo Scientific, catalog no. K0253). The thermal profile of the qPCR was: 95 °C 10 min, 45× (95 °C 30 s, 60 °C 30 s, 72 °C 30 s) (for primers see Supplementary Data 2).
Resazurin assay
Subsequent to editing, cells were grown in media containing ROCK inhibitor Y-27632 for 1 d, followed by normal media for 2 d before being supplied with fresh media containing 10% resazurin solution (Cell Signaling, catalog no. 11884) and grown for 5 h before fluorescence readings using a Typhoon 9410 imager (Amershamn Biosciences) and quantification using ImageJ and the ‘ReadPlate’ plugin (Fig. 4 and Extended Data Fig. 7), or grown for 2 h before fluorescence readings using a CLARIOstar imager (BMG Labtech) (Extended Data Figs. 1, 3 and 8). Resazurin is converted into fluorescent resorufin by cellular dehydrogenases and fluorescence (excitation: 530–570 nm, emission: 590–620 nm) reflects the amount of living cells85. Wells with media and resazurin but without cells were used as blank.
Brain organoids
We generated cortical organoids as previously described86 with minor changes. In brief, cells were detached using Accutase (Sigma, catalog no. A6964) for 3 min at 37 °C and 9,000 cells per clonal cell line were seeded in low-attachment 96-well plates (Corning) in 150 µl mTeSR1 media (StemCell Technologies, catalog no. 05851) with supplement (StemCell Technologies, catalog no. 05852) and 10 µM ROCK inhibitor (Calbiochem, catalog no. 688000) for the first two days after seeding. The plates were centrifuged 3 h after seeding at 200g for 1 min to concentrate the cells in the middle of the well. Starting 48 h past seeding, until day 5, the initial media was diluted out with human pluripotent stem (hPS) cell media by carefully aspirating 100 µl and adding 100 µl of fresh hPS cell media. hPS cell media consisted of DMEM/F12, knockout serum 20%, GlutaMax 1:200, nonessential amino acids 1:100, Pen-Strep 1:100 (all Life Technologies), and 2-mercaptoethanol 100 µM (Sigma, catalog no. M3148) supplemented with 10 µM SB-431542 (Abcam, catalog no. ab120163) and 5 µM dorsomorphin (Sigma, catalog no. P5499). On day 6, the medium was changed to neural medium (NM) consisting of Neurobasal A (Life Technologies, catalog no. 10888-022), B27 supplement (no vitamin A) (Life Technologies, catalog no. 12587010) and GlutaMax 1:100 (Life Technologies, catalog. no. 35050-061) supplemented with 20 ng ml−1 EGF (Millipore, catalog no. 01-102) and 20 ng ml−1 FGF2 (R&D Systems, catalog no. 233-FB). Organoids were cultured in this medium for the next 19 d with daily medium changes in the first 10 d and every second day for the remaining 9 d. From day 25 on, FGF2 and EGF2 were replaced with 20 ng ml−1 BDNF (PeproTech, catalog no. 450-02) and 20 ng ml−1 NT3 (PeproTech, catalog no. 450-03) in the NM with medium changes every second day. Starting at day 43, only NM without any growth factors was used with medium changes every second day. We acquired a time course of phase-contrast images of organoids, extracted the two-dimensional (2D) shapes using the polygonal lasso tool of Adobe Photoshop CS5 software and quantified 2D shape descriptors of each organoid using ImageJ.
Karyotyping
H9 hESCs that carry no repair gene mutation (wild type) or both DNA-PKcs K3753R and Polθ V896* (double mutant) were treated with different tenfold-diluted concentrations (1 µg ml−1 to 0.1 ng ml−1) of bleomycin to determine the highest concentration that still allows propagation of both cell lines. Both cell lines were then propagated in media containing 1 ng ml−1 bleomycin for 5 months before karyotyping. Trypsin-induced Giemsa staining (GTG) or spectral karyotyping (SKY) were carried out according to international quality guidelines (ISCN 2016: An International System for Human Cytogenetic Nomenclature87 by the ‘Sächsischer Inkubator für klinische’ (Leipzig).
