Abstract
We report the evolution of mScarlet3, a cysteine-free monomeric red fluorescent protein with fast and complete maturation, as well as record brightness, quantum yield (75%) and fluorescence lifetime (4.0 ns). The mScarlet3 crystal structure reveals a barrel rigidified at one of its heads by a large hydrophobic patch of internal residues. mScarlet3 behaves well as a fusion tag, displays no apparent cytotoxicity and it surpasses existing red fluorescent proteins as a Förster resonance energy transfer acceptor and as a reporter in transient expression systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data supporting this study are available within the article and the Supplementary Information, including the digital data underlying the figures. Other (raw) data supporting this study are available from the corresponding author upon reasonable request. Plasmids constructed in this study, their maps and sequences were deposited to the non-profit organization Addgene ID 189752-189779. The mScarlet3 crystal structure was deposited at the PDB under accession code 7ZCT. Source data are provided with this paper.
Code availability
Image analysis was performed in ImageJ/Fiji using macros described previously11. The codes are available at GitHub (https://github.com/molcyto/MC-FLIM-Petri-dish; https://github.com/molcyto/MC-Ratio-Petri-dish; https://github.com/molcyto/MC-FLIM-96-wells; https://github.com/molcyto/MC-Ratio-96-wells; https://github.com/molcyto/MC-Bleach-96-wells).
References
Matz, M. V. et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 17, 969–973 (1999).
Yarbrough, D., Wachter, R. M., Kallio, K., Matz, M. V. & Remington, S. J. Refined crystal structure of DsRed, a red fluorescent protein from coral, at 2.0-Å resolution. Proc. Natl Acad. Sci. USA 98, 462–467 (2001).
Campbell, R. E. et al. A monomeric red fluorescent protein. Proc. Natl Acad. Sci. USA 99, 7877–7882 (2002).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Merzlyak, E. M. et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods 4, 555–557 (2007).
Bajar, B. T. et al. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci. Rep. 6, 20889 (2016).
Shcherbo, D. et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009).
Shemiakina, I. I. et al. A monomeric red fluorescent protein with low cytotoxicity. Nat. Commun. 3, 1204 (2012).
Bindels, D. S. et al. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56 (2017).
Mukherjee, S. et al. Directed evolution of a bright variant of mCherry: suppression of nonradiative decay by fluorescence lifetime selections. J. Phys. Chem. B 126, 4659–4668 (2022).
Bindels, D. S., Postma, M., Haarbosch, L., van Weeren, L. & Gadella, T. W. J. Multiparameter screening method for developing optimized red-fluorescent proteins. Nat. Protoc. 15, 450–478 (2020).
Shaner, N. C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5, 545–551 (2008).
Costantini, L. M., Fossati, M., Francolini, M. & Snapp, E. L. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic 13, 643–649 (2012).
Costantini, L. M. et al. A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 6, 1–13 (2015).
Valbuena, F. M. et al. A photostable monomeric superfolder green fluorescent protein. Traffic 21, 534–544 (2020).
Gross, L. A., Baird, G. S., Hoffman, R. C., Baldridge, K. K. & Tsien, R. Y. The structure of the chromophore within DsRed, a red fluorescent protein from coral. Proc. Natl Acad. Sci. USA 97, 11990–11995 (2000).
Shagin, D. A. et al. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol. Biol. Evol. 21, 841–850 (2004).
Vermeer, J. E. M., van Munster, E. B., Vischer, N. O. & Gadella, T. W. J. Jr. Probing plasma membrane microdomains in cowpea protoplasts using lipidated GFP-fusion proteins and multimode FRET microscopy. J. Microsc. 214, 190–200 (2004).
Aslantürk, Ö. S. in Genotoxicity—A Predictable Risk to Our Actual World (InTech, 2018).
Dabbaghi, M., Hashemi, K., Oskuee, R. K. & Afkhami-Goli, A. Reverse relation between cytotoxicity and polyethylenimine/DNA ratio, the effect of using HEPES-buffered saline (HBS) medium in gene delivery. Toxicol. Vitr. 83, 105414 (2022).
Cipriani, F. et al. CrystalDirect: a new method for automated crystal harvesting based on laser-induced photoablation of thin films. Acta Crystallogr D Biol. Crystallogr. 68, 1393–1399 (2012).
McCarthy, A. A. et al. ID30B - a versatile beamline for macromolecular crystallography experiments at the ESRF. J. Synchrotron Radiat. 25, 1249–1260 (2018).
Delagenière, S. et al. ISPyB: an information management system for synchrotron macromolecular crystallography. Bioinformatics 27, 3186–3192 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Kawakami, K. Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 8 Suppl 1, S7 (2007).
Kawakami, K. et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004).
Wan, H. et al. Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp. Cell. Res. 312, 1526–1539 (2006).
Urasaki, A., Morvan, G. & Kawakami, K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174, 639–649 (2006).
Acknowledgements
We are grateful to R. Breedijk (Amsterdam) for assistance and maintenance of the LCAM microscopes and to S. Engilberge (Grenoble) for assistance in drawing mScarlet3 structural figures. Part of the work was performed in the UMCG Microscopy and Imaging Center. This work was supported by a grant from the French Agence Nationale de la Recherche (ANR-17-CE11-0013 to J.D.).
Author information
Authors and Affiliations
Contributions
T.W.J.G. designed and supervised the project. L.v.W. and J.S. performed the mutagenesis experiments. L.v.W. prepared bacteria, purified proteins, cultured and transfected mammalian cells. T.W.J.G. performed and analyzed the spectroscopic characterization and cellular imaging experiments. M.A.H. performed and analyzed the TCSPC experiments. A.H.G.W. and B.N.G.G. performed and analyzed the zebrafish experiments. S.A., J.D. and A.R. purified mScarlet3 for crystallization, crystallized mScarlet3 and solved and analyzed the crystal structure. T.W.J.G. and A.R. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The University of Amsterdam has applied for a European Patent (EP 33108) and international PCT Patent (WO 35192; patents pending) on ‘Improved variants of monomeric scarlet red fluorescent protein’ with T.W.J.G. and L.v.W. as inventors. All other authors have no competing interests.
Peer review
Peer review information
Nature Methods thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
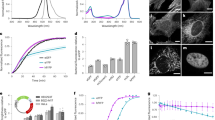
Extended Data Fig. 1 Brightness of RFPs in HeLa cells 24 h after transfection.
For each RFP an mTurquoise2 construct was coexpressed in cells in a 1:1 molecular ratio. The average red fluorescence and cyan fluorescence was quantified for each cell (red data points). a mScarlet3 n = 231, b mScarlet-I3 n = 303, c mScarlet n = 238, d mApple n = 266, e mCherry n = 272, f mScarlet-I n = 303, g mKate2 n = 294, h mRuby3 n = 275, I FusionRed n = 156, j TagRFP-T n = 320, and k dTomato n = 268. The red fluorescence values were corrected for spectral throughput of the microscope (correcting for instrument-dependent excitation and detection efficiencies of the different RFPs). The slopes of the linear regression line that is forced the origin was used for calculating the relative brightness of the RFPs (as compared to mScarlet) and indicated in Extended Data Table 1 and Fig. 1d. The slope was calculated as ∑RC/∑C2 in which R is the RFP intensity value of a single cell (vertical axes) and C is the CFP intensity of the same cell (horizontal axes). The standard error of the slope (indicated in Fig. 1d and Extended Data Table 1) was calculated as Sqrt((∑R2-(∑RC)2/∑C2)/((n-1)∑C2)). In which n is the number of cells.
Extended Data Fig. 2 Alkaline denaturation speed of diverse purified RFPs at pH 13.5.
a: logarithmic time axis, b: normal time axis.
Extended Data Fig. 3 Photostability of RFP variants.
a: Wide-field bleaching of mScarlet variants without time-axis correction, b: same data as a) but y-axis scaled according to cellular brightness; c: confocal bleaching of mScarlet variants; d: confocal bleaching of mScarlet variants and other RFPs. In order to fairly compare the photostability of different RFP variants under confocal imaging conditions, the time axis is corrected in c and d for spectral properties relative to mScarlet to mimic an identical overall emission rate (photons/s) for all RFPs at the start of the experiment. e: Blue light-induced photochromicity of RFP variants. The RFP variants were expressed in HeLa cells and continuously exposed with 550 nm excitation light. At t = 15, 31, 46 and 52 s, the 550 nm excitation light interrupted by a brief excitation at 470 nm for 1 s (not recorded on time axis) after which the 550 nm excitation was continued. The sudden increase after blue light excitation is caused by photochromic behavior.