Statistics and reproducibility
Bar graphs in figures were plotted and s.e.m. error bars were calculated using GraphPad Prism 6 software. The number of replicates is stated in the respective figure legends. No statistical method was used to predetermine sample size. The experiments were not randomized. Samples were prepared unblinded but in parallel. Analysis was performed on the basis of numerical sample names, without the identity of the samples being known during the analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data generated in this study have been deposited in the Dryad database under accession code dryad.fj6q5740f. Source data are provided with this paper. Data are also available on request from the authors.
References
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR–Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 (2013).
Shrivastav, M., De Haro, L. P. & Nickoloff, J. A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 (2008).
Sfeir, A. & Symington, L. S. Microhomology-mediated end joining: a back-up survival mechanism or dedicated pathway? Trends Biochem. Sci. 40, 701–714 (2015).
Campenhout, C. V. et al. Guidelines for optimized gene knockout using CRISPR/Cas9. Biotechniques 66, 295–302 (2019).
Adikusuma, F. et al. Large deletions induced by Cas9 cleavage. Nature 560, E8–E9 (2018).
Choi, P. S. & Meyerson, M. Targeted genomic rearrangements using CRISPR/Cas technology. Nat. Commun. 5, 3728 (2014).
Bhargava, R., Onyango, D. O. & Stark, J. M. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet. 32, 566–575 (2016).
Hussmann, J. A. et al. Mapping the genetic landscape of DNA double-strand break repair. Cell 184, 5653–5669.e25 (2021).
Richardson, C. D. et al. CRISPR–Cas9 genome editing in human cells occurs via the Fanconi anemia pathway. Nat. Genet. 50, 1132–1139 (2018).
Gallagher, D. N. et al. A Rad51-independent pathway promotes single-strand template repair in gene editing. PLoS Genet. 16, e1008689 (2020).
Gonzalez, F. et al. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 15, 215–226 (2014).
Weisheit, I. et al. Detection of deleterious on-target effects after HDR-mediated CRISPR editing. Cell Rep. 31, 107689 (2020).
Zhang, X. H., Tee, L. Y., Wang, X. G., Huang, Q. S. & Yang, S. H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 4, e264 (2015).
Höijer, I. et al. CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat. Commun. 13, 627 (2022).
Robert, F., Barbeau, M., Ethier, S., Dostie, J. & Pelletier, J. Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing. Genome Med. 7, 93 (2015).
Suzuki, K. et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149 (2016).
Riesenberg, S. & Maricic, T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun. 9, 2164 (2018).
Riesenberg, S. et al. Simultaneous precise editing of multiple genes in human cells. Nucleic Acids Res. 47, e116 (2019).
Shrivastav, M. et al. DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair 8, 920–929 (2009).
Neal, J. A., Xu, Y., Abe, M., Hendrickson, E. & Meek, K. Restoration of ATM expression in DNA-PKcs-deficient cells inhibits signal end joining. J. Immunol. 196, 3032–3042 (2016).
Chen, S., Chen, D., Liu, B. & Haisma, H. J. Modulating CRISPR/Cas9 genome-editing activity by small molecules. Drug Discov. Today 27, 951–966 (2022).
Black, S. J., Kashkina, E., Kent, T. & Pomerantz, R. T. DNA polymerase θ: a unique multifunctional end-joining machine. Genes 7, 67 (2016).
Roerink, S. F., van Schendel, R. & Tijsterman, M. Polymerase theta-mediated end joining of replication-associated DNA breaks in C. elegans. Genome Res. 24, 954–962 (2014).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 518, 258–262 (2015).
Kelso, A. A., Lopezcolorado, F. W., Bhargava, R. & Stark, J. M. Distinct roles of RAD52 and POLQ in chromosomal break repair and replication stress response. PLoS Genet. 15, e1008319 (2019).
Wienert, B. et al. Timed inhibition of CDC7 increases CRISPR-Cas9 mediated templated repair. Nat. Commun. 11, 2109 (2020).
Hanamshet, K., Mazina, O. M. & Mazin, A. V. Reappearance from obscurity: mammalian Rad52 in homologous recombination. Genes 7, 63 (2016).
Paulsen, B. S. et al. Ectopic expression of RAD52 and dn53BP1 improves homology-directed repair during CRISPR–Cas9 genome editing. Nat. Biomed. Eng. 1, 878–888 (2017).
Nagy, E. & Maquat, L. E. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem. Sci. 23, 198–199 (1998).
Kagawa, W. et al. Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. Mol. Cell 10, 359–371 (2002).
Vakulskas, C. A. et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 24, 1216–1224 (2018).
Zhang, L. et al. AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat. Commun. 12, 3908 (2021).
Mateos-Gomez, P. A. et al. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 518, 254–257 (2015).
Prasad, R. et al. Human DNA polymerase θ possesses 5’-dRP lyase activity and functions in single-nucleotide base excision repair in vitro. Nucleic Acids Res. 37, 1868–1877 (2009).
Huang, F. et al. Identification of specific inhibitors of human RAD51 recombinase using high-throughput screening. ACS Chem. Biol. 6, 628–635 (2011).
Zatreanu, D. et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat. Commun. 12, 3636 (2021).
Zhou, J. et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nat. Cancer 2, 598–610 (2021).
Tatiossian, K. J. et al. Rational selection of CRISPR–Cas9 guide RNAs for homology-directed genome editing. Mol. Ther. 29, 1057–1069 (2021).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Concordet, J. P. & Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46, W242–W245 (2018).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Nelson, J. W. et al. Engineered pegRNAs improve prime editing efficiency. Nat. Biotechnol. 40, 402–410 (2022).
Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184, 5635–5652.e29 (2021).
Habib, O., Habib, G., Hwang, G. H. & Bae, S. Comprehensive analysis of prime editing outcomes in human embryonic stem cells. Nucleic Acids Res. 50, 1187–1197 (2022).
Adikusuma, F. et al. Optimized nickase- and nuclease-based prime editing in human and mouse cells. Nucleic Acids Res. 49, 10785–10795 (2021).
Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR–Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34, 339–344 (2016).
Zhang, Y. et al. CRISPR–Cas9 mediated LAG-3 disruption in CAR-T cells. Front. Med. 11, 554–562 (2017).
Trujillo, C. A. et al. Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment. Science 371, 694 (2021).
Maricic, T. et al. Comment on ‘Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment’. Science 374, eabi6060 (2021).
Herai, R. H., Szeto, R. A., Trujillo, C. A. & Muotri, A. R. Response to comment on ‘Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment’. Science 374, eabi9881 (2021).
Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 9, 1911 (2018).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Lavrov, A. V., Varenikov, G. G. & Skoblov, M. Y. Genome scale analysis of pathogenic variants targetable for single base editing. BMC Med. Genomics 13, 80 (2020).
Liu, S. C. et al. Target residence of Cas9-sgRNA influences DNA double-strand break repair pathway choices in CRISPR/Cas9 genome editing. Genome Biol. 23, 165 (2022).
Brinkman, E. K. et al. Kinetics and fidelity of the repair of Cas9-induced double-strand DNA breaks. Mol. Cell 70, 801–813.e6 (2018).
Fernandez-Vidal, A. et al. A role for DNA polymerase θ in the timing of DNA replication. Nat. Commun. 5, 4285 (2014).
Sternberg, S. H., Redding, S., Jinek, M., Greene, E. C. & Doudna, J. A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507, 62–67 (2014).
Storici, F., Snipe, J. R., Chan, G. K., Gordenin, D. A. & Resnick, M. A. Conservative repair of a chromosomal double-strand break by single-strand DNA through two steps of annealing. Mol. Cell. Biol. 26, 7645–7657 (2006).
Bothmer, A. et al. Characterization of the interplay between DNA repair and CRISPR/Cas9-induced DNA lesions at an endogenous locus. Nat. Commun. 8, 13905 (2017).
Orthwein, A. et al. A mechanism for the suppression of homologous recombination in G1 cells. Nature 528, 422–426 (2015).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Onder, T. T. & Daley, G. Q. New lessons learned from disease modeling with induced pluripotent stem cells. Curr. Opin. Genet. Dev. 22, 500–508 (2012).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Rogers, G. L. et al. Optimization of AAV6 transduction enhances site-specific genome editing of primary human lymphocytes. Mol. Ther. Methods Clin. Dev. 23, 198–209 (2021).