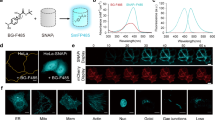
Extended Data Fig. 4 Evaluation of RFPs as fusion tag in HeLa cells.
a-h: representative images of selected Cyterm-RFP fusions in HeLa cells 24 h after transfection. a FusionRed, b mScarlet3; c TagRFP-T; d mCherry; e mScarlet-I3; f mScarlet-I3-NCwt; g mScarlet-I; h mScarlet. For a-h bar is 10 µm and the fluorescence intensities were pseudo-colored between minimum and maximum intensities according to the mScarlet lookup table as indicated in image d and subsequently gamma-corrected (value 0.5, including the lookup table) to better visualize the low intensity areas. For cell numbers imaged and evaluated see Extended Data Table 1, images shown are representative of these. i-l: mScarlet3 as fusion tag for cellular localization studies. mScarlet3 was fused to: i alpha-tubulin (microtubule cytoskeleton); j EB3 (+ends of microtubules); k Giantin (Golgi apparatus); and l a 3x repeated nuclear localization signal (NLS) (nuclei) and expressed in HeLa cells. For i-l bar is 10 µm and the fluorescence intensity was pseudo-colored between minimum and maximum fluorescence according to the mScarlet lookup table indicated in l. Micrographs shown in i-l are representative of at least 4 images made from 2 independent transfections.
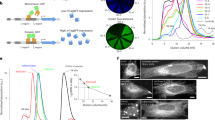
Extended Data Fig. 5 FRET in HeLa cells expressing SYFP2 alone or SYFP2-RFP fusion proteins.
a. Average FRET spectra from HeLa cells expressing SYFP2 alone or SYFP2-RFP fusion proteins. All spectra were normalized to the unquenched SYFP2 donor. b. Quantification of FRET spectra from a. E (blue bars) represent the energy transfer efficiency (average ± sd), and Qs (red bars) depict the sensitized emission quantum yield (average ± sd). Qs is defined as the number of sensitized emission quanta emitted by the RFP acceptor divided by the number of quanta absorbed by the SYFP2 donor and is calculated by Qs=E·Qa, in which Qa is the acceptor quantum yield of fluorescence. Qs is not dependent on the emission spectra and is proportional to the integrated acceptor sensitized emission signal. Each data point represents quantitative unmixing analysis of a single cell spectrum, 18–25 single cell spectra were recorded for each condition. c. SYFP2 fluorescence lifetime of HeLa cells expressing SYFP2 alone or SYFP2-RFP fusion proteins as determined by frequency-domain FLIM. Tau(phi) (red bars) is the average lifetime estimated from the phase shift (± sd), Tau(mod) (blue bars) is the average fluorescence lifetime estimated from the demodulation (± sd). For c each data point represents the average lifetime of an image with 4–20 individual cells, 4–6 FLIM recordings were done for each condition.
Extended Data Fig. 6 Relative cell viability of RFP transfected cells.
mEGFP and RFPs were transfected separately into HeLa cells at day 0. At day 2 each of the RFP transfected cells were split and mixed with mEGFP transfected cells. At days 4 and 6 the cell mixtures were resplit. At days 2, 4 and 6 after (4–5 h after splitting/mixing) the number of RFP and mEGFP expressing cells in the mixtures was scored. The ratio of RFP to mEGFP expressing cells was normalized to that obtained at day 2 to correct for bias in transfection efficiencies. For each RFP 8-12 technical replicates (depicted as individual data points) were measured. The average normalized ratio of red to green cell numbers ± sd is indicated.
Extended Data Fig. 7 Brightness of RFPs in mammalian cells after fixation relative to mScarlet.