Omer-Javed, A. et al. Mobilization-based chemotherapy-free engraftment of gene-edited human hematopoietic stem cells. Cell 185, 2248–2264.e21 (2022).
Brault, J. et al. CRISPR-targeted MAGT1 insertion restores XMEN patient hematopoietic stem cells and lymphocytes. Blood 138, 2768–2780 (2021).
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 53, 895–905 (2021).
Saito, S., Maeda, R. & Adachi, N. Dual loss of human POLQ and LIG4 abolishes random integration. Nat. Commun. 8, 16112 (2017).
Zelensky, A. N., Schimmel, J., Kool, H., Kanaar, R. & Tijsterman, M. Inactivation of Pol θ and C-NHEJ eliminates off-target integration of exogenous DNA. Nat. Commun. 8, 66 (2017).
Lees-Miller, J. P. et al. Uncovering DNA-PKcs ancient phylogeny, unique sequence motifs and insights for human disease. Prog. Biophys. Mol. Biol. 163, 87–108 (2021).
Yousefzadeh, M. J. & Wood, R. D. DNA polymerase POLQ and cellular defense against DNA damage. DNA Repair 12, 1–9 (2013).
Harnor, S. J., Brennan, A. & Cano, C. Targeting DNA-dependent protein kinase for cancer therapy. ChemMedChem 12, 895–900 (2017).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Mora-Bermudez, F. et al. Longer metaphase and fewer chromosome segregation errors in modern human than Neanderthal brain development. Sci. Adv. 8, eabn7702 (2022).
Johnston, A. D., Simoes-Pires, C. A., Suzuki, M. & Greally, J. M. High-efficiency genomic editing in Epstein–Barr virus-transformed lymphoblastoid B cells using a single-stranded donor oligonucleotide strategy. Commun. Biol. 2, 312 (2019).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
Meyer, M. & Kircher, M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010, pdb prot5448 (2010).
Renaud, G., Stenzel, U. & Kelso, J. leeHom: adaptor trimming and merging for Illumina sequencing reads. Nucleic Acids Res. 42, e141 (2014).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Pinello, L. et al. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat. Biotechnol. 34, 695–697 (2016).
O’Brien, J., Wilson, I., Orton, T. & Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426 (2000).
Sloan, S. A., Andersen, J., Pasca, A. M., Birey, F. & Pasca, S. P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 13, 2062–2085 (2018).
McGowan-Jordan, J. et al. ISCN 2016: an International System for Human Cytogenomic Nomenclature (Karger, 2016).
Acknowledgements
We thank A. Weihmann and B. Schellbach for DNA sequencing and H. Holland for karyotyping. Funding was provided by the Max Planck Society (S.P.) and the NOMIS foundation (S.P.).
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
S.R. conceived the idea. S.R., P.K. and D.M. performed editing experiments and analyzed data. D.M., P.K. and D.W. generated organoids. S.R., N.H., T.M. and S.P. gave input on study design. D.D. and J.K. generated the iPrime cell line. S.R., S.P. and T.M. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Related patent applications on repair gene modified cell lines (patent applicant: Max Planck Society; inventors: S.R. and T.M.; application number: EP17203591.7 and PCT/EP2018/059173; publication: 2018-10-17 WO2018189186; status: pending) and compounds for transient HDR increase (patent applicant: Max Planck Society; inventors: S.R. and T.M.; application number: EP18215071.4; publication: 2020-06-25 WO2020127738A1; status: pending) have been filed. All other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Matthew Porteus, Fyodor Urnov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Lei Tang and Madhura Mukhopadhyay, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cell population growth.
Number of living wild type H9 hESCs and repair mutant variants for 6 days of cell culture. 50,000 cells each were seeded on day 0. The lines correspond to wild type (black) or repair gene mutants (colored). The amount of living cells was quantified by a fluorescence resazurin assay. Absolute cell number was estimated by comparing resazurin assay fluorescence and cell counting using the Countess Automated Cell Counter (Invitrogen) on day 1. Error bars show the s.e.m. of replicates (n = 2).
Extended Data Fig. 2 Deletion patterns after editing with single-stranded DNA donors.
(a) Deletion pattern shapes after editing in hESCs with Cas9D10A double nicking (corresponding to Fig. 1b). (b) Deletion pattern shapes after editing in K562 cells with Cas9D10A double nicking, Cas9-HiFi, and Cas12a (Cpf1-Ultra) (corresponding to Fig. 1c). The lines correspond to wild type (black) or repair gene mutants (colored). Each line is the mean of independent biological replicates (n = 3). The vertical dotted line indicates the position of the nicking or cleavage sites.
Extended Data Fig. 3 Indel efficiencies and cell survival after editing without DNA donors.
(a) Genome editing efficiencies using Cas9D10A double nicking without a DNA donor in H9 hESCs that carry either no repair gene mutation or combinations of DNA-PKcs K3753R, Polϴ V896*, and RAD52 K152A/R153A/R156A (K/R152-156A). Frequencies of deletions are presented based on microhomology (MH) length. For insertions, replicates are depicted by dots. (b) Deletion pattern shapes of editing from panel a. The lines correspond to wild type (black) or repair gene mutants (colored). Each line is the mean of replicates. (c) Cell survival corresponding to edits from panel a. Cell survival was quantified by a fluorescence resazurin assay with respect to editing in wild type cells. Independent biological replicates were performed (n = 3) and error bars show the s.e.m.
Extended Data Fig. 4 Transient microhomology-mediated end-joining (MMEJ) inhibition by siRNAs.
(a) Editing efficiencies of the VCAN target using Cas9D10A double nicking in H9 hESCs carrying the K3753R mutation, and with additional POLQ mutations (V896* full knockout, DE2540AA polymerase knockout, 6x silent SNPs that do not change the encoding amino acids), transient POLQ inhibition with siRNAs, or transient RAD51 inhibition with the small molecule B02. The K3753R mutation will prevent backup NHEJ repair when MMEJ is inhibited. Frequencies of deletions are presented based on microhomology (MH) length. For HDR, replicates are depicted by dots. The mean outcome purities (percentage HDR of all editing events) are given. Independent biological replicates were performed (n = 2, except DNA-PKcs K3753R / DE2540AA / B02 and DNA-PKcs K3753R / 6x silent SNP n = 3, DNA-PKcs K3753R / DE2540AA n = 5, and DNA-PKcs K3753R n = 6) and error bars show the s.e.m. (b) A scheme of the different siRNAs targeting the POLQ mRNA to induce Ago2/RISC assisted cleavage as well as a site of silent mutations that do not change the encoded amino acids, and translated Polϴ is shown below. Polϴ motifs described to be detrimental for homologous recombination/HDR are colored rose, Polϴ inhibitory mutations are red. (c) Time course of POLQ mRNA knock down with siRNA 765. POLQ expression was normalized with GAPDH expression and is given relative to untreated cells. Independent biological replicates were performed (n = 2) and error bars show the s.e.m.
Extended Data Fig. 5 Transient microhomology-mediated end-joining (MMEJ) inhibition by small molecules.
Editing efficiencies of the VCAN target using Cas9D10A double nicking in H9 hESCs carrying the K3753R mutation and additional small molecules to inhibit the MMEJ repair proteins Polϴ, PARP or Ligase I/III: (a) ART558, (b) Novobiocin, (c) AG-14361, (d) Rucaparib, (e) L67, (f) L189. Frequencies of deletions are presented based on microhomology (MH) length. For HDR, replicates are depicted by dots. The mean outcome purities (percentage HDR of all editing events) are given. Independent biological replicates were performed (n = 6, except n = 3 for mock c-f) and error bars show the s.e.m.
Extended Data Fig. 6 Indel signatures of no donor control edits with Cas9.