Indicated is the average relative brightness (± sd), corrected for spectral throughput determined from single cell red to cyan ratios and linear regression (though the origin) like in Extended Data Fig. 1.
Extended Data Fig. 8 Illustration of the potential van der Waals interactions between 9 residues of mScarlet3 near the surface of one head of the β-barrel.
Residues belong to the central α-helix traversing the β-barrel (Thr74), to the α-helix capping one head of the β-barrel (Trp84), to the loop linking the latter helices (His76), to the 7th strand (Leu151), to the 8th strand (Leu158), to the loop bridging the 9th and 10th strands (Pro191), to the 10th strand (Phe194, Ile196), to the 11th strand (Ala220). Potential van der Waals interactions (dashed black lines) are highlighted by inter-carbon atom distances below 4.0 Å. a. View from the side of the β-barrel. The set of vdW interactions forms a crown-like structure.b. 90° view (from the barrel head) showing residues organized in three layers: the main one is formed of residues Thr74, Trp84, Leu158, Phe194 and Ala220, with residues His76 and Pro191 ahead of this layer, and residues Leu151 and Ile196 behind it.
Extended Data Fig. 9 Evolution path for mScarlet3 (seq-id #15) and mScarlet-I3 (#16) from mScarlet (#1) and mScarlet-I (#2), respectively.
The numbers correspond with the sequence IDs in Supplementary Fig. 1. The horizontal axis depicts the (modulation) fluorescence lifetime as determined in live HeLa cells ~ 24 h after transfection and corresponds with intrinsic brightness (more to the right is better), and the vertical axis depicts the cellular brightness (higher is better) normalized to mScarlet-I. F. Vertical error bars are SD of the slope of the line fitted to the red to cyan cellular ratios (of 22–77 cells, for example see Extended Data Fig. 1) or the average modulation lifetime of the mScarlet variant in HeLa cells (from 3 individual FLIM recordings with > 5 cells each).
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2, Tables 1 and 2 and Fig. 1.
Confocal timelapse of mScarlet3-Cyterm imaged in U2Os cells 48 h after transfection. In addition to ER and nuclear envelope labeling, motile punctate endomembrane structures appear that are possibly involved in removal of mScarlet3-Cyterm from the ER. This is particularly apparent in the top right cell. Total video duration 42 s at 0.32 s frame rate
Prolonged confocal imaging of actin dynamics using Lifeact-mScarlet3. Actin dynamics was imaged in HeLa cells transfected with pLifeact-mScarlet3. One thousand frames were captured each with two scans averaged. No noticeable bleaching was observed. Total video duration is 16 h at 1 min frame rate.
Microtubule polymerization visualized by EB3-mScarlet3 in HeLa cells. Total video duration is 2 min in real time, bar is 10 µm.
Dynamic microtubules visualized by expression of mScarlet3-α tubulin in HeLa cells. Total video duration is 2 min in real time, bar is 10 µm.
mScarlet3 applied for prolonged live-cell imaging of nuclei in pancreatic cells in zebrafish larvae. H2A-mScarlet3 was expressed under an elastase promotor and examined on a lightsheet microscope. Nuclear localization is found only in the exocrine cells of the pancreas. Movement is caused by motion of the larva. Total video duration is 12 h in real time at 15 min frame rate.
Lightsheet microscopy of H2B-mScarlet3-labeled cell nuclei in a developing zebrafish larva (full larva.) Total video duration is 355 min.
Lightsheet microscopy of H2B-mScarlet3-labeled cell nuclei in a developing zebrafish larva (zoom on mitotic cells). Total video duration is 355 min (zoom). Note several dividing cells.
Source data
Source Data Fig. 1
Source data for Fig.1a–i.
Source Data Fig. 2
Source data for Fig. 2 i.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2a,b.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3a–e.
Source Data Extended Data Fig. 5
Source data for Extended Data Fig. 5a–c.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 9
Source data for Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gadella, T.W.J., van Weeren, L., Stouthamer, J. et al. mScarlet3: a brilliant and fast-maturing red fluorescent protein. Nat Methods 20, 541–545 (2023). https://doi.org/10.1038/s41592-023-01809-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01809-y