Indel signatures after genome editing of thirty targets using iCRISPR-Cas9 in 409B2 hiPSCs without a DNA donor. Indels are plotted in two ways: (a) Insertions are indicated in orange, and deletions with various microhomology (MH) lengths are indicated with various shades of blue and purple. For insertions, replicates are depicted by dots. (b) Deletions with sizes equal or bigger 3 bp are indicated in yellow, while all other indels are indicated in petrol with replicates as dots. The former and latter have been described as proxies for NHEJ and MMEJ after Cas9 editing, respectively, and a strong proxy for MMEJ is predictive of tendency for HDR if a DNA donor would be present40. These no donor controls are related to Fig. 2d. Independent biological replicates were performed (n = 3) and error bars show the s.e.m.
Extended Data Fig. 7 Cell survival after Cas9 editing for indel generation without a DNA donor and precise editing with HDRobust.
Cell survival after Cas9 edits of thirty targets in 409B2 hiPSCs without DNA donors (related sequencing data Extended Data Fig. 6) (a), and with both DNA donors and HDRobust (related sequencing data Fig. 2d) (b). Cell survival was quantified by a fluorescence resazurin assay with respect to mock electroporation without editing. Independent biological replicates are depicted by dots (n = 3) and error bars show the s.e.m. Each dot in the right panels indicate the mean of one target, the box the 25th to 75th percentile, the line the median and whiskers extend to the minimum and maximum values.
Extended Data Fig. 8 Repeated editing using HDRobust to enrich for HDR edited cells.
(a) Genome editing efficiencies using repeated Cas9D10A double nicking or Cas9-HiFi in combination with HDRobust in H9 hESCs that carry no repair gene mutation. Frequencies of deletions are presented based on microhomology (MH) length. For HDR, replicates are depicted by dots. The mean outcome purity given below is the percentage of HDR of all editing events. (b) Cell survival corresponding to edits from panel a. Cell survival was quantified by a fluorescence resazurin assay with respect to editing in wild type cells. Independent biological replicates were performed (n = 3) and error bars show the s.e.m.
Extended Data Fig. 9 Deletion events at the OSBP2 off-target O-OT-1 in cell lines with repair gene mutant combinations.
(a) Top five deletions at the OSBP2 off-target O-OT-1 H9 hESCs that carry no repair gene mutation or combinations of DNA-PKcs K3753R, Polϴ V896*, and RAD52 K152A/R153A/R156A (K/R152-156A). Sequence similarity flanking deletions indicated in purple and the numbers of similar bases (‘homology length’) are given. (b) Deletion frequency at the OSBP2 off-target O-OT-1 H9 hESCs that carry no repair gene mutation or combinations of DNA-PKcs K3753R, Polϴ V896*, and RAD52 K/R152-156A. The purple deletion line is the mean of independent biological replicates (n = 3). The vertical dotted line indicates the position of the off-target cut. Data shown is related to Fig. 4c.
Extended Data Fig. 10 NOVA1 brain organoid images used for area and shape analysis.
Phase-contrast images and shapes of brain organoids derived from cellular clones carrying ancestral (a) or human (b) NOVA1. The time course of organoid development from d7 to d33 (top to bottom) of three different cellular clones for and ancestral (Anc1-3, green circles and image frames) and human (Hum1-3, gray circles and image frames) NOVA1 is shown for four different organoids for each day and clone. The size bar is 200 µm.
Supplementary information
Supplementary Information
Supplementary discussion and Fig. 1.
Supplementary Data 1
CRISPResso cut site plots related to Extended Data Fig. 6.
Supplementary Data 2
List of oligonucleotides.
Source data
Source Data Fig. 1
Numerical source data for plotting graphs.
Source Data Fig. 2
Numerical source data for plotting graphs.
Source Data Fig. 3
Numerical source data for plotting graphs.
Source Data Fig. 4
Numerical source data for plotting graphs.
Source Data Fig. 5
Numerical source data for plotting graphs.
Source Data Fig. 6
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 1
Numerical source data for plotting graphs
Source Data Extended Data Fig. 2
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 3
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 4
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 5
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 6
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 7
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 8
Numerical source data for plotting graphs.
Source Data Extended Data Fig. 10
Numerical source data for plotting graphs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riesenberg, S., Kanis, P., Macak, D. et al. Efficient high-precision homology-directed repair-dependent genome editing by HDRobust. Nat Methods 20, 1388–1399 (2023). https://doi.org/10.1038/s41592-023-01949-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01949-1